Abstract
Context:
Little is known about practice patterns in thyroid cancer, a cancer that is increasing in incidence.
Objective:
We sought to identify aspects of thyroid cancer management that have the greatest variation.
Design/Setting/Participants:
We surveyed 944 physicians involved in thyroid cancer care from 251 hospitals affiliated with the US National Cancer Database. Physicians were asked questions in the following four domains: thyroid surgery, radioactive iodine use, thyroid hormone replacement postsurgery, and long-term thyroid cancer management. We calculated the ratio of observed variation to hypothetical maximum variation under the assumed distribution of the response. Ratios closer to 1 indicate greater variation.
Results:
We had a 66% response rate. We found variation in multiple aspects of thyroid cancer management, including the role of central lymph node dissections (variation, 0.99; 95% confidence interval [CI], 0.98–1.00), the role of pretreatment scans before radioactive iodine treatment (variation, 1.00; 95% CI, 0.98–1.00), and all aspects of long-term thyroid cancer management, including applications of ultrasound (variation, 0.97; 95% CI, 0.93–0.99) and radioactive iodine scans (variation, 0.99; 95% CI, 0.97–1.00). For the management of small thyroid cancers, variation exists in all domains, including optimal extent of surgery (variation, 0.91; 95% CI, 0.88–0.94) and the role of both radioactive iodine treatment (variation, 0.91; 95% CI, 0.89–0.93) and suppressive doses of thyroid hormone replacement (variation, 1.00; 95% CI, 0.99–1.00).
Conclusion:
We identified areas of variation in thyroid cancer management. To reduce the variation and improve the management of thyroid cancer, there is a need for more research and more research dissemination.
Thyroid cancer is now one of the 10 most common cancers in the United States (1, 2). For more than a decade, the incidence of thyroid cancer has been rising at a faster rate than that of any other malignancy, (3), and there is no indication that this rise in incidence is going to plateau in the near future.
Despite being an increasingly common malignancy, little is known about current treatment practices in thyroid cancer. The recommended management of well-differentiated thyroid cancer, which includes the most common form of thyroid malignancy, is surgery, sometimes followed by radioactive iodine and suppressive doses of thyroid hormone replacement. However, because of limited and conflicting data (4–9), much of the management is left to physician discretion (10). It is clear that treatment intensity has escalated over time (11–13) and that there is variation in treatment according to nonclinical factors (12). However, consistency in physician knowledge, attitudes, and beliefs has not been assessed. It is not clear whether there is variation in specific aspects of thyroid surgery, radioactive iodine use, thyroid hormone replacement postsurgery, or long-term thyroid cancer management.
To determine the aspects of thyroid cancer management with the greatest variation, we surveyed 944 physicians involved in thyroid cancer care.
Materials and Methods
Data source and study sample
We selected the 1159 Commission on Cancer–accredited programs that reported treating thyroid cancer patients for a minimum of 4 years between 2004 and 2008 to the National Cancer Data Base (NCDB), a joint project of the American College of Surgeons and the American Cancer Society. We eliminated the hospitals with the lowest thyroid cancer case volume (n = 235). We then had a cohort of 924 hospitals, which represented 96% of the original patient sample. We randomly sampled 589 of these 924 hospitals based on previous power calculations. We then contacted the registrars and searched the web sites of the randomly sampled hospitals to identify the surgeons who performed most of the thyroid cancer procedures at each hospital. We surveyed 850 surgeons.
These surgeons were asked to “Please list the names, specialties, and hospital affiliations of the physicians who provide care to your thyroid cancer patients or administer radioactive iodine when needed.” As shown in Figure 1, the surgeon respondents identified 903 endocrinologists, nuclear medicine providers, and other physicians involved in thyroid cancer management. This cohort was the subject of a second survey. Identical in each survey were descriptions of several clinical vignettes and questions.
Figure 1.
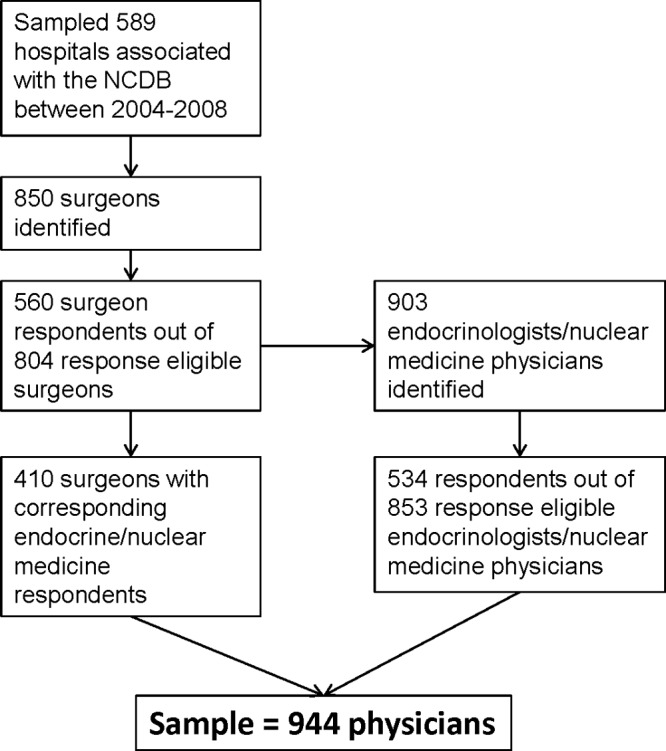
Sampling method and subject flow.
For both surveys, we used the modified Dillman survey method for dissemination (14). This includes an initial mailing with an introductory letter, the survey instrument with a promise of anonymity in our reports, a postage-paid return envelope, and a small gift. Three weeks later, we mailed a postcard reminder. Two weeks after the postcard reminder, we mailed a second identical survey with postage-paid return envelopes to all nonresponders.
Data from the surveys were deidentified and entered in a database. Physicians from hospitals that had a minimum of 1 respondent from the surgeon survey and 1 respondent from the endocrine/nuclear medicine survey were included in the cohort for analyses. Surgeon and endocrine/nuclear medicine surveys were merged by hospital identifier to create the analytic dataset. This study was granted exemption by the University of Michigan Institutional Review Board.
Measures
Before either survey was administered, the instruments were designed and pilot tested for pertinence, clarity, and consistency in a multidisciplinary group of providers.
Survey items and clinical vignettes (which included 6-point Likert scales) covered the following four domains: thyroid surgery, radioactive iodine use, thyroid hormone replacement postsurgery, and long-term thyroid cancer management. Aspects of thyroid cancer management included in both surveys consisted of the following: extent of resection for an 0.8-cm cancer, extent of resection for an 0.8-cm cancer with worrisome lymph nodes seen preoperatively, central lymph node dissection regardless of tumor size, central lymph node dissection with tumor size >1 cm, central lymph node dissection with suspicious lymph nodes seen preoperatively, radioactive iodine for an 0.8-cm cancer, radioactive iodine for a 1.9-cm cancer without lymph node metastases, and radioactive iodine for a 1.9-cm cancer with lymph node metastases. Aspects of thyroid cancer management included only in the endocrinology/nuclear medicine survey were the following: pretreatment scan for patients with thyroid cancer, pretreatment scan for an 0.8-cm thyroid cancer, treatment method for distant metastases (fixed dose, dosimetry, or no radioactive iodine), and the opinion of respondents on use of thyroid hormone replacement to suppress TSH levels below the normal range for an 0.8-cm cancer, a 1.1- to 2-cm cancer, or a 1.9-cm cancer with lymph node involvement and when distant metastases are present. Also included in the endocrine/nuclear medicine survey were aspects of long-term management. Physicians were asked which of the following tests they routinely schedule during the first year after the initial thyroid surgery and radioactive iodine treatment for all patients with well-differentiated thyroid cancer and then specifically for patients with American Joint Committee on Cancer (AJCC) stage I thyroid cancer with an undetectable serum thyroglobulin level when the TSH concentration is below the normal range. Options included a serum thyroglobulin level when the serum TSH concentration is below normal range (ie, random thyroglobulin), a stimulation of thyroglobulin (includes recombinant human thyrotropin or withdrawal of thyroid hormone replacement), ultrasound examination of the neck (bedside or with in the radiology department), and a diagnostic whole-body radioactive iodine scan.
Statistical analyses
Frequency distributions of survey items and clinical vignettes covered under each of the four domains were evaluated. We analyzed aspects of thyroid cancer management that were included in both surveys as well as those that were included only in the endocrine/nuclear medicine survey. To identify aspects of thyroid cancer management that have the greatest variation, we calculated observed variation relative to hypothetical maximum variation under the assumed distribution of the response. For binary responses, observed variation was calculated as np(1 − p), where n is the number of survey responses for the specific item and p is the proportion of “yes” response on the specific survey item. Because for a binomial distribution, the variance is maximized at P = .5, the ratio of observed to hypothetical maximum variation was calculated as 4p(1 − p) for the binary response items.
For a multinomial response with K categories, the observed dispersion matrix is given by
where n again is the number of survey responses for the specific item, and pi is the proportion of responses in the ith category of the specific multinomial item, i = 1, 2, … K. Variation in the specific aspect of management for such a response is maximized at pi = 1/K. Thus, the dispersion matrix under maximum variation is given by
Because we were interested in capturing the total variability in each marginal, we used the ratio of the traces of Dobs and Dmax as the criterion for identifying items that have the greatest variation. Ratios closer to 1 indicate higher variation. In addition to the point estimates, we obtained 95% confidence intervals (CIs) for the trace ratios using the bootstrap resampling technique. Five hundred random bootstrap samples with replacement and of the same size as the original sample were drawn from the original dataset for each response item. Trace ratios were calculated based on the above method for each bootstrap run. The 25th and 97th percentile points of the empirical distribution of the trace ratios were used to construct the lower and upper limits of the corresponding 95% CI. All analyses were performed using R.
In a subgroup analysis, we used χ2 tests to compare answers from surgeons vs nonsurgeons, physicians in academic practice settings vs physicians in nonacademic practices settings, and physicians who read the 2009 revised American Thyroid Association guidelines vs those who had not read them.
Results
We had a 70% (560 of 804) response rate for the surgeon survey and a 63% (534 of 853) response rate for the endocrinology/nuclear medicine survey (the total response rate was 66% [1094 of 1657]). We evaluated the distribution of the responders vs nonresponders, and there was no significant difference based on region. Of the survey respondents, 19% were from the East North Central region, 8% from the East South Central region, 15% from the Mid Atlantic region, 6% from the Mountain region, 7% from the New England region, 12% from the Pacific region, 19% from the South Atlantic region, 9% from the West North Central, and 5% from the West South Central region. Of the 560 respondent surgeons, 410 had at least 1 corresponding endocrine/nuclear medicine/other respondent. As shown in Figure 1, the analyses cohort included 944 physicians from 251 hospitals.
Table 1 shows the respondent characteristics. Surgeons represented 43% of the respondents, endocrinologists 38%, nuclear medicine providers 9%, and other providers 10%. Most of the respondents worked in private practice (59%) but 25% worked in academic practices and 16% in community-based academic affiliate practices. Although 76% had read the 2009 Revised American Thyroid Association (ATA) Management Guidelines for Patients with Thyroid Nodules and Differentiated Thyroid Cancer (10), only 31% had read the 2010 National Comprehensive Cancer Network (NCCN) Thyroid Carcinoma Clinical Practice Guidelines in Oncology (15).
Table 1.
Respondent Characteristics (n = 944 From 251 Hospitals)
| Characteristic | Value |
|---|---|
| Age, y, mean ± SD | 51.47 ± 10.64 |
| Time in practice, mean ± SD | 19.33 ± 10.36 |
| Sex, n (%) | |
| Male | 747 (81) |
| Female | 179 (19) |
| Race, n (%) | |
| White | 731 (81) |
| Black | 15 (2) |
| American Indian/Alaska Native | 2 (0) |
| Asian | 130 (14) |
| Other | 31 (3) |
| Ethnicity, n (%) | |
| Hispanic | 35 (4) |
| Specialization, n (%) | |
| Surgery | 410 (43) |
| Endocrinology | 359 (38) |
| Nuclear medicine | 81 (9) |
| Other | 92 (10) |
| Practice setting, n (%) | |
| Academic | 222 (25) |
| Community-based academic affiliate | 143 (16) |
| Private practice | 527 (59) |
| Read clinical guidelines, n (%) | |
| 2009 ATAa | 716 (75.9) |
| 2010 NCCNb | 292 (30.9) |
Table 2 shows the distribution of answers within the domains of thyroid cancer surgery, radioactive iodine use, thyroid hormone replacement postsurgery, and long-term thyroid cancer management. Fewer than 30% of survey respondents recommend thyroid lobectomy for an 0.8-cm cancer. Regarding the use of central lymph node dissection, 20% recommend this procedure regardless of tumor size, 47% recommend it when the tumor size is >1 cm, and 85% recommend it when suspicious lymph nodes are seen preoperatively. Close to half of respondents recommend pretreatment scans for any patient with thyroid cancer (48%), but a smaller proportion (23%) recommend a pretreatment scan when the tumor is 0.8 cm and intrathyroidal. Although answers crossed the spectrum of response options, more physicians are against use of radioactive iodine for an 0.8-cm intrathyroidal cancer and more are in favor of radioactive iodine for a 1.9-cm intrathyroidal cancer. The proportion strongly in favor of radioactive iodine increases from 5% for an 0.8-cm intrathyroidal cancer to 35% for a 1.9-cm intrathyroidal cancer without lymph node metastases to 83% for a 1.9-cm cancer with lymph node metastases. Of the endocrinologists/nuclear medicine/other providers surveyed, 68% recommend a fixed dose of radioactive iodine for a patient with pulmonary metastases and extensive local disease, and 31% recommend dosimetry-guided radioactive iodine. Whereas close to half of relevant respondents recommend keeping the TSH level below the normal range (ie, suppress TSH) for an 0.8-cm intrathyroidal cancer (50%) and a 1.1- to 2-cm intrathyroidal cancer (57%), the overwhelming majority would suppress the TSH level if there is lymph node involvement (92%) or distant metastases (97%).
Table 2.
Response Distribution
| Thyroid Cancer Surgery |
|||
|---|---|---|---|
| Total Thyroidectomy | Total Thyroidectomy + CLND | Thyroid Lobectomy | |
| Extent of resection for 0.8-cm cancer | 483 (52.7) | 206 (22.4) | 228 (24.9) |
| Extent of resection for 0.8-cm cancer with suspicious LNs seen preoperatively | 686 (72.9) | 255 (27.1) | |
| No CLND | + CLND | |
|---|---|---|
| CLND regardless of tumor size | 750 (79.7) | 191 (20.3) |
| CLND with tumor size >1 cm | 503 (53.4) | 438 (46.6) |
| CLND with suspicious LNs preoperatively | 145 (15.4) | 796 (84.6) |
| RAI for Thyroid Cancer |
||
|---|---|---|
| Yes | No | |
| Pretreatment scan for thyroid cancer patientsa | 234 (48.4) | 250 (51.6) |
| Pretreatment scan specifically for 0.8-cm thyroid cancera | 115 (22.7) | 392 (77.3) |
| Strongly Against | Moderately Against | Weakly Against | Weakly for | Moderately for | Strongly for | |
|---|---|---|---|---|---|---|
| RAI for 0.8-cm cancer | 266 (29.6) | 305 (34.0) | 89 (9.9) | 99 (11.0) | 95 (10.6%) | 44 (4.9%) |
| RAI for 1.9-cm cancer | 28 (3.1) | 62 (6.9) | 49 (5.4) | 118 (13.1) | 328 (36.4%) | 317 (35.1%) |
| RAI for 1.9-cm cancer +LN metastasis | 2 (0.2) | 12 (1.3) | 6 (0.7) | 20 (2.2) | 116 (12.8%) | 751 (82.8%) |
| Fixed Dose of RAI | Dosimetry | None | |
|---|---|---|---|
| Treatment method for distant metastases* | 350 (68.0) | 161 (31.2) | 4 (0.8) |
| Thyroid Hormone Replacement for Thyroid Cancer |
||
|---|---|---|
| Yes | No | |
| Suppress TSH for 0.8-cm cancera | 254 (50) | 254 (50) |
| Suppress TSH for 1.1- to 2-cm cancera | 300 (56.5) | 231 (43.5) |
| Suppress TSH for 1.9-cm cancer and LN involvementa | 465 (91.9) | 41 (8.1) |
| Suppress TSH if distant metastasesa | 493 (97.2) | 14 (2.8) |
| Long-Term Management of Thyroid Cancer |
||
|---|---|---|
| Yes | No | |
| Tests routinely scheduled during the first year after thyroid surgery | ||
| Random tgba | 371 (70) | 159 (30) |
| Stimulated tgba | 196 (37.0) | 334 (63.0) |
| Ultrasounda | 215 (41.4) | 305 (58.6) |
| Ultrasound for stage I thyroid cancer with tgb <0.5a | 237 (45.4) | 283 (54.6) |
| Diagnostic whole-body RAI scana | 286 (54.0) | 244 (46.0) |
| Diagnostic whole-body RAI scan for stage I thyroid cancer with tgb <0.5a | 157 (29.6) | 373 (70.4) |
Data are n (%). Abbreviations: CLND, central lymph node dissection; LN, lymph node; RAI, radioactive iodine; tgb, thyroglobulin.
Only in endocrine/nuclear medicine survey (n = 534).
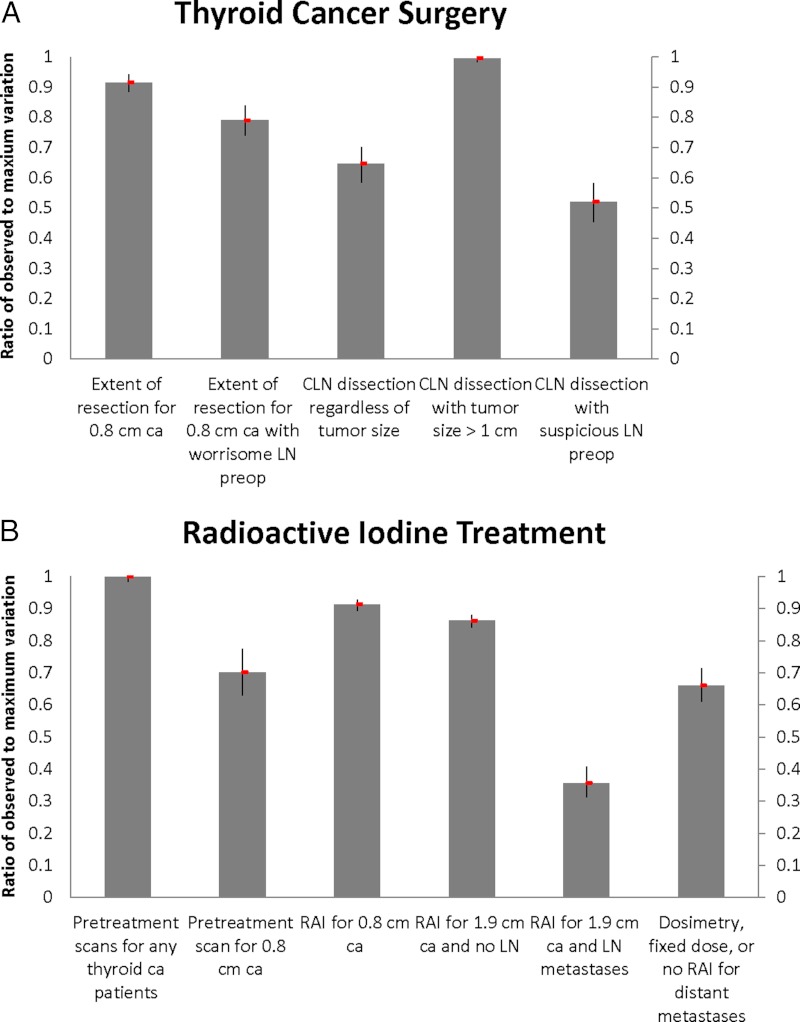
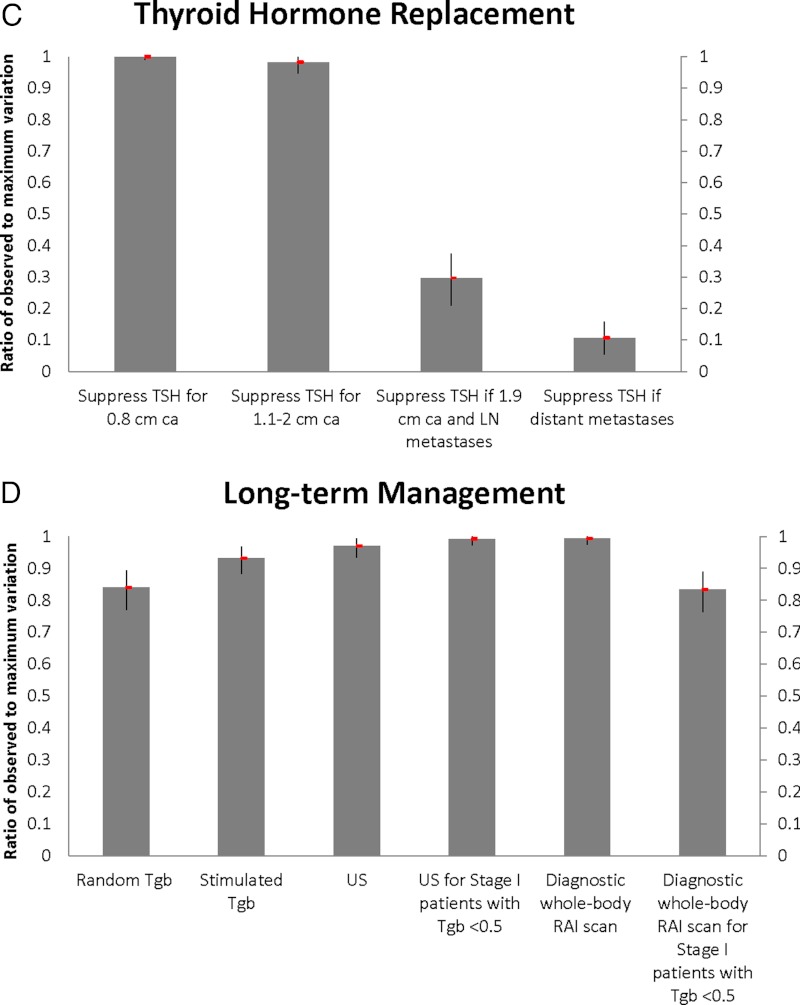
Figure 2 demonstrates the observed-to-maximum variation ratios for aspects of thyroid cancer management within the four specified domains. There is marked variation over the role of central lymph node dissection, especially in patients with tumor size >1 cm (0.99; 95% CI, 0.98–1.00), and extent of resection for an 0.8-cm intrathyroidal cancer (0.91; 95% CI, 0.88–0.94). There is also large variation in the use of pretreatment scans for any patient with thyroid cancer (1.00; 95% CI, 0.98–1.00) and radioactive iodine use for an 0.8-cm intrathyroidal cancer (0.91; 95% CI, 0.89–0.93) and a 1.9-cm intrathyroidal cancer (0.86; 95% CI, 0.84–0.88). Far less variation is seen for a 1.9-cm cancer with lymph node metastases (0.36; 95% CI, 0.31–0.40), but there is some variation in physician recommendations for fixed dose vs dosimetry-guided radioactive iodine for patients with distant metastases (0.66; 95% CI, 0.61–0.71). There seems to be a consensus regarding keeping the TSH level below normal when there are distant metastases (0.10; 95% CI, 0.05–0.16) but not for 0.8-cm intrathyroidal tumors (1.00; 95% CI, 0.99–1.00). Physicians vary across all long-term management options. Variation in recommendations for ultrasound use for patients with stage I thyroid cancer with undetectable thyroglobulin is high (0.99; 95% CI, 0.97–1.00) as is variation over the role of diagnostic whole-body scans in all patients (0.99; 95% CI, 0.97–1.00). There is also some variation in physician recommendations for random thyroglobulin (0.84; 95% CI, 0.77–0.89) and stimulated thyroglobulin (includes withdrawal of thyroid hormone or recombinant TSH stimulation) (0.93; 95% CI, 0.88–0.97).
Figure 2.
The observed-to-maximum variation ratios for aspects of thyroid cancer management within the four specified domains: thyroid surgery, radioactive iodine use (RAI), thyroid hormone replacement postsurgery, and long-term thyroid cancer management. CLN, central lymph node; LN, lymph node.
In subgroup analysis, we found that nonsurgeons were more likely to recommend total thyroidectomy instead of lobectomy if concerning lymph nodes are seen preoperatively (76% vs 68%, P = .004). When there is an isolated unifocal 0.8-cm papillary thyroid cancer, the same proportion of surgeons and nonsurgeons are strongly against radioactive iodine, but more nonsurgeons are moderately against radioactive iodine (38% vs 28%, P = .0138). In addition, in a patient with a 1.9-cm cancer and lymph node involvement, surgeons are more likely to be strongly for radioactive iodine (87% vs 81%, P = .027).
When we evaluated differences in practice setting across items included in the surgery domain, there were no significant differences between academic vs nonacademic physicians. However, there were differences between physicians from academic practice settings vs those from nonacademic practice settings when the questions focused on radioactive iodine use. We found that physicians in academic practice settings were more likely to use pretreatment scans (57% vs 46%, P = .043). There was a pattern of nonacademic physicians favoring radioactive iodine more strongly than academic physicians. For example, physicians from an academic practice setting were more likely to be strongly against radioactive iodine in patients with an 0.8-cm isolated papillary thyroid cancer (38% vs 26%, P = .02). Physicians from a nonacademic practice setting were more likely to be strongly for radioactive iodine when a patient has a 1.9-cm papillary thyroid cancer and no known lymph node involvement (37% vs 27%, P = .004) and when a patient has a 1.9-cm papillary thyroid cancer and central lymph node involvement (85% vs 79%, P = .011).
We found that physicians who read the 2009 ATA guidelines were more likely to recommend total thyroidectomy over lobectomy when concerning lymph nodes were seen preoperatively (79% vs 63%, P < .001). Likewise, those who read the ATA guidelines were more likely to recommend central lymph node dissections when concerning lymph nodes were seen preoperatively (76% vs 68%, P = .03) and when the patient's tumor is >1 cm (50% vs 34%, P < .001).
With regard to radioactive iodine use, those who read the guidelines were less likely to recommend a pretreatment scan to a patient with an 0.8-cm papillary thyroid cancer (19% vs 43%, P < .001) and more likely to be strongly against radioactive iodine for an 0.8-cm papillary thyroid cancer (32% vs 18%, P < .001). Physicians who read the 2009 ATA guidelines were also more likely to favor dosimetry over a fixed dose of radioactive iodine for a patient with pulmonary metastases from papillary thyroid cancer (33% vs 22%, P = .03).
When we looked at surgical recommendations for subcentimeter cancers, there was no difference across regions. Likewise, when we looked at low-risk cancers (an 0.8-cm cancer and a 1.9-cm cancer without lymph node metastases), there was no significant difference between region of practice and likelihood of recommending radioactive iodine.
Discussion
Variation exists in each domain of thyroid cancer management: thyroid surgery, radioactive iodine use, thyroid hormone replacement postsurgery, and long-term thyroid cancer management. Large variation exists in the management of small thyroid cancers, including optimal surgery and the role of radioactive iodine treatment and suppressive doses of thyroid hormone replacement. There is also substantial variation regarding prophylactic central lymph node dissections, the role of pretreatment scans before radioactive iodine treatment, the use a previously determined fixed dose of radioactive iodine vs dosimetry-guided radioactive iodine for treatment of distant metastases, and all aspects of long-term thyroid cancer management. There is more consensus when the patient has higher risk cancer. For example, there is very little variation over the use of radioactive iodine in the treatment of patients with lymph node metastases and the role of TSH suppression when there are either lymph node metastases or distant metastases.
Because of both a paucity of randomized control trials in thyroid cancer management and conflicting observational studies (4–9, 16), the most recent clinical guidelines for thyroid cancer frequently rely on expert opinion and often leave much of the management to physician discretion (10). Previous studies have found that when clinical guideline recommendations are based on expert consensus instead of strong evidence, clinician compliance with guideline-directed management is lower (17, 18). Thus, it is not surprising that we found marked variation among physicians regarding prophylactic central lymph node dissections, the role of pretreatment scans before radioactive iodine treatment, the use of a fixed dose of radioactive iodine vs dosimetry for distant metastases, and long-term thyroid cancer management, given that there are no strong guideline recommendations regarding these aspects of thyroid cancer management and/or that data are limited (10).
In contrast, the most recent guidelines are clear about the management of small, intrathyroidal cancers. For intrathyroidal, subcentimeter thyroid cancer, lobectomy is adequate, and radioactive iodine is not recommended (10). In addition, suppressive doses of thyroid hormone replacement are not beneficial in the lowest risk patients (10, 19). Thus, we expected that the degree of variation in the management of low-risk thyroid cancer would be lower.
The reason for the variation in the management of these small thyroid cancers is not known. Given that more than three fourths of respondents read the 2009 ATA guidelines and close to one third read the 2010 NCCN guidelines, lack of knowledge is not likely to be the sole reason for the variation. A more likely explanation for variation in the management of small thyroid cancers is a lack of acceptance of the existing data and of the recent clinical guidelines. Based on our previous work, it is possible that some physicians prefer more intensive management across multiple domains (20, 21).
Although there are some topics, such as management of higher risk patients, associated with a low level of variation in provider treatment preferences, on the whole, marked variation was seen across all domains: surgery, radioactive iodine use, thyroid hormone replacement, and long-term management. The variation in management that we identified is indicative of clinical uncertainty in our field, and it has clear implications with regard to patient health and health care costs. If two clinically identical patients are receiving different surgical and/or medical management, then one may be “overtreated” or the other “undertreated.” Thyroid cancer treatments are not without risks; yet, in some patients there are clear benefits. It is critical that we understand which patients should and should not receive specific treatments to maximize treatment benefit and minimize risks.
Strengths of this study include the clinically relevant research question, the large sample size, the representation of multiple specialists involved in thyroid cancer management, the method of identifying a cohort of physicians from each hospital, and the method of quantifying variation. An additional strength is that, by asking each physician to assess the same clinical vignettes and answer the same questions, we essentially “adjusted” for physician case mix. However, although pilot testing was performed, there was no formal validation of the survey. Additional limitations are similar to those restraining other survey studies and include nonresponse bias and the possibility that physicians' reports may not reflect their practices.
Despite these limitations, it is clear that the identified areas of clinical variation in thyroid cancer management reflect variation among physicians' knowledge, attitudes, and beliefs. It is likely that some areas of variation are due to lack of data and others may be related to lack of acceptance of the newly defined standards of care. In particular, because small thyroid cancers represent the largest proportion of thyroid cancers and are the subgroup of thyroid cancer with the greatest rise in incidence (22–24), if left unaddressed, the uncertainties in management we identified will become more of an issue with time. We infer a need for both more thyroid cancer research and improved research dissemination. The results of this study could help to define research agendas for the management of this increasingly common cancer.
Acknowledgments
We thank Brittany Gay, Barbara Salem, Kathryn Schuessler, and Ashley Gay for their work in data collection and processing. Cornell University Survey Research Institute scanned the surgeon surveys for data file. The endocrine/nuclear medicine survey was endorsed by the American Thyroid Association.
This work was supported by the National Institutes of Health (Grant K07CA154595-02 to M.R.H.), a University of Michigan Comprehensive Cancer Center Idea Award, the Cancer Surveillance and Outcomes Research Team (CanSORT) Pilot of Feasibility Fund, and the Elizabeth Caroline Crosby Fund.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ATA
- American Thyroid Association
- CI
- confidence interval
- NCCN
- National Comprehensive Cancer Network.
References
- 1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29 [DOI] [PubMed] [Google Scholar]
- 2. National Cancer Institute Common cancer types. www.cancer.gov/cancertopics/types/commoncancers Accessed May 21, 2012
- 3. National Cancer Institute Surveillance Epidemiology and End Results.www.seer.cancer.gov Accessed November 1, 2011
- 4. Hay ID. Managing patients with a preoperative diagnosis of AJCC/UICC stage I (T1N0M0) papillary thyroid carcinoma: East versus West, whose policy is best? World J Surg. 2010;34:1291–1293 [DOI] [PubMed] [Google Scholar]
- 5. Mazzaferri EL. What is the optimal initial treatment of low-risk papillary thyroid cancer (and why is it controversial)? Oncology (Williston Park). 2009;23:579–588 [PubMed] [Google Scholar]
- 6. Grant CS, Stulak JM, Thompson GB, Richards ML, Reading CC, Hay ID. Risks and adequacy of an optimized surgical approach to the primary surgical management of papillary thyroid carcinoma treated during 1999–2006. World J Surg. 2010;34:1239–1246 [DOI] [PubMed] [Google Scholar]
- 7. Durante C, Attard M, Torlontano M, et al. Identification and optimal postsurgical follow-up of patients with very low-risk papillary thyroid microcarcinomas. J Clin Endocrinol Metab. 2010;95:4882–4888 [DOI] [PubMed] [Google Scholar]
- 8. Doi SA, Engel JM, Onitilo AA. Total thyroidectomy followed by postsurgical remnant ablation may improve cancer specific survival in differentiated thyroid carcinoma. Clin Nucl Med. 2010;35:396–399 [DOI] [PubMed] [Google Scholar]
- 9. Mendelsohn AH, Elashoff DA, Abemayor E, St John MA. Surgery for papillary thyroid carcinoma: is lobectomy enough? Arch Otolaryngol Head Neck Surg. 2010;136:1055–1061 [DOI] [PubMed] [Google Scholar]
- 10. Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214 [DOI] [PubMed] [Google Scholar]
- 11. Bilimoria KY, Bentrem DJ, Linn JG, et al. Utilization of total thyroidectomy for papillary thyroid cancer in the United States. Surgery. 2007;142:906–913; discussion 13 e1–2 [DOI] [PubMed] [Google Scholar]
- 12. Haymart MR, Banerjee M, Stewart AK, Koenig RJ, Birkmeyer JD, Griggs JJ. Use of radioactive iodine for thyroid cancer. JAMA. 2011;306:721–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim EY, Eisele DW, Goldberg AN, Maselli J, Kezirian EJ. Neck dissections in the United States from 2000 to 2006: volume, indications, and regionalization. Head Neck. 2011;33:768–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dillman DA, ed. 2007 Mail and Internet Surveys: The Tailored Design Method, 2nd ed New York, NY: Wiley, 2007 [Google Scholar]
- 15. Tuttle RM, Ball DW, Byrd D, et al. Thyroid carcinoma. J Natl Compr Canc Netw. 2010;8:1228–1274 [DOI] [PubMed] [Google Scholar]
- 16. Bilimoria KY, Bentrem DJ, Ko CY, et al. Extent of surgery affects survival for papillary thyroid cancer. Ann Surg. 2007;246:375–381; discussion 81–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Greenberg CC, Lipsitz SR, Neville B, et al. Receipt of appropriate surgical care for Medicare beneficiaries with cancer. Arch Surg. 2011;146:1128–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. In H, Neville BA, Lipsitz SR, Corso KA, Weeks JC, Greenberg CC. The role of National Cancer Institute-designated cancer center status: observed variation in surgical care depends on the level of evidence. Ann Surg. 2012;255:890–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jonklaas J, Sarlis NJ, Litofsky D, et al. Outcomes of patients with differentiated thyroid carcinoma following initial therapy. Thyroid. 2006;16:1229–1242 [DOI] [PubMed] [Google Scholar]
- 20. Haymart M, Banerjee M, Yang D, et al. The relationship between extent of thyroid cancer surgery and use of radioactive iodine. Ann Surg. 2012;35:396–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Haymart MR, Banerjee M, Yang D, Stewart AK, Koenig RJ, Griggs JJ. The role of clinicians in determining radioactive iodine use for low-risk thyroid cancer. Cancer. 2013;119:259–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295:2164–2167 [DOI] [PubMed] [Google Scholar]
- 23. Chen AY, Jemal A, Ward EM. Increasing incidence of differentiated thyroid cancer in the United States, 1988–2005. Cancer. 2009;115:3801–3807 [DOI] [PubMed] [Google Scholar]
- 24. Hughes DT, Haymart MR, Miller BS, Gauger PG, Doherty GM. The most commonly occurring papillary thyroid cancer in the United States is now a microcarcinoma in a patient older than 45 years. Thyroid. 2011;21:231–236 [DOI] [PubMed] [Google Scholar]