Abstract
Context:
The relationships among cortical volumetric bone mineral density (CortBMD) and comprehensive measures of mineral metabolism have not been addressed in chronic kidney disease (CKD).
Objective:
The aim of the study was to identify the determinants of CortBMD in childhood CKD. A secondary objective was to assess whether CortBMD was associated with subsequent fracture.
Design and Participants:
This prospective cohort study included 171 children, adolescents, and young adults (aged 5–21 years) with CKD stages 2–5D at enrollment and 89 1 year later.
Outcomes:
Serum measures included vitamin D [25-hydroxyvitamin D (25[OH]D), 1,25-dihydroxyvitamin D (1,25(OH)2D), 24,25-dihydroxyvitamin D], vitamin D-binding protein, intact PTH, fibroblast growth factor 23, calcium, and phosphorus. Tibia quantitative computed tomography measures of CortBMD were expressed as sex-, race-, and age-specific Z-scores based on 675 controls. Multivariable linear regression identified the independent correlates of CortBMD Z-scores and the change in CortBMD Z-scores.
Results:
Lower calcium (β = .31/1 mg/dL, P = .01) and 25(OH)D (β = .18/10 ng/mL, P = .04) and higher PTH (β = −.02/10%, P = .002) and 1,25(OH)2D (β = −.07/10%, P < .001) were independently associated with lower CortBMD Z-scores at baseline. The correlations of total, free, and bioavailable 25(OH)D with CortBMD did not differ. Higher baseline 1,25(OH)2D (P < .05) and greater increases in PTH (P < .001) were associated with greater declines in CortBMD Z-scores. Greater increases in calcium concentrations were associated with greater increases in CortBMD Z-scores in growing children (interaction P = .009). The hazard ratio for fracture was 1.75 (95% confidence interval 1.15–2.67; P = .009) per SD lower baseline CortBMD.
Conclusions:
Greater PTH and 1,25(OH)2D and lower calcium concentrations were independently associated with baseline and progressive cortical deficits in childhood CKD. Lower CortBMD Z-score was associated with increased fracture risk.
Chronic kidney disease (CKD) poses multiple threats to bone accrual during growth. We reported that secondary hyperparathyroidism was associated with declines in peripheral quantitative computed tomography (pQCT) measures of cortical volumetric bone mineral density (CortBMD) in childhood CKD (1). Although the fracture implications of these findings are not known, a study in adult hemodialysis patients demonstrated that a low pQCT CortBMD was strongly associated with prevalent fractures (2).
Recent cross-sectional bone histomorphometry studies in children demonstrated that defective mineralization was present as early as stage 2 CKD, and the prevalence increased with progressive CKD severity, exceeding 90% in CKD 5D (dialysis) (3). In contrast, a mineralization defect was observed in only 3% of adults on dialysis (4). Therefore, the growing skeleton may be uniquely vulnerable to impaired mineralization in CKD.
In children with predialysis CKD, lower serum calcium and higher PTH concentrations were associated with defective mineralization (3), whereas higher fibroblast growth factor 23 (FGF-23) was associated with better mineralization in children on dialysis. The development of treatments for CKD mineral and bone disorder requires a better understanding of the impact of mineral metabolism on CortBMD as well as interactions with growth.
Recent studies highlight the need to consider vitamin D-binding protein (DBP) and vitamin D catabolism in CKD. Bhan et al (5) demonstrated that bioavailable 25-hydroxyvitamin D [25(OH)D], ie, the fraction not bound to DBP, was better correlated with measures of mineral metabolism than total 25(OH)D in adults on hemodialysis. In addition, reduced vitamin D catabolism, as reflected by lower serum concentrations of 24,25-dihydroxyvitamin D [24,25(OH)2D], has also been identified in CKD (6).
This prospective cohort study is the first to examine associations between CortBMD and serum calcium, phosphorus, PTH, FGF-23, and vitamin D concentrations [including 24,25(OH)2D, free and bioavailable 25(OH)D] in a large cohort of children, adolescents, and young adults with CKD. CortBMD reference data were generated in 675 concurrent healthy participants. The primary objectives were to identify determinants of baseline CortBMD Z-scores and changes in CortBMD Z-scores in childhood CKD. Secondary objectives were to compare the associations of total, free, and bioavailable 25(OH)D concentrations with CortBMD and to assess whether baseline CortBMD Z-score was associated with subsequent fracture.
Materials and Methods
Study participants
This study was part of a larger study of bone health in 205 participants with CKD, ages 5–21 years, conducted at the Children's Hospital of Philadelphia (CHOP) and Cincinnati Children's Hospital Medical Center (CCHMC) (1, 7–10). Our prior study of total 25(OH)D and 1,25-dihydroxyvitamin D [1,25(OH)2D] concentrations included 182 participants (8). Of these, 171 had a pQCT scan performed within 1 month of their blood sample and are included in this cross-sectional analysis. Longitudinal analyses were performed in the subset of 89 with both CortBMD and vitamin D measured 12 ± 4 months later. Of the 82 participants excluded from the longitudinal analyses, 25 had no further follow-up, 33 underwent renal transplantation during the follow-up interval, and 24 did not have a usable pQCT scan or vitamin D in this time frame. The 170 participants with a baseline CortBMD measure and 1 or more follow-up visits were included in the fracture analysis.
The reference population of 675 healthy participants was recruited from practices in the Philadelphia area (1, 8–11). The Institutional Review Boards at CHOP and CCHMC approved the study protocol. Informed consent was obtained from participants 18 years of age or older and assent with parental consent from subjects younger than 18 years.
Anthropometry
Height was measured with a stadiometer and weight with a digital scale. Height and body mass index (BMI; kilograms per square meter) Z-scores were calculated using national reference data (12).
Laboratory measurements
Serum 25(OH)D and 1,25(OH)2D concentrations were measured by I125 RIA (DiaSorin Inc, Stillwater, Minnesota) (13). Intraassay coefficients of variation (CVs) were 2.2% and 7%–11%, respectively (14). Serum 24,25(OH)2D was measured by combined tandem mass spectrometry (6) with an interassay CV of 8.6%.
Serum DBP was measured in duplicate (CV < 10%) using an ELISA (R&D Systems, Minneapolis, MN). The interassay CV was 1.6%–3.6%, and recovery was 98%–103%. Intact (iPTH) and bioactive PTH (1–84PTH) concentrations were measured by RIA with CVs of 3%–10% (Scantibodies Laboratory, Santee, California) (15). Intact FGF-23 was measured via an ELISA (Kainos Laboratories Inc, Tokyo, Japan; CV 6.7%–12.4%). Serum albumin and creatinine were measured by spectrophotometric enzymatic assay (Vitros; Johnson & Johnson Co, Rochester, New York) with CVs of 1%–2% and 1%–5%, respectively. Serum calcium and phosphorus were measured using standard clinical methods with CVs of 1.3% and 2.1% or less, respectively; calcium was corrected for albumin (16). Estimated glomerular filtration rate (eGFR) was calculated based on height and serum creatinine using the Chronic Kidney Disease in Children Study equation (17). Participants were categorized as CKD stages 2–3 (eGFR 30–89 mL/min per 1.73 m2), stages 4–5 (<30 mL/min per 1.73 m2), and CKD stage 5D (dialysis) (18).
Free and bioavailable 25(OH)D
Serum free and bioavailable 25(OH)D [free + albumin bound 25(OH)D] was calculated using total 25(OH)D, DBP, and albumin concentrations and the equations adapted by Powe et al from those for free testosterone (19, 20).
Peripheral quantitative computed tomography
This study was limited to measures of CortBMD to examine associations with measures of mineral metabolism. Kovanlikaya et al (21) demonstrated that rickets resulted in marked decreases in quantitative computed tomography measures of femur CortBMD. In contrast, pQCT measures of trabecular BMD include the marrow space and cannot distinguish between disease effects on trabecular structure vs mineralization. Bone measures were obtained in the midshaft of the tibia, 38% proximal to the distal growth plate, using a Stratec XCT2000 device (Orthometrix, White Plains, New York) as described (9, 10). The 38% site was used to minimize partial-volume effects, which may result in an underestimation of CortBMD in disorders characterized by cortical thinning. Analyses demonstrating absence of partial-volume effects were provided previously (10). The radiation effective dose equivalent was less than 0.01 μSv. The manufacturer's hydroxyapatite phantom was scanned daily. A test-retest precision study in 60 children and adolescents yielded an in vivo CV for CortBMD of 0.5%.
We reported that CortBMD in healthy children increased with age and was greater in females and blacks, and the sex and race effects varied with age (11). Therefore, CortBMD results were converted to sex- and race (black vs other)-specific Z-scores relative to age using the LMS method (1, 9, 10). This method accounts for the nonlinearity, heteroscedasticity, and skew of bone data in growing children (22).
Disease characteristics and medications
Medical charts were reviewed for disease and treatment characteristics. Information on current medications and sources of vitamin D supplementation were obtained by questionnaire and confirmed in the medical record. Participants/guardians were interviewed at all study visits regarding details of new fractures, and all fractures that occurred after the baseline visit were confirmed by review of radiology reports.
Statistical analyses
All analyses were performed using STATA 11.0 (Stata Corp, College Station, Texas). A 2-sided P < .05 was considered statistically significant. Continuous variables were reported as mean ± SD values or as median and interquartile range (IQR) if skewed. Group differences were assessed using the Student's t test, ANOVA, Wilcoxon rank sum, Kruskal-Wallis, or χ2 tests as appropriate, and the nptrend command in STATA as the nonparametric test for trend across ordered groups. Correlations were assessed by Pearson product moment or Spearman's rank correlations. The corcor program was used to test the equality of 2 dependent correlations. Skewed variables were natural log transformed (23).
Multivariable linear regression analysis was used to evaluate correlates of the CortBMD Z-score at enrollment, testing age, sex, race (black vs other), Tanner stage, BMI Z-score, study location (CHOP vs CCHMC), underlying diagnosis, CKD stage, acidosis (serum bicarbonate < 19 mmol/L), glucocorticoid and vitamin D sterol therapy at the time of the visit, and serum concentrations of PTH (iPTH and 1–84PTH), FGF-23, 25(OH)D (total, free, and bioavailable), 1,25(OH)2D, 24,25(OH)2D, phosphorus, and calcium. Model assumptions were assessed via graphical checks, eg, to assess linearity of relationships and normality of residuals.
Multivariable linear regression was used to evaluate the correlates of change in the CortBMD Z-score. In our recent study in this cohort (1), we demonstrated that the baseline CortBMD Z-score, baseline age, change in tibia length, and study location (CHOP) were inversely related to the change in the CortBMD Z-score. Therefore, a base model with these covariates was used to test the associations between change in the CortBMD Z-score and the following: sex, race, baseline and change in the BMI Z-score, the underlying diagnosis, CKD severity at baseline and change in renal function (decline in eGFR > 10 mL/min per 1.73 m2 or starting dialysis vs all others), acidosis, baseline glucocorticoid and vitamin D sterol therapy, initiating sterol therapy, and the baseline value of and change in all measures of mineral metabolism. Cox regression analysis, adjusted for sex and age, was used to determine whether the baseline CortBMD Z-score was associated with fracture [hazard ratio (HR) for time to first fracture].
Results
Participant characteristics (Table 1)
Table 1.
Baseline Characteristics of the 171 Children and Adolescents With CKD Included in This Study
| Study Site | |
|---|---|
| The Children's Hospital of Philadelphia | 105 (61%) |
| Cincinnati Children's Hospital Medical Center | 66 (39%) |
| Age category (at time of study), y | |
| 5–8 | 18 (11%) |
| 9–11 | 33 (19%) |
| 12–14 | 41 (24%) |
| 15–21 | 79 (46%) |
| Age at CKD diagnosis, y | 5.0 (0, 11.0) |
| Male | 101 (59%) |
| Race | |
| White | 117 (68%) |
| Black | 44 (26%) |
| Other | 10 (6%) |
| Hispanic ethnicity | 8 (5%) |
| Height Z-score | −0.80 ± 1.32 |
| BMI Z-score | 0.23 ± 1.24 |
| Underlying etiology of renal disease | |
| Congenital anomalies of the kidney and urinary tract | 96 (56%) |
| Glomerulonephritis | 24 (14%) |
| Focal segmental glomerulosclerosis | 32 (19%) |
| Other | 19 (11%) |
| CKD stage | |
| 2–3 | 68 (40%) |
| 4–5 | 51 (30%) |
| Dialysis | 52 (30%) |
| Dialysis modality | |
| Hemodialysis | 34 (65%) |
| Peritoneal dialysis | 18 (35%) |
| Vitamin D supplement usage ≥ 400 IU/d | 28 (16%) |
| Receiving vitamin D sterol therapy by CKD stage | |
| 2–3 | 16 (24%) |
| 4–5 | 38 (75%) |
| Dialysis | 45 (87%) |
| On calcium supplement/phosphate binder | 78 (46%) |
| On non-calcium-based phosphate binder | 22 (13%) |
| On glucocorticoid therapy | 21 (12%) |
| Acidosis | 16 (10%) |
Data are presented as n (%), median (IQR), or mean ± SD.
The baseline characteristics in Table 1 were comparable in participants with vs without a follow-up visit, with the exception of sex (66% vs 51% male) and CKD severity (CKD stages 2–3: 53% vs 26%; CKD stages 4–5: 26% vs 34%; and CKD stage 5D: 21% vs 40%), which can be explained by the 33 participants excluded for intervening transplantation. Vitamin D sterol use was more common than nutritional vitamin D supplementation, because participants were enrolled before the study sites measured 25(OH)D as part of clinical care. The vitamin D assays were performed in batches often well after sample collection, and neither participants nor their providers were given the results. Only 11 of 28 participants on supplemental vitamin D had 25(OH)D concentrations less than 20 ng/mL.
Measures of mineral metabolism by CKD stage (Table 2)
Table 2.
Measures of Mineral Metabolism and CortBMD Z-score According to CKD Stage
| CKD 2–3 | CKD 4–5 | Dialysis | P Value | |
|---|---|---|---|---|
| Corrected calcium, mg/dL | 9.4 (9.2, 9.8) | 9.3 (9.1, 9.6) | 9.4 (8.8, 9.7) | ≥.09a |
| Phosphorus, mg/dL | 4.2 (3.8, 4.9) | 5.2 (4.6, 5.9) | 5.5 (4.4, 6.7) | <.001a |
| Total 25(OH)D, ng/mL | 31.4 (18.9, 35.9) | 22.0 (10.4, 31.9) | 14.4 (8.7, 20.5) | <.001a |
| Free 25(OH)D, pg/mL | 9.9 (6.7, 14.4) | 8.0 (4.8, 11.6) | 5.7 (3.9, 9.6) | <.001a |
| Bioavailable 25(OH)D, ng/mL | 3.6 (2.5, 5.1) | 2.6 (1.7, 3.7) | 1.8 (1.0, 2.8) | <.001a |
| 1,25(OH)2D, pg/mL | 36.5 (29, 45.2) | 30.5 (22.2, 41.2) | 18.6 (13.2, 22.7) | <.001a |
| 24,25(OH)2D, ng/mL | 2.5 (1.7, 3.9) | 1.0 (0.5, 2.2) | 0.5 (0.3, 0.8) | <.001a |
| iPTH, pg/mL | 46 (26, 72) | 140 (73, 409) | 252 (104, 617) | <.001a |
| 1–84PTH, pg/mL | 26 (16, 39) | 87 (35, 270) | 164 (54, 359) | <.001a |
| FGF-23, pg/mL | 52 (37, 72) | 127 (53, 269) | 349 (152, 2759) | <.001a |
| Vitamin D deficiency [25(OH)D < 20 ng/mL] | 26% | 49% | 73% | <.001b |
| CortBMD Z-score | 0.27 ± 0.99 | −0.56 ± 1.26 | 0.00 ± 1.57 | .002c |
Results presented as median (IQR), mean ± SD, or percentage.
Kruskal-Wallis test and test for trend among groups.
χ2 test.
ANOVA.
Calcium did not differ by CKD stage. Greater CKD severity was associated with greater concentrations of serum phosphorus, iPTH, 1–84PTH, and FGF-23, and with lower total, free, and bioavailable 25(OH)D, 1,25(OH)2D, and 24,25(OH)2D (all P < .001).
Association between total vs free and bioavailable 25(OH)D and CortBMD Z-score
CortBMD Z-score was associated with total, free and bioavailable 25(OH)D (Table 3). There were no significant differences among the correlation coefficients for total, free, and bioavailable 25(OH)D with CortBMD Z-score (all P ≥ .3).
Table 3.
Unadjusted Correlations Among Measures of Mineral Metabolism and CortBMD Z-score
| Calcium | Phos | iPTH | FGF-23 | 25(OH)D | Free 25(OH)D | Bioavailable 25(OH)D | 1,25(OH)2D | 24,25(OH)2D | |
|---|---|---|---|---|---|---|---|---|---|
| CortBMD Z-score | 0.33a | −0.18c | −0.32a | −0.05 | 0.28a | 0.24b | 0.22b | −0.07 | 0.25b |
| Calcium | −0.25b | −0.33a | −0.11 | 0.20b | 0.13 | 0.10 | 0.08 | 0.26a | |
| Phos | 0.56a | 0.41a | −0.24b | −0.25b | −0.27a | −0.18c | −0.31a | ||
| iPTH | 0.43a | −0.45a | −0.35a | −0.38a | −0.30a | −0.58a | |||
| FGF-23 | −0.17c | −0.27a | −0.29a | −0.44a | −0.22b | ||||
| 25(OH)D | 0.47a | 0.79a | |||||||
| 1,25(OH)2D | 0.42a |
Abbreviation: Phos, Phosphorus. Results presented as Spearman's ρ. P > 0.1 for nonsignificant correlations.
P ≤ .001.
P ≤ .01.
P < .05.
CortBMD Z-score
The mean CortBMD Z-score was −0.06 ± 1.31. Table 2 summarizes CortBMD Z-score according to CKD stage. Table 3 shows the bivariate correlation matrix for CortBMD Z-score and measures of mineral metabolism. Greater concentrations of calcium, 25(OH)D, and 24,25(OH)2D were associated with greater CortBMD Z-score, and higher concentrations of phosphorus and iPTH with lower CortBMD Z-score. In bivariate analysis, older age (−0.08 SD per year, P = .002), black race (−0.46 SD, P < .05), study site of CHOP (−0.64 SD, P = .002), acidosis (−0.71 SD, P = .04), focal segmental glomerulosclerosis (vs other diagnoses, −0.56 SD, P = .03), CKD stages 4–5 (vs stages 2–3, −0.84 SD, P = .001), and vitamin D sterol therapy (−0.55 SD, P = .007) were associated with lower CortBMD Z-score; glucocorticoid exposure (0.65 SD, P = .03) was associated with greater CortBMD Z-score. There was no difference in mean CortBMD Z-score by dialysis modality (hemodialysis −0.01 vs peritoneal dialysis 0.01, P = .97).
In multivariable analyses, calcium, 25(OH)D, and glucocorticoid exposure were positively associated, and iPTH and 1,25(OH)2D concentrations were negatively associated with CortBMD Z-score. The sequential models in Table 4 demonstrate how the relations among vitamin D metabolites, calcium, and iPTH influenced their independent associations with CortBMD Z-score. Adjusting for the positive association between 25(OH)D and 1,25(OH)2D (substrate dependence) strengthened their independent and opposing effects on CortBMD Z-score (model 3). The associations between these 2 vitamin D metabolites and CortBMD Z-score did not change with adjustment for calcium (model 4). Adjustment for iPTH attenuated the positive associations of 25(OH)D and calcium with CortBMD Z-score (model 4 vs 5). Being on vitamin D sterol therapy was associated with lower CortBMD Z-score, but this was explained by higher iPTH concentrations in those on sterols (model 6 vs 7). The variance inflation factor for these models was ≤ 1.35 indicating that there was no problematic collinearity. 24,25(OH)2D, phosphorus, race, Tanner stage, acidosis, and underlying disease were not associated with CortBMD Z-score in multivariable analysis. Sex, BMI Z-score, and FGF-23 were not associated with CortBMD Z-score in bivariate or multivariable analyses. Table 5 shows the final model with the difference in CortBMD Z-score associated with each independent determinant displayed in clinically interpretable measures. Substituting 1–84PTH for iPTH had no effect on the model findings. Adjustment for CKD stage did not impact model findings except for modestly attenuating the β-coefficient for 25(OH)D (0.18 to 0.16 per 10 ng/mL difference, P = .06). The β-coefficients and P values for 1,25(OH)2D and calcium did not change significantly. In multivariate analyses, CKD stages 4–5 were associated with lower CortBMD Z-score (vs CKD stages 2–3, −0.51 SD, P = .03).
Table 4.
Multivariate Regression Analyses of Correlates of CortBMD-Z
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5a | Model 6 | Model 7 | |
|---|---|---|---|---|---|---|---|
| Model R2 | 0.17 | 0.19 | 0.25 | 0.31 | 0.35 | 0.34 | 0.36 |
| On glucocorticoid | 1.01 (0.001) | 1.09 (<0.001) | 0.94 (0.001) | 0.78 (0.006) | 0.76 (0.006) | 0.88 (0.002) | 0.83 (0.003) |
| 25(OH)D | 0.02 (0.06) | 0.03 (0.002) | 0.03 (0.002) | 0.02 (0.04) | 0.02 (0.005) | 0.02 (0.04) | |
| ln[1,25(OH)2D] | −0.68 (<0.001) | −0.69 (<0.001) | −0.72 (<0.001) | −0.71 (<0.001) | −0.73 (<0.001) | ||
| Calcium | 0.42 (<0.001) | 0.31 (0.01) | 0.37 (0.002) | 0.29 (0.01) | |||
| ln(PTH) | −0.24 (0.002) | −0.19 (0.02) | |||||
| On sterol | −0.48 (0.008) | −0.34 (0.07) |
Cell contents are difference in Z-score (SD), with P value in parentheses. All models are adjusted for age and study site.
Final model.
Table 5.
Final Multivariate Model of Determinants of CortBMD Z-score
| Covariate | Difference in CortBMD-Z | 95% CI | P Value |
|---|---|---|---|
| Calcium, per 1 mg/dL | 0.31 | 0.08, 0.54 | .01 |
| PTH, per 10% | −0.02 | −0.04, −0.01 | .002 |
| 25(OH)D, per 10 ng/mL | 0.18 | 0.008, 0.34 | .04 |
| 1,25(OH)2D, per 10% | −0.07 | −0.10, −0.04 | <.001 |
| Glucocorticoid therapy | 0.76 | 0.23, 1.30 | .006 |
Also adjusted for age and study site. 1,25(OH)2D and PTH are log transformed.
Changes in CortBMD Z
The median time between visits was 1 year (IQR 1.0, 1.1). The median change in CortBMD Z-score was −0.07 (IQR −0.55, 0.40). Although the magnitude of the median changes was small, there were participants who had both substantial increases and decreases in all measures of mineral metabolism, facilitating our ability to detect associations with change in CortBMD Z-score. The medians and total ranges for changes in measures of mineral metabolism were as follows: calcium, 0.01 mg/dL (−1.92 to 3.02); phosphorus, 0.2 mg/dL (−4.4 to 5.5); total 25(OH)D, 2.1 ng/mL (−28.7 to 28.9); 1,25(OH)2D, −3.0 pg/mL (−32.1 to 22.5); 24,25(OH)2D, −0.01 ng/mL (−3.32 to 8.27); iPTH, 2.25 pg/mL (−505 to 1076); and FGF-23, 9.04 pg/mL (−6940 to 17 546).
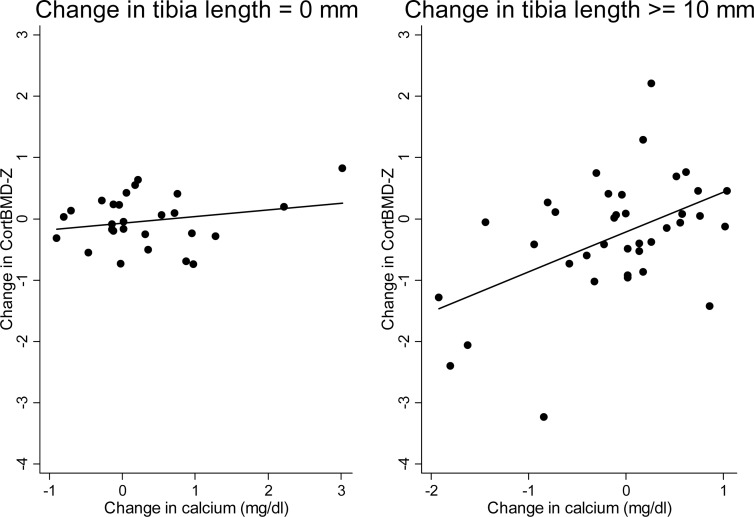
Each of the following variables when added to the base model was associated with the change in CortBMD Z-score: baseline calcium (β = −.50, P = .008); change in calcium (β = .71, P = .002); change in iPTH (β = −.28, P < .001); change in 24,25(OH)2D (β = −.15, P = .03); and worsening renal function (β = 0.10, P = .01). Given the strong positive association between CortBMD Z-score and calcium both cross-sectionally and longitudinally, we hypothesized that calcium may have a greater impact on the growing skeleton. We confirmed that the change in serum calcium was significantly associated with the change in CortBMD Z-score among participants with greater growth (P for interaction = .02). The Spearman's rho for the association between change in CortBMD Z-score and change in calcium was 0.04 (P = .83) if the change in the tibia length was 0 as compared with 0.39 (P = .02) if the change in the tibia length was 10 mm or greater (Figure 1). In multivariable analysis, this interaction and the opposing effects of calcium and iPTH persisted; increases in serum calcium were associated with increases in CortBMD Z-score among those participants who were growing (Table 6). Increases in iPTH and higher baseline 1,25(OH)2D were associated with decreases in CortBMD Z-score in all participants. Change in 24,25(OH)2D was not significant after adjustment for renal function. The R2 of this model was 0.61, indicating that it explained 61% of the variability in change in CortBMD Z-score remaining after the normal variability explained by age, race, and sex was captured by the Z-score. Substituting change in 1–84 PTH for iPTH did not impact model findings.
Figure 1.
Association between change in CortBMD Z-score and change in calcium differs by change in tibia length.
Table 6.
Final Multivariate Model of Determinants of Change in CortBMD Z-scorea
| Covariate | β | 95% CI | P Value |
|---|---|---|---|
| Change in tibia lengtha | −1.21 | −2.06, −0.37 | .006 |
| Change in calciuma | −0.78 | −1.58, 0.01 | .053 |
| Change in calcium by change in tibia length interaction | 0.45 | 0.12, 0.79 | .009 |
| Change in PTHa | −0.26 | −0.37, −0.14 | <.001 |
| Baseline 1,25(OH)2Da | −0.07 | −0.13, −0.0003 | .049 |
Also adjusted for age, study site, baseline CortBMD Z-score, baseline calcium, and change in renal function.
Natural log transformed.
Fracture
Eleven of 170 participants (6.5%) sustained a fracture after their baseline visit (incidence of 556 per 10 000 person-years). Median follow-up time was 1.1 years (IQR 1.0, 1.2). Cox regression analysis adjusted for age and sex demonstrated that lower baseline CortBMD Z-score was associated with fracture; the HR per SD decrease in CortBMD was 1.75 [95% confidence interval (CI) 1.15–2.67; P = .009]. The mean CortBMD Z-score was −0.93 in those who subsequently fractured, compared with 0.08 in those who did not (P = .02). Fracture sites included clavicle (n = 1), tibia (n = 3), foot (n = 3), toes (n = 2), and radius/ulna (n = 2). Associated activities included snowboarding, dancing, running, soccer, scooter racing, fall with foot caught in chair, football, struck by a slow-moving car tire, roughhousing, car accident, and fall downstairs. Given that a prior population-based study demonstrated that dual-energy x-ray absorptiometry (DXA) results contributed to fracture risk in children, regardless of trauma severity, all fractures were included in the primary analysis (24). Excluding the 2 events involving cars, the HR per SD decrease in CortBMD increased to 1.89 (95% CI 1.19–2.99; P = .007).
Discussion
This is the first study to assess the independent relations between comprehensive measures of mineral metabolism and volumetric cortical BMD in CKD, and the first to assess whether lower CortBMD was associated with subsequent fractures. In cross-sectional and longitudinal analyses, lower calcium and greater iPTH and 1,25(OH)2D were the main determinants of CortBMD Z-score deficits. Furthermore, lower baseline CortBMD Z-score was associated with an increased risk of subsequent fracture. The incidence of fracture in our cohort was 4-fold higher than that reported in a large population-based study of fracture epidemiology in children and adolescents (133 per 10 000 person-years) (25).
The strong positive association between serum calcium and CortBMD Z-score is a novel finding with potentially important clinical implications. Our study corroborates and complements the recent histomorphometry findings of Wesseling-Perry et al (3); in 52 children with CKD, defective mineralization was associated with lower serum calcium and higher iPTH concentrations. Furthermore, we demonstrated an interaction between changes in calcium and linear growth, ie, that increases in serum calcium were strongly associated with increases in CortBMD Z-score, but only among children who were growing.
Observational studies (26, 27) and 2 randomized trials (28, 29) have linked the use of calcium-based phosphate binders with increased arterial calcification in patients with end-stage renal disease and raised concern about excess calcium load in this population (30). In the 1 study that included children, none of the patients younger than 20 years of age had evidence of coronary artery calcification (26). Our findings provide a cautionary note regarding the calcium requirements of the growing skeleton for optimal long-term bone health. In healthy children, positive calcium balance results in average peak calcium accretion rates of 359 and 284 mg/d in boys and girls, respectively (31). As previously reported in this cohort, greater increases in iPTH were associated with greater declines in CortBMD Z-score as anticipated, given the high bone turnover state of hyperparathyroidism (32, 33). Importantly, the positive association between CortBMD Z-score and calcium persisted after adjustment for the inverse relationship (lower calcium results in greater iPTH) between calcium and iPTH.
Although 1,25(OH)2D has both anabolic and catabolic actions on bone, its primary effect in preclinical models is resorptive (34). Increased 1,25(OH)2D concentrations in mice with intestine-specific vitamin D receptor inactivation were shown to promote bone turnover, leading to osteopenia, and suppress matrix mineralization (35). Compelling clinical evidence is provided by the recent report that the increased fracture risk observed after yearly high-dose oral vitamin D3 was associated with the 1,25(OH)2D level 3 months after its administration (36). Our findings of an inverse association between 1,25(OH)2D levels and CortBMD Z-score, independent of PTH, are consistent with a resorptive effect. After adjustment for its strong positive association with 1,25(OH)2D (as its substrate), greater 25(OH)D concentrations were associated with higher CortBMD Z-score cross-sectionally, and this was partially explained by iPTH and calcium. Another important finding was the comparable relations between CortBMD Z-score and total vs free and bioavailable 25(OH)D. Recently a study in healthy young adults reported that free and bioavailable 25(OH)D were more strongly correlated with DXA spine BMD than total 25(OH)D (20). Using the same DBP assay and formulae to estimate free and bioavailable 25(OH)D, we found no significant difference in the correlation coefficients between CortBMD Z-score and total, free, and bioavailable 25(OH)D, suggesting that at least in terms of CortBMD, total 25(OH)D provides an adequate assessment of nutritional vitamin D status in CKD.
This is the fourth pediatric chronic disease population in which we have demonstrated that glucocorticoid therapy is associated with greater CortBMD Z-score (9, 23, 37). We attributed this to glucocorticoid actions on osteoblasts and osteocytes to suppress bone formation (38) with resultant accumulation of older cortical bone of greater density. Although not the focus of this paper, we recently examined associations between concurrent glucocorticoid dose and CortBMD Z-score in children with nephrotic syndrome and normal renal function and demonstrated that greater glucocorticoid exposure, lesser linear growth, and lesser expansion of cortical area were independently associated with greater increases in CortBMD Z-score (39). These findings support our hypothesis that glucocorticoid-induced suppression of bone formation results in accumulation of older cortical bone of greater material density independent of gains in bone length and dimensions. Given that participants on glucocorticoids at baseline had lesser increases in tibia length over the follow-up interval, the association between glucocorticoids and CortBMD Z-score may have been partially confounded by growth.
The lack of bone biopsy data is a limitation of this study. Without histomorphometry, it cannot be determined whether cortical deficits are secondary to increased porosity and/or decreased mineralization. However, a prior quantitative computed tomography study demonstrated reduced CortBMD in children with rickets, suggesting our observations are consistent with impaired mineralization (21). The lack of measures of ionized calcium is an additional limitation; however, total calcium was corrected for serum albumin. The longitudinal analysis may have been subject to bias; however, comparison of baseline characteristics revealed that those without longitudinal data differed only in the distribution of sex and CKD stage, both of which were accounted for in the analysis. There was substantial heterogeneity in participant characteristics, but the predialysis study population was representative based on comparison to the Chronic Kidney Disease in Children Study (40). Finally, although the longitudinal component strengthens the cross-sectional findings, the observational nature of the study limits conclusions regarding causality.
This study has several important strengths. It is the largest longitudinal study of bone structure and mineral metabolism in childhood CKD. Unlike DXA, the volumetric outcome of CortBMD generated by pQCT is not confounded by bone size; this is critical in pediatric CKD, given the burden of short stature. The large sample of healthy reference participants allowed for the generation of age-, sex-, and race-specific Z-scores. This study is unique in its comprehensive assessment of mineral metabolism with simultaneous and longitudinal measures of vitamin D metabolites, iPTH, FGF-23, calcium, and phosphorus. Furthermore, the measurement of DBP allowed for the novel analyses of the relations between free and bioavailable 25(OH)D and CortBMD Z-score. Finally, the prospective collection of fracture data allowed us to relate cortical BMD to this relevant outcome.
In summary, we demonstrated that calcium, iPTH, and 1,25(OH)2D are important and independent correlates of CortBMD Z-score and that calcium is of particular importance in the growing skeleton. Cortical bone comprises 80% of skeletal mass, and children are more likely to fracture the cortical-rich appendicular skeleton. This is the first pediatric study to demonstrate that lower cortical density is associated with increased risk of fracture in CKD, making it an important therapeutic target.
Acknowledgments
We thank the study participants and their families for their time and dedication. We greatly appreciate the assistance of the Clinical Research Coordinators and the staff at the Clinical Translational Research Centers at CCHMC and CHOP in the conduct of this study, particularly Samir Sayed, BS, in the core laboratory at CHOP for his work on the DBP assay. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This work was supported by National Institutes of Health Grants R01-DK060030 (to M.B.L.), R01-HD040714 (to M.B.L.), and K24-DK076808 (to M.B.L.) and by the National Center for Research Resources Grant UL1RR024134, which is now at the National Center for Advancing Translational Sciences, Grant UL1TR000003. M.R.D. was supported by a National Kidney Foundation/Amgen Kidney Disease Outcomes Quality Initiative Research Fellowship, The Nephcure Foundation-American Society of Nephrology Research Grant, and Grant K23DK093556.
Disclosure Summary: I.H.d.B. receives research funding from Abbott Laboratories. The remaining authors have nothing to disclose.
Footnotes
- BMI
- body mass index
- CCHMC
- Cincinnati Children's Hospital Medical Center
- CHOP
- Children's Hospital of Philadelphia
- CI
- confidence interval
- CKD
- chronic kidney disease
- CortBMD
- cortical volumetric bone mineral density
- CV
- coefficient of variation
- 5D
- CKD stage 5D (dialysis)
- DBP
- vitamin D-binding protein
- DXA
- dual-energy x-ray absorptiometry
- eGFR
- estimated glomerular filtration rate
- FGF-23
- fibroblast growth factor 23
- HR
- hazard ratio
- iPTH
- intact PTH
- IQR
- interquartile range
- 24,25(OH)2D
- 24,25-dihydroxyvitamin D
- 1,25(OH)2D
- 1,25-dihydroxyvitamin D
- 25(OH)D
- 25-hydroxyvitamin D
- 1–84PTH
- bioactive PTH
- pQCT
- peripheral quantitative computed tomography.
References
- 1. Tsampalieros A, Kalkwarf HJ, Wetzsteon RJ, et al. Changes in bone structure and the muscle-bone unit in children with chronic kidney disease. Kidney Int. 2013;83(3):495–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jamal SA, Gilbert J, Gordon C, Bauer DC. Cortical pQCT measures are associated with fractures in dialysis patients. J Bone Miner Res. 2006;21:543–548 [DOI] [PubMed] [Google Scholar]
- 3. Wesseling-Perry K, Pereira RC, Tseng CH, et al. Early skeletal and biochemical alterations in pediatric chronic kidney disease. Clin J Am Soc Nephrol. 2012;7:146–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Malluche HH, Mawad HW, Monier-Faugere MC. Renal osteodystrophy in the first decade of the new millennium: analysis of 630 bone biopsies in black and white patients. J Bone Miner Res. 2011;26:1368–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bhan I, Powe CE, Berg AH, et al. Bioavailable vitamin D is more tightly linked to mineral metabolism than total vitamin D in incident hemodialysis patients. Kidney Int. 2012;82:84–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bosworth CR, Levin G, Robinson-Cohen C, et al. The serum 24,25-dihydroxyvitamin D concentration, a marker of vitamin D catabolism, is reduced in chronic kidney disease. Kidney Int. 2012;82:693–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Foster BJ, Kalkwarf HJ, Shults J, et al. Association of chronic kidney disease with muscle deficits in children. J Am Soc Nephrol. 2011;22:377–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kalkwarf HJ, Denburg MR, Strife CF, et al. Vitamin D deficiency is common in children and adolescents with chronic kidney disease. Kidney Int. 2012;81:690–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Terpstra AM, Kalkwarf HJ, Shults J, et al. Bone density and cortical structure after pediatric renal transplantation. J Am Soc Nephrol. 2012;23:715–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wetzsteon RJ, Kalkwarf HJ, Shults J, et al. Volumetric bone mineral density and bone structure in childhood chronic kidney disease. J Bone Miner Res. 2011;26:2235–2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Leonard MB, Elmi A, Mostoufi-Moab S, et al. Effects of sex, race, and puberty on cortical bone and the functional muscle bone unit in children, adolescents, and young adults. J Clin Endocrinol Metab. 2010;95:1681–1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ogden CL, Kuczmarski RJ, Flegal KM, et al. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002;109:45–60 [DOI] [PubMed] [Google Scholar]
- 13. Hollis BW. Quantitation of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D by radioimmunoassay using radioiodinated tracers. Methods Enzymol. 1997;282:174–186 [DOI] [PubMed] [Google Scholar]
- 14. Clive DR, Sudhaker D, Giacherio D, et al. Analytical and clinical validation of a radioimmunoassay for the measurement of 1,25 dihydroxy vitamin D. Clin Biochem. 2002;35:517–521 [DOI] [PubMed] [Google Scholar]
- 15. Gao P, Scheibel S, D'Amour P, et al. Development of a novel immunoradiometric assay exclusively for biologically active whole parathyroid hormone 1–84: implications for improvement of accurate assessment of parathyroid function. J Bone Miner Res. 2001;16:605–614 [DOI] [PubMed] [Google Scholar]
- 16. Serum-calcium. Lancet. 1979;1:858–859 [PubMed] [Google Scholar]
- 17. Schwartz GJ, Munoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266 [PubMed] [Google Scholar]
- 19. Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666–3672 [DOI] [PubMed] [Google Scholar]
- 20. Powe CE, Ricciardi C, Berg AH, et al. Vitamin D-binding protein modifies the vitamin D-bone mineral density relationship. J Bone Miner Res. 2011;26:1609–1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kovanlikaya A, Loro ML, Hangartner TN, Reynolds RA, Roe TF, Gilsanz V. Osteopenia in children: CT assessment. Radiology. 1996;198:781–784 [DOI] [PubMed] [Google Scholar]
- 22. Cole TJ. The LMS method for constructing normalized growth standards. Eur J Clin Nutr. 1990;44:45–60 [PubMed] [Google Scholar]
- 23. Wetzsteon RJ, Shults J, Zemel BS, et al. Divergent effects of glucocorticoids on cortical and trabecular compartment BMD in childhood nephrotic syndrome. J Bone Miner Res. 2009;24:503–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Clark EM, Ness AR, Tobias JH. Bone fragility contributes to the risk of fracture in children, even after moderate and severe trauma. J Bone Miner Res. 2008;23:173–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cooper C, Dennison EM, Leufkens HG, Bishop N, van Staa TP. Epidemiology of childhood fractures in Britain: a study using the general practice research database. J Bone Miner Res. 2004;19:1976–1981 [DOI] [PubMed] [Google Scholar]
- 26. Goodman WG, Goldin J, Kuizon BD, et al. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med. 2000;342:1478–1483 [DOI] [PubMed] [Google Scholar]
- 27. London GM, Marty C, Marchais SJ, Guerin AP, Metivier F, de Vernejoul MC. Arterial calcifications and bone histomorphometry in end-stage renal disease. J Am Soc Nephrol. 2004;15:1943–1951 [DOI] [PubMed] [Google Scholar]
- 28. Block GA, Spiegel DM, Ehrlich J, et al. Effects of sevelamer and calcium on coronary artery calcification in patients new to hemodialysis. Kidney Int. 2005;68:1815–1824 [DOI] [PubMed] [Google Scholar]
- 29. Chertow GM, Burke SK, Raggi P. Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int. 2002;62:245–252 [DOI] [PubMed] [Google Scholar]
- 30. Moe SM, Chertow GM. The case against calcium-based phosphate binders. Clin J Am Soc Nephrol. 2006;1:697–703 [DOI] [PubMed] [Google Scholar]
- 31. Bailey DA, Martin AD, McKay HA, Whiting S, Mirwald R. Calcium accretion in girls and boys during puberty: a longitudinal analysis. J Bone Miner Res. 2000;15:2245–2250 [DOI] [PubMed] [Google Scholar]
- 32. Parfitt AM. A structural approach to renal bone disease. J Bone Miner Res. 1998;13:1213–1220 [DOI] [PubMed] [Google Scholar]
- 33. Schober HC, Han ZH, Foldes AJ, et al. Mineralized bone loss at different sites in dialysis patients: implications for prevention. J Am Soc Nephrol. 1998;9:1225–1233 [DOI] [PubMed] [Google Scholar]
- 34. Haussler MR, Whitfield GK, Kaneko I, et al. Molecular Mechanisms of Vitamin D Action. Calcif Tissue Int. 2013;92:77–98 [DOI] [PubMed] [Google Scholar]
- 35. Lieben L, Masuyama R, Torrekens S, et al. Normocalcemia is maintained in mice under conditions of calcium malabsorption by vitamin D-induced inhibition of bone mineralization. J Clin Invest. 2012;122:1803–1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sanders K, Duque G, Ebeling P, et al. The efficacy of high-dose oral vitamin D3 administered once a year: increased fracture risk is associated with 1,25 vitamin D level at 3-months post dose. J Bone Miner Res. 2012;27(suppl 1). Available at http://www.asbmr.org/Meetings/AnnualMeeting/AbstractDetail.aspx?aid=6beb2500–4b8e–472b-8b38–d881cadb80e3 (Abstract) Accessed January 17, 2013 [Google Scholar]
- 37. Dubner SE, Shults J, Baldassano RN, et al. Longitudinal assessment of bone density and structure in an incident cohort of children with Crohn's disease. Gastroenterology. 2009;136:123–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. O'Brien CA, Jia D, Plotkin LI, et al. Glucocorticoids act directly on osteoblasts and osteocytes to induce their apoptosis and reduce bone formation and strength. Endocrinology. 2004;145:1835–1841 [DOI] [PubMed] [Google Scholar]
- 39. Tsampalieros A, Gupta P, Denburg MR, et al. Glucocorticoid effects on changes in bone mineral density and cortical structure in childhood nephrotic syndrome. J Bone Miner Res. 2013;28(3):480–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wong CS, Pierce CB, Cole SR, et al. Association of proteinuria with race, cause of chronic kidney disease, and glomerular filtration rate in the chronic kidney disease in children study. Clin J Am Soc Nephrol. 2009;4:812–819 [DOI] [PMC free article] [PubMed] [Google Scholar]