Abstract
Context:
Aging in men is associated with reduced testosterone (T) levels and physiological changes leading to frailty, but the benefits of T supplementation are inconclusive.
Objective:
We studied the effects of T supplementation with and without progressive resistance training (PRT) on functional performance, strength, and body composition.
Design, Setting, and Participants:
We recruited 167 generally healthy community-dwelling older men (66 ± 5 years) with low-normal baseline total T levels (200–350 ng/dL).
Intervention:
Subjects were randomized to placebo or transdermal T gel [2 doses targeting either a lower (400–550 ng/dL) or higher (600–1000 ng/dL) T range] and to either PRT or no exercise for 12 months.
Main Outcome Measure:
The primary outcome was functional performance, whereas secondary outcomes were strength and body composition.
Results:
A total of 143 men completed the study. At 12 months, total T was 528 ± 287 ng/dL in subjects receiving any T and 287 ± 65 ng/dL in the placebo group. In the PRT group, function and strength were not different between T- and placebo-treated subjects, despite greater improvements in fat mass (P = .04) and fat-free mass (P = .01) with T. In the non-PRT group, T did not improve function but improved fat mass (P = .005), fat-free mass (P = .03), and upper body strength (P = .03) compared with placebo. There were fewer cardiovascular events in the T-treated groups compared with placebo.
Conclusions:
T supplementation was well tolerated and improved body composition but had no effect on functional performance. T supplementation improved upper body strength only in nonexercisers compared with placebo.
Aging in men is associated with unfavorable changes in body composition, strength, and physical function (1–3), which may increase the risk of frailty and disability, loss of independence, reduced quality of life, and increased mortality (4). Similar alterations in young hypogonadal men are largely reversed with testosterone (T) replacement (5), suggesting that low T levels may be responsible, in part, for the physiological alterations that occur with advancing age. Decreased T production with aging is partially offset by reduced clearance, frequently resulting in low-normal T levels. Although the physiological importance and clinical approach to treatment of T levels in this low-normal range is unclear, there is considerable interest in T replacement or supplementation in aging men to preserve or improve function and prevent frailty.
A consistent finding of T treatment in older men is an increase in lean mass and a decline in fat mass (FM) (6–14). To date, most studies have shown little to no effect of T supplementation on measures of strength or physical function (6, 8–14). An important limitation of previous studies is the lack of a concurrent exercise intervention. Exercise improves body composition, strength, and physical function, and this response is maintained even into very old age (15, 16). T levels may be an important determinant of the anabolic response of muscle to exercise, creating a favorable hormonal environment that may augment exercise-induced responses in strength, power, and physical function. Although a positive association has been observed between exercise-induced increases in strength and serum T in older men (17), little is known about the potential interaction between T supplementation and strength training.
We sought to determine the independent and combined effects of 12 months of T supplementation and progressive resistance exercise training (PRT) on physical function, strength, and body composition in older men with low-normal baseline T levels (200–350 ng/dL). We also sought to determine differences in the frequency of adverse effects between lower-range (400–550 ng/dL) and higher-range (600–1000 ng/dL) T supplementation.
Subjects and Methods
Subjects
Subjects were untrained community dwelling men ≥60 years of age with an average of 2 separate baseline fasting morning total T samples between 200 and 350 ng/dL; stable medication regimen for ≥3 months; body mass index <35 kg/m2; stable weight for the past 6 months; and a Mini Mental State Examination (18) score ≥24. All volunteers were screened using a graded maximal exercise test to eliminate subjects with evidence of active coronary artery disease. Additional exclusion criteria included exercise-limiting conditions, an abnormal digital rectal examination or transrectal ultrasound, a prostate-specific antigen (PSA) above the age-adjusted normal level, an American Urological Association (AUA) symptom score (19) >20, a history of prostate or breast cancer, uncontrolled hypertension, diabetes, untreated dyslipidemia, hematocrit (HCT) >52%, use of androgenic steroids or other drugs that could affect T levels, and other clinically important illnesses or conditions that could affect the outcome measures.
Study design
The study was approved by the Colorado Multiple Institutional Review Board, and all subjects provided written informed consent. The study was designed and conducted by the study team, with industry support limited to provision of 1% T (Androgel; Abbott Laboratories, Chicago, Illinois) and matching placebo gel. The study was a 12-month, randomized, placebo-controlled trial of T supplementation crossed with PRT (3 times/wk vs none). Randomization was performed using a documented permuted block randomization with random block sizes. Subjects and investigators (except the study pharmacist and statistician) were blinded to drug treatment. Outcome measures were assessed at baseline and 26 and 52 weeks by research personnel blinded to treatment group.
The T gel and matching placebo were provided in 2.5- or 5.0-g packets. All subjects were initiated on two 2.5-g packets daily (2 placebo packets in the placebo group, 1 T gel and 1 placebo packet in the lower-range T group, and 2 T gel packets in the higher-range T group). Serum T levels were monitored by the pharmacist approximately every 2 weeks for the first 12 weeks with dose titrations made in 2.5-g increments to achieve a level of 400 to 550 ng/dL in the lower-range group and 600 to 1000 ng/dL in the higher-range group; sham adjustments were made in the placebo group. The maximum dose of T gel used was 10 g/d.
Safety monitoring included assessment of PSA, AUA score, HCT, alanine amino transferase and aspartate amino transferase at baseline and 4, 12, 26, and 52 weeks. Subjects with a confirmed increase in PSA ≥0.75 ng/mL over baseline or an AUA score ≥20 stopped the study drug until medically cleared by the study urologist and their primary healthcare provider if appropriate. Subjects with a HCT ≥54% underwent phlebotomy to lower HCT to ≤52%. If the HCT persisted at ≥54%, or was >55% at any time, the study drug dose was reduced. The Epworth Sleepiness Score (20) and arterial oxygenation by pulse oximetry were measured at baseline and 12, 26, 36, and 52 weeks to monitor for hypoxemia.
The PRT intervention included 4 upper-body (bench press, incline press, overhead pull-down, and seated row), and 3 lower-body (knee extension, knee flexion, and seated leg press) exercises. The maximal weight a participant could lift once [1-repetition maximum (1-RM)] for each exercise was assessed at baseline and 2 weeks and then monthly in the PRT groups to define the appropriate training progression. Resistance was progressively increased from 50% to 80% of the last 1-RM whenever 3 full sets could be completed. Subjects were asked to attend supervised exercise sessions 3 d/wk. Overall physical activity level was measured in all participants using the Yale Physical Activity Survey for Older Adults (21).
Outcome measures
The primary outcome was the change from baseline in the total score on the continuous-scale physical functional performance test (CS-PFP) between the placebo plus PRT group compared with any-T (lower- and higher-range groups combined) plus PRT groups. The CS-PFP comprises 15 everyday tasks requiring upper and lower body strength and flexibility, balance, coordination, and endurance (22). The CS-PFP was developed to measure performance in higher-functioning adults with minimal floor or ceiling effects and is valid, reliable, and sensitive to change (22, 23). Total and individual domain scores are scaled from 0 to 100. The stair climbing and 6-minute walk tasks of the CS-PFP were evaluated as individual measures of power and endurance, respectively.
Muscle strength was assessed by 1-RM measurements for each of the 7 exercises. Average (bilateral) grip strength was assessed with a hand dynamometer. Leg extensor power was evaluated using a Nottingham leg extensor power rig (watts). Isokinetic strength at the knee and elbow (flexion and extension at 120°), ankle (dorsiflexion and extension at 60°), and shoulder (internal and external rotation at 120°) was measure by dynamometry (newton-meters). Joints were tested in a fixed order. Body composition was measured using dual-energy x-ray absorptiometry (Hologic Discovery, Bedford, Massachusetts).
Total serum T and SHBG were measured by ELISA using a Beckman Coulter (Brea, California) Access II analyzer as previously described (24).
Adverse events were categorized as prespecified, serious (the subject sought medical attention), or nonserious. Serious events were subcategorized into cardiovascular and noncardiovascular based on review of adverse event reports and medical records by 2 study physicians blinded to treatment assignment.
Statistical analysis
The study was designed to provide >90% power with 25 subjects in each of 6 groups to detect a 0.8-SD difference between the effects of any-T vs placebo in the no-PRT group or the PRT group on the primary outcome at the 2.5% level using a 1-sided t test because the hypothesis was 1-sided. The effect size translates to a between-group difference in change over 12 months of 6.4 on the CS-PFP, 7.36 kg of lean mass, or 9.6 kg on the bench press. As prespecified, with the exception of the adverse events analysis, the lower- and higher-range T groups were combined (any-T).
The effect of any-T plus PRT on physical function at 52 weeks was tested using linear regression with a robust sandwich variance estimator to allow for heteroscedasticity. The primary analysis was conducted in the PRT groups and compared CS-PFP at 52 weeks with any-T to placebo, adjusted for baseline CS-PFP. A 1-sided P value < .025 was used to define statistical significance. Secondary analyses were planned as confirmatory and were evaluated using the model described above, with 2-sided P values < 0.05 used to define statistical significance. To address whether the effects of any-T on physical function are the same without PRT, the model was applied to the non-PRT groups and the same contrast estimated.
Because the variability in T levels may have affected the results of the study, exploratory analyses replaced the indicator variable for group with serum T levels (in 10-ng/dL increments) at 52 weeks to evaluate exposure to T with a more sensitive measure. The model included baseline and an indicator for exercise.
Baseline characteristics of the treatment groups were compared using the global F test from a 1-way ANOVA for continuous measures or a χ2 test for independent proportions for categorical measures. SAS version 9.2 (SAS Institute Inc, Cary, North Carolina) was used for all statistical analyses. All analyses were intention-to-treat. We made no adjustments for multiple testing. To avoid data-driven conclusions and control the type I error risk, all analyses were prespecified and prioritized in a statistical analysis plan filed before any data analysis.
Results
Subjects
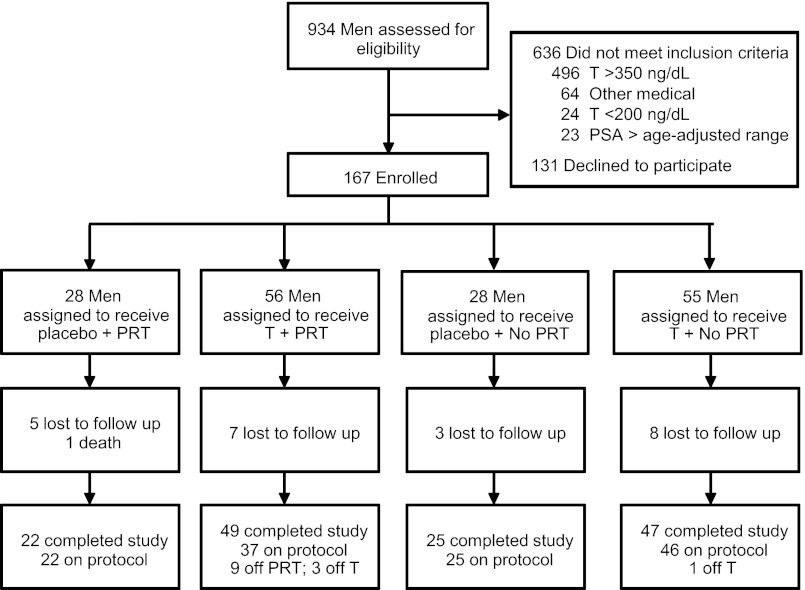
A total of 167 volunteers were randomized. Twenty-three subjects dropped out of the study and refused follow-up, 1 subject died, and 143 (86%) completed the study and were included in the analysis (Figure 1). There were no differences in baseline characteristics between subjects in the any-T and placebo groups (Table 1) or between the PRT and non-PRT groups (data not shown).
Figure 1.
The flow of subjects through the study. A total of 167 men were randomized, and 143 men completed follow-up testing and were included in the analysis.
Table 1.
Baseline Characteristics of Participants by Drug Assignmenta
| Characteristic | Placebo (n = 47) | T (any) (n = 96) |
|---|---|---|
| Age, y | 66.5 ± 5.2 | 66.5 ± 5.8 |
| Race | ||
| White | 54 (96.4%) | 103 (92.8%) |
| African-American | 1 (1.8%) | 5 (4.5%) |
| Asian | 0 (0.0%) | 1 (0.9%) |
| Other | 1 (1.8%) | 2 (1.8%) |
| Ethnicity | ||
| Non-Hispanic | 54 (96.4%) | 110 (99.1%) |
| Hispanic | 2 (3.6%) | 1 (0.9%) |
| Education, y | ||
| 8–12 | 3 (5.4%) | 5 (4.5%) |
| 13–20 | 53 (94.6%) | 106 (95.5%) |
| Systolic BP, mm Hg | 128.2 ± 16.5 | 126.1 ± 14.8 |
| Diastolic BP, mm Hg | 75.8 ± 8.5 | 75.8 ± 7.8 |
| VO2 max, ml/kg/min | 25.1 ± 4.9 | 25.3 ± 5.7 |
| Yale Physical Activity Survey, kcal/wk | 5607 ± 3031 | 5969 ± 3553 |
| Total T, ng/dL | 294.3 ± 38.8 | 297.7 ± 43.4 |
| Calculated free T, nmol/Lb | 0.2 ± 0.1 | 0.2 ± 0.0 |
| PSA, ng/mL | 1.5 ± 1.0 | 1.4 ± 0.9 |
| Hematocrit, % | 46.7 ± 3.0 | 46.3 ± 3.0 |
| AUA symptom score | 5.4 ± 4.0 | 5.5 ± 4.5 |
| Fasting glucose, mg/dL | 95.0 ± 8.9 | 95.8 ± 10.4 |
| Fasting insulin, μIU/mL | 14.5 ± 8.3 | 13.9 ± 7.4 |
| Cholesterol, mg/dL | 179.4 ± 31.8 | 173.8 ± 34.3 |
| HDL cholesterol, mg/dL | 45.4 ± 9.4 | 44.0 ± 11.9 |
| LDL cholesterol, mg/dL | 106.4 ± 28.9 | 100.3 ± 29.2 |
| Triglycerides, mg/dL | 138.1 ± 58.8 | 147.8 ± 79.0 |
| AST, U/L | 25.4 ± 5.2 | 26.2 ± 5.2 |
| ALT, U/L | 26.6 ± 7.8 | 27.6 ± 9.5 |
| TSH, μIU/mL | 2.2 ± 1.1 | 2.2 ± 1.1 |
| Chronic conditions, number | 0.41 ± 1.77 | 0.25 ± 1.32 |
| Prescription medications, number | 3.21 ± 2.39 | 2.49 ± 1.55 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; AUA, American Urologic Association; BP, blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; PSA, prostate specific antigen; VO2 max, maximal oxygen uptake.
SI conversion factors: total T, 0.0347; glucose, 0.0555; insulin, 6.945; total, HDL, and LDL cholesterol, 0.0259; and triglycerides, 0.0113.
Data are presented as mean ± SD or number (percent).
Calculated free T according to Södegård et al (41).
Treatment adherence
Adherence to the drug intervention was similar among groups. The mean possession ratio (days of gel dispensed/days in the study) was 88% ± 9% for placebo, 85% ± 15% for the lower-range T group, and 88% ± 12% for the higher-range T group. The average final T dose was 5.0 ± 2.0 g/d (interquartile range = 2.5–7.5) in the lower-range group and 8.7 ± 1.8 g/d (interquartile range = 7.5–10.0) in the higher-range group. The T dose remained stable after 12 weeks unless required by an adverse event. In the lower-range group, the average T level was 401 ± 268 ng/dL at 12 weeks and 457 ± 278 ng/dL at 52 weeks. Corresponding levels in the higher-range group were 583 ± 286 and 598 ± 385 ng/dL (P = .001 for lower- vs. higher-range T at 52 weeks).
Adherence to exercise was higher at 6 than 12 months and was not different between any-T and placebo groups at either time point. Mean attendance for the placebo plus PRT group was 9.4 ± 2.1 and 6.4 ± 3.2 sessions per month (P < .001; 78% and 53% of sessions) at 6 and 12 months, respectively, and for the any-T plus PRT group was 8.9 ± 2.9 and 7.0 ± 2.7 sessions per month (P < .001; 74% and 58% of sessions). Maximal oxygen uptake and Yale Physical Activity Survey scores did not change in any group.
Physical function
Among non-PRT subjects, those randomized to placebo had greater improvement in lower body strength compared with any-T (Table 2 and Figure 2). There was no significant improvement in physical function with any-T in either PRT or non-PRT subjects and no improvement in physical function with PRT in either any-T–treated or placebo-treated subjects.
Table 2.
Change From Baseline to 12 Months in Physical Function, Strength, and Power by Exercise Groupa
| Placebo (n = 22 PRT and 25 No PRT) |
Any-T (n = 49 PRT and 47 No PRT) |
Δ Change (95% CI) | P Value | |||
|---|---|---|---|---|---|---|
| Baseline | Change | Baseline | Change | |||
| PRT | ||||||
| Physical function (Range 0–100) | ||||||
| CS-PFP total | 69.1 ± 9.9 | 3.6 ± 8.0 | 70.5 ± 14.6 | 3.3 ± 7.4 | −0.2 (−4.2, 3.8) | .93 |
| Upper body strength | 75.8 ± 10.8 | 1.8 ± 10.9 | 78.6 ± 14.8 | 0.3 ± 11.5 | −0.7 (−6.1, 4.6) | .79 |
| Upper body flexibility | 78.2 ± 12.2 | −2.2 ± 11.0 | 75.4 ± 12.7 | 0.9 ± 9.7 | 2.3 (−2.6, 7.1) | .36 |
| Lower body strength | 64.1 ± 11.8 | 4.4 ± 9.4 | 67.3 ± 15.9 | 3.5 ± 9.4 | −0.5 (−5.5, 4.4) | .84 |
| Balance | 68.9 ± 10.8 | 4.7 ± 8.8 | 69.7 ± 15.3 | 4.6 ± 7.8 | 0.2 (−4.1, 4.5) | .93 |
| Endurance | 69.0 ± 10.5 | 4.7 ± 8.6 | 70.0 ± 15.1 | 4.5 ± 7.5 | 0.1 (−4.0, 4.3) | .95 |
| Stair climb speed, s | 4.8 ± 1.1 | −0.0 ± 0.9 | 4.7 ± 1.7 | −0.2 ± 0.8 | −0.3 (0.7, 0.1) | .16 |
| 6-min walk, m | 536.4 ± 65.0 | 24.3 ± 88.8 | 560.1 ± 132.6 | 25.6 ± 79.2 | 3.2 (−39.5, 46.0) | .88 |
| Strength (1-RM, kg) | ||||||
| Upper body | ||||||
| Bench press | 58.4 ± 17.7 | 26.9 ± 11.4 | 58.7 ± 15.9 | 25.7 ± 14.5 | −1.5 (−8.7, 5.6) | .67 |
| Incline press | 50.7 ± 15.4 | 24.9 ± 12.5 | 50.6 ± 14.6 | 23.6 ± 12.7 | −1.9 (−8.5, 4.8) | .58 |
| Overhead pulldown | 80.5 ± 20.2 | 28.7 ± 14.9 | 79.0 ± 16.6 | 26.2 ± 15.6 | −3.4 (−11.3, 4.6) | .41 |
| Seated row | 53.8 ± 14.3 | 21.3 ± 13.1 | 53.6 ± 12.3 | 20.6 ± 11.3 | −1.4 (−7.6, 4.7) | .65 |
| Avg upper body | 61.0 ± 15.6 | 25.5 ± 11.3 | 60.5 ± 14.0 | 24.3 ± 11.6 | −1.5 (−7.5, 4.5) | .63 |
| Grip strength | 41.7 ± 10.9 | 3.4 ± 6.7 | 40.5 ± 11.0 | 3.2 ± 10.3 | −1.9 (−6.3, 2.5) | .40 |
| Lower body | ||||||
| Knee extension | 65.6 ± 23.8 | 30.1 ± 18.8 | 63.1 ± 20.2 | 31.5 ± 15.2 | 0.5 (−8.0, 9.0) | .90 |
| Knee flexion | 47.6 ± 14.6 | 20.8 ± 11.3 | 49.4 ± 12.2 | 18.5 ± 12.0 | −2.6 (−8.3, 3.2) | .38 |
| Seated leg press | 135.7 ± 43.9 | 31.8 ± 27.8 | 129.3 ± 35.4 | 38.3 ± 30.5 | −0.1 (−14.0, 13.7) | .99 |
| Avg lower body | 83.9 ± 27.1 | 27.0 ± 17.0 | 80.3 ± 20.3 | 28.0 ± 19.7 | −1.6 (−10.9, 7.7) | .74 |
| Power (Power Rig, watts) | ||||||
| Leg extensor | 229.3 ± 50.8 | 24.3 ± 62.0 | 245.3 ± 68.8 | 5.1 ± 51.1 | −18.1 (−46.7, 10.5) | .21 |
| No PRT | ||||||
| Physical function (Range 0–100) | ||||||
| CS-PFP total | 68.4 ± 16.1 | 3.1 ± 6.7 | 71.6 ± 11.9 | 0.8 ± 7.3 | −2.2 (−5.8, 1.4) | .23 |
| Upper body strength | 75.2 ± 14.2 | 3.6 ± 9.0 | 77.2 ± 12.8 | 0.0 ± 8.8 | −3.5 (−7.9, 0.8) | .11 |
| Upper body flexibility | 76.5 ± 11.8 | −2.4 ± 9.5 | 78.6 ± 9.6 | −2.1 ± 9.2 | 0.7 (−3.8, 5.1) | .76 |
| Lower body strength | 64.3 ± 18.2 | 5.4 ± 7.0 | 68.4 ± 14.2 | 0.7 ± 8.7 | −4.5 (−8.7, −0.4) | .03 |
| Balance | 68.5 ± 17.5 | 2.5 ± 7.4 | 71.7 ± 12.5 | 1.4 ± 7.8 | −0.8 (−4.6, 3.0) | .68 |
| Endurance | 68.2 ± 17.0 | 2.8 ± 7.8 | 71.8 ± 12.3 | 1.5 ± 7.5 | −1.1 (−4.9, 2.7) | .57 |
| Stair climb speed, s | 5.0 ± 1.4 | −0.1 ± 0.9 | 4.7 ± 1.2 | 0.1 ± 1.0 | 0.1 (−0.4, 0.5) | .78 |
| 6-min walk, m | 536.1 ± 106.0 | 7.5 ± 93.7 | 564.5 ± 89.1 | 8.5 ± 64.9 | 0.8 (−39.0, 40.6) | .97 |
| Strength (1-RM, kg) | ||||||
| Upper body | ||||||
| Bench press | 56.5 ± 19.8 | 3.5 ± 8.9 | 60.1 ± 15.8 | 7.3 ± 9.2 | 4.7 (0.5, 9.0) | .03 |
| Incline press | 50.9 ± 19.2 | 1.9 ± 7.9 | 52.0 ± 15.0 | 8.1 ± 8.9 | 6.9 (2.7, 11.1) | .001 |
| Overhead pulldown | 77.4 ± 23.0 | 6.3 ± 11.0 | 82.5 ± 15.5 | 9.3 ± 9.6 | 3.9 (−1.2, 8.9) | .13 |
| Seated row | 50.5 ± 12.5 | 5.7 ± 7.4 | 56.1 ± 11.9 | 7.0 ± 9.6 | 2.4 (−1.9, 6.6) | .27 |
| Avg upper body | 58.8 ± 17.3 | 4.3 ± 7.7 | 62.8 ± 13.2 | 7.8 ± 6.9 | 3.9 (0.5, 7.4) | .03 |
| Grip strength | 56.5 ± 19.8 | 2.9 ± 8.0 | 39.1 ± 12.7 | 6.0 ± 12.4 | 4.1 (0.0, 8.3) | .05 |
| Lower body | ||||||
| Knee extension | 60.1 ± 25.3 | 11.0 ± 10.1 | 64.9 ± 20.4 | 12.4 ± 10.6 | 1.9 (−3.1, 6.9) | .45 |
| Knee flexion | 46.1 ± 11.8 | 5.5 ± 5.6 | 49.0 ± 10.3 | 7.1 ± 7.4 | 1.8 (−1.6, 5.2) | .30 |
| Seated leg press | 125.7 ± 38.8 | 9.1 ± 21.5 | 130.4 ± 35.0 | 11.6 ± 23.7 | 4.5 (−6.3, 15.3) | .41 |
| Avg lower body | 77.3 ± 23.7 | 9.4 ± 11.6 | 81.8 ± 20.6 | 10.5 ± 10.9 | 1.9 (−3.4, 7.3) | .48 |
| Power (Power Rig, watts) | ||||||
| Leg extensor | 212.3 ± 70.6 | 4.5 ± 34.6 | 233.3 ± 64.8 | 0.8 ± 36.9 | −1.5 (−19.1, 16.1) | .87 |
Abbreviation: CI, confidence interval.
Data are mean ± SD. Δ Change = change from baseline (any-T) minus change from baseline (placebo). P values are for placebo vs any-T, conditioned on baseline values.
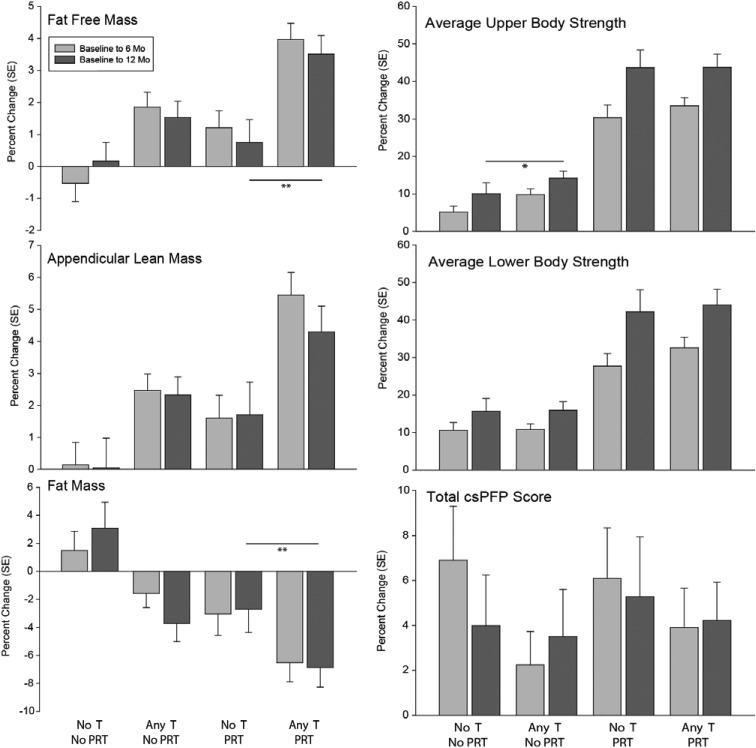
Figure 2.
Unadjusted relative mean changes from baseline at 6 and 12 months. Error bars represent SE. **, P < .05 on comparison of absolute adjusted means (Tables 2 and 3).
Muscle strength and power
Among PRT subjects, changes in 1-RM and power were not different between placebo- and any-T–treated subjects. (Table 2 and Figure 2). Among non-PRT subjects, any-T treatment was associated with greater increments in 1-RM for bench press (12.1% vs 6.2%), incline press (15.6% vs 3.7%), average upper body strength (12.4% vs 7.3%), and grip strength (15.3% vs 7.8%) compared with placebo. Changes in lower-body 1-RM, isokinetic strength, and power were not different between any-T and placebo in the non-PRT subjects.
Body composition
Among subjects randomized to PRT, those receiving any-T averaged a 1.2-kg greater decrement in FM, a 1.7-kg greater increment in fat-free mass (FFM), and a 0.3-kg greater increment in arm FFM than placebo subjects (Table 3 and Figure 2). Among non-PRT subjects, those receiving any-T averaged a 1.7-kg greater decrease in FM, a 0.9-kg greater decrease in trunk FM, a 0.6-kg greater increase in appendicular FFM, and a 0.3-kg greater increase in arm FFM compared with those receiving placebo.
Table 3.
Change from Baseline to 12 Months in Body Composition by Exercise Groupa
| Placebo (n = 22 PRT and 25 No PRT) |
Any-T (n = 49 PRT and 47 No PRT) |
Δ Change (95% CI) | P Value | |||
|---|---|---|---|---|---|---|
| Baseline | Change | Baseline | Change | |||
| PRT | ||||||
| Weight, kg | 90.6 ± 13.8 | −0.1 ± 2.8 | 90.0 ± 11.2 | 0.3 ± 2.8 | 0.4 (−1.0, 1.7) | .61 |
| Body mass index, kg/m2 | 29.5 ± 3.6 | −0.8 ± 2.9 | 28.6 ± 3.0 | 0.1 ± 0.9 | 0.7 (−0.2, 1.6) | .11 |
| FM, kg | 27.4 ± 7.1 | −0.6 ± 1.7 | 26.6 ± 6.3 | −1.8 ± 2.5 | −1.2 (−2.3, 0.0) | .04 |
| FFM, kg | 63.2 ± 8.6 | 0.4 ± 2.1 | 63.4 ± 7.0 | 2.1 ± 2.4 | 1.5 (0.4, 2.6) | .01 |
| FM trunk, kg | 16.3 ± 4.4 | −0.4 ± 1.2 | 15.6 ± 4.1 | −0.9 ± 1.7 | −0.5 (−1.3, 0.3) | .19 |
| FFM, kg | ||||||
| Appendicular | 27.8 ± 4.2 | 0.4 ± 1.3 | 28.0 ± 3.6 | 1.1 ± 1.4 | 0.6 (−0.1, 1.2) | .09 |
| Arms | 7.3 ± 1.2 | 0.2 ± 0.4 | 7.5 ± 1.1 | 0.5 ± 0.5 | 0.3 (0.0, 0.5) | .03 |
| Legs | 20.5 ± 3.1 | 0.2 ± 1.0 | 20.6 ± 2.5 | 0.6 ± 1.0 | 0.3 (−0.2, 0.8) | .20 |
| Waist circumference, cm | 105.2 ± 9.4 | −2.2 ± 4.3 | 103.7 ± 9.1 | −2.1 ± 4.4 | −0.3 (−2.5, 1.8) | .77 |
| Hip circumference, cm | 104.3 ± 8.0 | −0.6 ± 5.2 | 105.0 ± 8.1 | −2.8 ± 7.0 | −2.1 (−5.0, 0.8) | .15 |
| No PRT | ||||||
| Weight, kg | 86.9 ± 13.4 | 0.8 ± 3.3 | 90.8 ± 12.5 | −0.1 ± 3.1 | −1.0 (−2.6, 0.6) | .22 |
| Body mass index, kg/m2 | 28.3 ± 3.7 | 0.3 ± 1.1 | 29.2 ± 3.3 | −0.3 ± 1.7 | −0.5 (−1.3, 0.3) | .19 |
| FM, kg | 24.5 ± 7.6 | 0.7 ± 2.3 | 26.3 ± 6.9 | −1.0 ± 2.3 | −1.6 (−2.8, −0.5) | .005 |
| FFM, kg | 62.4 ± 7.2 | 0.1 ± 1.7 | 64.5 ± 7.4 | 1.0 ± 2.2 | 0.8 (−0.2, 1.9) | .12 |
| FM trunk, kg | 14.4 ± 4.3 | 0.5 ± 1.7 | 15.5 ± 4.2 | −0.4 ± 1.4 | −0.9 (−1.6, −0.1) | .03 |
| FFM, kg | ||||||
| Appendicular | 27.6 ± 3.3 | 0.0 ± 1.1 | 28.6 ± 3.73 | 0.6 ± 1.0 | 0.6 (0.1, 1.1) | .03 |
| Arms | 7.3 ± 1.0 | 0.0 ± 0.3 | 7.6 ± 1.1 | 0.3 ± 0.5 | 0.3 (0.0, 0.5) | .02 |
| Legs | 20.3 ± 2.4 | 0.0 ± 0.9 | 21.0 ± 2.8 | 0.3 ± 0.8 | 0.3 (−0.1, 0.7) | .13 |
| Waist circumference, cm | 102.3 ± 9.8 | −0.2 ± 4.5 | 106.3 ± 19.2 | −2.4 ± 3.6 | −2.0 (−4.1, 0.0) | .05 |
| Hip circumference, cm | 102.7 ± 11.4 | 1.6 ± 7.0 | 105.1 ± 15.0 | −1.3 ± 5.6 | −2.4 (−4.9, 0.2) | .07 |
Abbreviation: CI, confidence interval.
Data are mean ± SD. Δ Change = change from baseline (any-T) minus change from baseline (placebo). P values are for placebo vs any-T, conditioned on baseline values.
Adverse events
There were no differences in the frequency of prespecified adverse events between placebo- and any-T–treated groups except for HCT (Table 4). Compared with the lower-range group, subjects in the higher-range group were more likely to have an elevated HCT.
Table 4.
Adverse Events
| Category/Event | Number of Subjects (No. of Events) |
Pa | Pb | |||
|---|---|---|---|---|---|---|
| Placebo (n = 47) | Any-T (n = 96) | T (Lower Range) (n = 47) | T (Higher Range) (n = 49) | |||
| Prespecified adverse events | ||||||
| HCTa | 3 (3) | 13 (17) | 2 (4) | 11 (13) | .27 | .008 |
| PSAb | 9 (14) | 12 (12) | 5 (9) | 7 (13) | .46 | .57 |
| AUA symptom scorec | 2 (2) | 1 (1) | 1 (1) | 0 (0) | .55 | 1.00 |
| Liver function testsd | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Somnolence/hypoxiae | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Serious adverse events | ||||||
| Cardiovascular | ||||||
| Acute coronary syndrome | 3 (3) | 1 (1) | 0 (0) | 1 (1) | .30 | .50 |
| Arrhythmia | 4 (4) | 0 (0) | 0 (0) | 0 (0) | .01 | |
| Aortic aneurysm | 1 (1) | 0 (0) | 0 (0) | 0 (0) | .34 | |
| Syncope/presyncope | 2 (3) | 2 (3) | 2 (3) | 0 (0) | .60 | .50 |
| Total cardiovascular | 10 (11) | 3 (4) | 2 (3) | 1 (1) | .001 | 1.00 |
| Other (noncardiovascular) | 20 (53) | 68 (119) | 31 (55) | 37 (64) | .003 | .24 |
| All other nonserious adverse events | ||||||
| Total | 9 (12) | 13 (13) | 6 (6) | 7 (7) | .47 | .78 |
Pa = exact likelihood ratio χ2 test for equal proportions placebo vs any-T, number of subjects. Pb = exact likelihood ratio χ2 test for equal proportions lower-range T vs higher-range T, number of subjects.
HCT ≥54%.
Increase of ≥0.75 ng/mL over baseline at any time point (confirmed by a second measurement in 2 weeks).
AUA symptom score ≥20.
Alanine aminotransferase or aspartate aminotransferase >2 times the upper limit of normal.
Somnolence = Epworth Sleepiness Score (20) >16; hypoxia is defined as arterial oxygen saturation by pulse oximetry <88%.
We observed no significant increases in PSA in any group. At 12 months, the mean PSA was 1.49 (range 0.21–4.70) ng/mL in the placebo groups, 1.56 (0.21–4.50) ng/mL in the lower-range T group, and 1.78 (0.50–4.60) ng/mL in the higher-range T group. Thirty subjects were referred for urological evaluation; 2 of these had a prostate biopsy. There were no cases of prostate cancer.
More subjects in the any-T–treated groups reported serious adverse events. However, there were fewer cardiovascular events among any-T–treated than among placebo-treated subjects (P value = .001).
Discussion
In otherwise healthy older men with low-normal baseline T levels, any-T supplementation and PRT independently decreased FM and increased FFM and upper body strength, but neither had an effect on physical function. Although the combination of any-T plus PRT produced greater changes in body composition than PRT alone, there were no additive effects of any-T plus PRT on strength or physical function.
Physical function
Physical function assessed by CS-PFP total score did not improve with any-T supplementation, either alone or combined with PRT. This is consistent with previous studies of T supplementation alone reporting no improvements in physical function (measured via stair climb, timed up-and-go, or walking speed) in healthy (7, 8, 25) and frail older men (12) and with studies of T combined with resistance exercise in younger HIV-positive men with serum T <349 ng/dL (26) and in frail elderly men with serum T <480 ng/dL (27). In contrast, improvement in physical function has been demonstrated with 6 months of T gel (10 g/d) in men ≥65 years with mobility limitations and serum T 100 to 350 ng/dL (28), with 6 months of T gel (0.05 g/d) in frail men ≥75 years with serum T <346 ng/dL (11) and with 13 months of im T alone or combined with finasteride in healthy men ≥65 years with serum T < 350 ng/dL (9). The reasons for the lack of improvements in the present study and the inconsistencies in the literature are unclear. In the present study, PRT alone did not improve CS-PFP scores. PRT alone and combined with endurance training has previously been shown to have modest effects on physical function, as measured by improved gait speed and chair rise and CS-PFP scores in older adults with and without functional limitations (22, 29). This may reflect the lack of an endurance component and/or suboptimal adherence to the PRT intervention. Participants in our study also had a high baseline level of physical function, with CS-PFP total scores close to those reported in healthy adults 35 to 44 years of age (30). By comparison, scores of 54.2 ± 11.0 and 23.6 ± 8.7 have been reported for community-dwelling older adults and long-term care residents with minimal dependency, respectively (22). The CS-PFP has not previously been used in studies of T treatment and may not measure aspects of physical function responsive to T treatment. This test may also lack sufficient sensitivity to detect small changes associated with T treatment.
Muscle strength and power
We observed improvements in strength with both any-T and PRT but found no additional improvements in strength with T plus PRT compared with either intervention alone. The improvements in strength with any-T alone compared with placebo were limited to the upper body, which supports other controlled trials of T supplementation in healthy older men (9, 12, 25, 31). This may reflect the higher level of regular exercise of the lower body in healthy older adults, However, Bhasin et al (32) found improvements in leg press strength in healthy older men that were correlated with both T dose and level. In the present study, exploratory analyses revealed a similar effect of serum T level achieved on the improvement in leg press strength but no apparent effect of T dose or serum T level on any other strength measures (Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org).
Although the effects of PRT on muscle strength in older adults are well established (29, 33), to our knowledge, this is the first trial to examine the effects of T plus PRT in generally healthy older men with low-normal serum T. An association between exercise-induced increases in strength and serum T in older men (17) suggests that T administration may augment muscle hypertrophy with PRT in older men, producing greater gains in strength. In young men, supraphysiological T supplementation plus weight training produced greater improvements in upper and lower body strength than either T or exercise alone (34). However, in a population of young HIV-infected men with weight loss and serum T <349 ng/dL, both im T and resistance exercise increased muscle mass and strength, but the combination resulted in no additional gains (26). Sullivan et al (27) also found a trend toward greater strength with 12 weeks of im T supplementation in frail elderly men with serum T <480 ng/dL but no augmentation of these effects with resistance exercise. In the present study, the increases in strength with PRT were comparable to those seen with similar PRT programs in older adults (33); however, T treatment did not augment the effects of PRT on strength. Although the primary analyses were not set up for a direct test of interaction between T and PRT, a model combining all 6 groups to test for main effects of T, PRT, and their interaction for bench press showed no interaction (data not shown). The lack of an additive effect of T with PRT raises the possibility of a common mechanistic pathway (eg, inhibition of myostatin) that should be investigated in future studies (35, 36).
Body composition
In the present study, older men randomized to any-T alone achieved an average 1.0-kg decrease in FM and 1.0-kg increase in FFM, compared with an average 2.0-kg decrease and 2.7-kg increase, respectively, reported in a systematic review of T therapy in older men (37). Participants randomized to placebo plus PRT had a 0.6-kg decrease in FM and a 0.4-kg increase in FFM, somewhat less than found in a recent meta-analysis of PRT in older adults (38). The combination of T plus PRT produced greater improvements in FM and FFM than placebo plus PRT. The effects of T with or without PRT were more pronounced in upper body FFM, which supports our finding with respect to strength. Previous studies of the combined effects of T plus PRT have been limited to young healthy men, chronically ill HIV-positive men, and older men in combination with megesterol acetate, with inconclusive results (26, 34, 39). To our knowledge, this is the first study to show that T treatment may augment PRT-induced changes in body composition in generally healthy older men.
It is unclear why the favorable changes in body composition consistently demonstrated with T supplementation in older men have not reliably translated into gains in overall strength or physical function. A threshold level of T may be needed to achieve increases in FFM sufficient to enhance strength and physical function (14). Because only 32% of men in the lower-range and 20% of men in the higher-range T group achieved target T levels at 52 weeks, we may not have reached the threshold for sufficient increases in FFM. Furthermore, the measured T levels were highly variable, indicating significant variability in absorption and/or adherence. The lack of ongoing regular dosage titration makes actual T exposure difficult to assess. Although the study was not powered to examine lower- and higher-range T groups separately, exploratory analyses by T group and with T level as a continuous variable did not affect the primary conclusions (Supplemental Tables 1 and 2).
Adverse events
Other than dose-dependent elevations in HCT, there were no differences in adverse events with lower-range compared with higher-range T. There were more serious adverse events reported in the any-T–treated groups compared with placebo. These were defined as any complaint for which the subject sought any medical attention; thus, the clinical significance is uncertain. In contrast to the Testosterone in Older Men with Mobility Limitations (TOM) trial (40), we observed fewer cardiovascular events in the any-T–treated groups compared with placebo, which may reflect the healthier population in the present study. T supplementation may be of most interest in older, frail men likely to have cardiovascular risk factors, and the safety of long-term use in this population remains unclear.
Conclusions
In this study of generally healthy older men with low-normal T levels, 52 weeks of T supplementation, PRT, and T plus PRT improved body composition and upper body strength but not physical function. Although T plus PRT produced greater improvements in body composition than either intervention alone, this did not translate into enhanced strength or physical function. T supplementation was well tolerated and associated with a lower rate of cardiovascular endpoints in this population. Whether T supplementation with or without PRT improves physical function in specific populations of older men requires further study.
Acknowledgments
This work was supported by the National Institute on Aging (NIA) R01 AG019339.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AUA
- American Urological Association
- CS-PFP
- continuous-scale physical functional performance test
- FFM
- fat-free mass
- FM
- fat mass
- HCT
- hematocrit
- PRT
- progressive resistance exercise training
- PSA
- prostate-specific antigen
- 1-RM
- 1-repetition maximum
- T
- testosterone.
References
- 1. Porter MM, Vandervoort AA, Lexell J. Aging of human muscle: structure, function and adaptability. Scand J Med Sci Sports. 1995;5:129–142 [DOI] [PubMed] [Google Scholar]
- 2. Schwartz RS, Shuman WP, Bradbury VL, et al. Body fat distribution in healthy young and older men. J Gerontol. 1990;45:M181–M185 [DOI] [PubMed] [Google Scholar]
- 3. Larsson L, Grimby G, Karlsson J. Muscle strength and speed of movement in relation to age and muscle morphology. J Appl Physiol. 1979;46:451–456 [DOI] [PubMed] [Google Scholar]
- 4. Fried L. The Principles of Geriatric Medicine and Gerontology. 3rd ed New York, NY: McGraw-Hill; 1994 [Google Scholar]
- 5. Bhasin S, Storer TW, Berman N, et al. Testosterone replacement increases fat-free mass and muscle size in hypogonadal men. J Clin Endocrinol Metab. 1997;82:407–413 [DOI] [PubMed] [Google Scholar]
- 6. Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in adult men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2006;91:1995–2010 [DOI] [PubMed] [Google Scholar]
- 7. Emmelot-Vonk MH, Verhaar HJ, Nakhai Pour HR, et al. Effect of testosterone supplementation on functional mobility, cognition, and other parameters in older men: a randomized controlled trial. JAMA. 2008;299:39–52 [DOI] [PubMed] [Google Scholar]
- 8. Storer TW, Woodhouse L, Magliano L, et al. Changes in muscle mass, muscle strength, and power but not physical function are related to testosterone dose in healthy older men. J Am Geriatr Soc. 2008;56:1991–1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Page ST, Amory JK, Bowman FD, et al. Exogenous testosterone (T) alone or with finasteride increases physical performance, grip strength, and lean body mass in older men with low serum T. J Clin Endocrinol Metab. 2005;90:1502–1510 [DOI] [PubMed] [Google Scholar]
- 10. Sattler FR, Castaneda-Sceppa C, Binder EF, et al. Testosterone and growth hormone improve body composition and muscle performance in older men. J Clin Endocrinol Metab. 2009;94:1991–2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Srinivas-Shankar U, Roberts SA, Connolly MJ, et al. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2010;95:639–650 [DOI] [PubMed] [Google Scholar]
- 12. Kenny AM, Kleppinger A, Annis K, et al. Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels, low bone mass, and physical frailty. J Am Geriatr Soc. 2010;58:1134–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. O'Connell MD, Roberts SA, Srinivas-Shankar U, et al. Do the effects of testosterone on muscle strength, physical function, body composition, and quality of life persist six months after treatment in intermediate-frail and frail elderly men? J Clin Endocrinol Metab. 2011;96:454–458 [DOI] [PubMed] [Google Scholar]
- 14. Sattler F, Bhasin S, He J, et al. Testosterone threshold levels and lean tissue mass targets needed to enhance skeletal muscle strength and function: the HORMA trial. J Gerontol A Biol Sci Med Sci. 2011;66:122–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jozsi AC, Campbell WW, Joseph L, Davey SL, Evans WJ. Changes in power with resistance training in older and younger men and women. J Gerontol A Biol Sci Med Sci. 1999;54:M591–M596 [DOI] [PubMed] [Google Scholar]
- 16. Hasten DL, Pak-Loduca J, Obert KA, Yarasheski KE. Resistance exercise acutely increases MHC and mixed muscle protein synthesis rates in 78–84 and 23–32 yr olds. Am J Physiol Endocrinol Metab. 2000;278:E620–E626 [DOI] [PubMed] [Google Scholar]
- 17. Hakkinen K, Pakarinen A. Serum hormones and strength development during strength training in middle-aged and elderly males and females. Acta Physiol Scand. 1994;150:211–219 [DOI] [PubMed] [Google Scholar]
- 18. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198 [DOI] [PubMed] [Google Scholar]
- 19. Barry MJ, Fowler FJ, Jr, O'Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148:1549–1557 [DOI] [PubMed] [Google Scholar]
- 20. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545 [DOI] [PubMed] [Google Scholar]
- 21. Dipietro L, Caspersen CJ, Ostfeld AM, Nadel ER. A survey for assessing physical activity among older adults. Med Sci Sports Exerc. 1993;25:628–642 [PubMed] [Google Scholar]
- 22. Cress ME, Buchner DM, Questad KA, Esselman PC, deLateur BJ, Schwartz RS. Exercise: effects on physical functional performance in independent older adults. J Gerontol A Biol Sci Med Sci. 1999;54:M242–248 [DOI] [PubMed] [Google Scholar]
- 23. Cress ME, Buchner DM, Questad KA, Esselman PC, deLateur BJ, Schwartz RS. Continuous-scale physical functional performance in healthy older adults: a validation study. Arch Phys Med Rehab. 1996;77:1243–1250 [DOI] [PubMed] [Google Scholar]
- 24. Jankowski CM, Gozansky WS, Kittelson JM, Van Pelt RE, Schwartz RS, Kohrt WM. Increases in bone mineral density in response to oral dehydroepiandrosterone replacement in older adults appear to be mediated by serum estrogens. J Clin Endocrinol Metab. 2008;93:4767–4773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Snyder PJ, Peachey H, Hannoush P, Berlin JA, Loh L, Lenrow DA, Holmes JH, Dlewati A, Santanna J, Rosen CJ, Strom BL. Effect of testosterone treatment on body composition and muscle strength in men over 65 years of age. J Clin Endocrinol Metab. 1999;84:2647–2653 [DOI] [PubMed] [Google Scholar]
- 26. Bhasin S, Storer TW, Javanbakht M, et al. Testosterone replacement and resistance exercise in HIV-infected men with weight loss and low testosterone levels. JAMA. 2000;283:763–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sullivan DH, Roberson PK, Johnson LE, et al. Effects of muscle strength training and testosterone in frail elderly males. Med Sci Sports Exerc. 2005;37:1664–1672 [DOI] [PubMed] [Google Scholar]
- 28. Travison TG, Basaria S, Storer TW, et al. Clinical meaningfulness of the changes in muscle performance and physical function associated with testosterone administration in older men with mobility limitation. J Gerontol A Biol Sci Med Sci. 2011;66:1090–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu CJ, Latham NK. Progressive resistance strength training for improving physical function in older adults. Cochrane Database Syst Rev. 2009:CD002759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hearty TM, Schenkman ML, Kohrt WM, Cress ME. Continuous scale physical functional performance test: appropriateness for middle-aged adults with and without Parkinson's disease. J Neurol Phys Ther. 2007;31:64–70 [DOI] [PubMed] [Google Scholar]
- 31. Kenny AM, Prestwood KM, Gruman CA, Marcello KM, Raisz LG. Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels. J Gerontol A Biol Sci Med Sci. 2001;56:M266–M272 [DOI] [PubMed] [Google Scholar]
- 32. Bhasin S, Woodhouse L, Casaburi R, et al. Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J Clin Endocrinol Metab. 2005;90:678–688 [DOI] [PubMed] [Google Scholar]
- 33. Peterson MD, Rhea MR, Sen A, Gordon PM. Resistance exercise for muscular strength in older adults: a meta-analysis. Ageing Res Rev. 2010;9:226–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bhasin S, Storer TW, Berman N, et al. The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N Engl J Med. 1996;335:1–7 [DOI] [PubMed] [Google Scholar]
- 35. Philp A, Hamilton DL, Baar K. Signals mediating skeletal muscle remodeling by resistance exercise: PI3-kinase independent activation of mTORC1. J Appl Physiol. 2011;110:561–568 [DOI] [PubMed] [Google Scholar]
- 36. Kovacheva EL, Hikim AP, Shen R, Sinha I, Sinha-Hikim I. Testosterone supplementation reverses sarcopenia in aging through regulation of myostatin, c-Jun NH2-terminal kinase, Notch, and Akt signaling pathways. Endocrinology. 2010;151:628–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bhasin S, Calof OM, Storer TW, et al. Drug insight: Testosterone and selective androgen receptor modulators as anabolic therapies for chronic illness and aging. Nat Clin Pract Endocrinol Metab. 2006;2:146–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Peterson MD, Sen A, Gordon PM. Influence of resistance exercise on lean body mass in aging adults: a meta-analysis. Med Sci Sports Exerc. 2011;43:249–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lambert CP, Sullivan DH, Freeling SA, Lindquist DM, Evans WJ. Effects of testosterone replacement and/or resistance exercise on the composition of megestrol acetate stimulated weight gain in elderly men: a randomized controlled trial. J Clin Endocrinol Metab. 2002;87:2100–2106 [DOI] [PubMed] [Google Scholar]
- 40. Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363:109–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Södergård R, Bäckström T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17β to human plasma proteins at body temperature. J Steroid Biochem. 1982;16:801–810 [DOI] [PubMed] [Google Scholar]