Abstract
Context:
Graves ophthalmopathy (GO) is an autoimmune disorder characterized by increased adipogenesis and hyaluronan (HA) production by orbital fibroblasts. Circulating autoantibodies (thyroid-stimulating antibodies [TSAbs]) directed at the thyrotropin receptor (TSHR) on these cells stimulate or augment these cellular processes. A recently developed drug-like small molecule inverse agonist of TSHR, NCGC00229600, termed 1, binds to TSHR and blocks basal and stimulated signal transduction.
Objective:
The purpose of this article was to determine whether 1 might inhibit HA production and relevant signaling pathways in orbital fibroblasts cultured in the presence of monoclonal TSAbs or bovine TSH (bTSH).
Design:
Primary cultures of undifferentiated GO orbital fibroblasts (n = 13) were untreated or treated with a TSAb (M22 or MS-1) or bTSH in serum-free medium, with or without 1 or a TSHR neutral antagonist, NCGC00242595, termed 2, which does not inhibit basal signaling but does inhibit stimulated signaling.
Main Outcome Measures:
cAMP production, Akt phosphorylation (Ser473pAkt in media and immunoblotting for pAkt/total Akt), and HA production were analyzed.
Results:
Compound 1 inhibited basal cAMP, pAkt, and HA production and that stimulated by M22 in undifferentiated orbital fibroblasts. Inhibition of HA production was dose-dependent, with a half-maximal inhibitory dose of 830 nM. This compound also inhibited MS-1- and bTSH-stimulated cAMP, pAkt, and HA production. Compound 2 did not inhibit basal HA production but did inhibit M22-stimulated HA production.
Conclusions:
Because cAMP, pAkt, and HA production are fibroblast functions that are activated via TSHR signaling and are important in the pathogenesis of GO, small molecule TSHR antagonists may prove to be effective in the treatment or prevention of the disease in the future.
Graves ophthalmopathy (GO) is an autoimmune disorder of the orbit characterized by inflammation and expansion of the orbital adipose tissues and extraocular muscles. Orbital fibroblasts are the target cells of this autoimmune process, and expansion of the orbital tissues is in part attributable to increased adipogenesis and production of hyaluronan (HA, hyaluronic acid) by these cells (1, 2). Our recent studies suggest that a monoclonal stimulatory thyrotropin receptor (TSHR) autoantibody (thyroid-stimulating antibody, TSAb), termed M22, engages the receptor expressed on orbital fibroblasts and enhances both adipogenesis (3) and HA production (4) primarily via activation of the phosphoinositol 3-kinase (PI3K)/phospho-Akt/mammalian target of rapamycin signaling cascade. Other investigators have shown similarly increased HA production in differentiated orbital fibroblasts activated by immunoglobulin G from the sera of patients with Graves disease (GD-IgG) (5) or transfected with an activating mutant TSHR (6).
Small molecule antagonists of TSHR bind within the transmembrane region of the receptor, acting in an allosteric manner to block signaling but not the binding of TSH or TSAb (7). These compounds are emerging as a novel class of therapeutic agents, having great potential in the treatment of patients with GD or GO (8, 9). In contrast to the already existing treatment options, TSHR antagonists might specifically target the underlying pathogenic mechanisms. Both our group (10) and that of van Zeijl et al (11) have previously shown that M22 stimulates cAMP production by GO orbital fibroblasts and that this stimulation can be inhibited by TSHR small molecule antagonists (11, 12). We undertook the current study to determine whether TSH or another TSAb might stimulate cAMP production, phosphorylation of Akt, or HA production in undifferentiated orbital fibroblasts. We also investigated whether the small molecule TSHR antagonist NCGC00229600 (13), termed 1, might inhibit these TSAb-induced orbital fibroblast functions thought to be important in the development of GO.
Materials and Methods
Cell culture
Orbital adipose tissue specimens were obtained from euthyroid patients with GO undergoing orbital decompression surgery for severe disease (n = 13). Of these patients, 5 were treated with corticosteroids before undergoing orbital decompression surgery. Seven patients received radioactive iodine treatment, 3 had taken antithyroid medication, 1 underwent thyroidectomy, and 2 received no treatment for hyperthyroidism. Seven patients were current smokers. Individual experiments used cells derived from 1 of 2 different sets of patients (either n = 6 or n = 7). The tissues were minced and placed directly in plastic culture dishes, allowing preadipocyte fibroblasts to adhere and proliferate as we described previously (14). The cells were initially grown in a humidified 5% CO2 incubator at 37°C in medium 199 containing 20% fetal bovine serum (FBS) (HyClone Laboratories, Inc, Logan, Utah), gentamicin (20 μg/mL), and penicillin (100 U/mL). They were subsequently maintained in 75-mm2 flasks in medium 199 containing antibiotics and 10% FBS, without the nutrients necessary for adipocyte differentiation. The Mayo Clinic institutional review board approved these studies, which were carried out according to official guidelines.
Some of the experiments were designed to assess the impact of the small molecule TSHR antagonist 1 (13) on adenylate cyclase or PI3K/Akt signaling in GO orbital cell cultures treated with the monoclonal TSAb M22 or MS-1 or with bovine TSH (bTSH) (T8931; Sigma-Aldrich, St Louis, Missouri). M22 was obtained from Kronus (M22–1b; Boise, Idaho) (15). MS-1 was kindly supplied by Dr. Terry Davies (Mount Sinai School of Medicine, New York, New York) (16). Because 1 has inverse agonist properties on TSHR signaling, as a control we used the small molecule TSHR neutral antagonist NCGC00242595 (17), termed 2, which also inhibits agonist-dependent TSHR signaling but has no effect on constitutive TSHR signaling in thyrocytes. The use of both ligands in comparison allowed us to demonstrate that the inhibition of basal activity by 1 is specific and caused by the inverse agonist properties. Compounds 1 and 2 were initially dissolved as a 10 mM solution in dimethyl sulfoxide (DMSO). Accordingly, the culture media were adjusted to contain the same DMSO concentration as was present in the parallel cultures treated with the various doses of 1 or 2. For these studies, GO orbital cells were grown to confluence in medium 199 containing 10% FBS in 24-well plates. Cells were seeded into 6-well plates, grown to confluence, and serum-starved for 24 hours. Cultures were pretreated with 1 (1.0 nM–30 μM), 2 (1.0–30 μM), or vehicle (0.1%–0.3% DMSO) for 30 to 60 minutes to allow receptor binding and then were incubated for between 10 and 60 minutes in serum-free medium containing 1, 2, or vehicle and M22 (100 ng/mL; 0.667 pM), MS-1 (10 μg/mL), or bTSH (10 U/L). Some experiments also contained the isotype control IgG2 (10 μg/mL; BD Biosciences, San Diego, California).
Other experiments measured the effect of 1 on the production of HA in cultures treated with M22, MS-1, or bTSH. For these studies, GO orbital cells were grown to confluence in medium 199 containing 10% FBS in 24-well plates. Cultures were deprived of serum for 24 to 48 hours before the start of experiments, with wells receiving 1, 2, or vehicle at the indicated concentrations for the final 24 hours of preincubation. Cells were cultured for an additional 48 hours in serum-free medium containing M22, MS-1, or bTSH, with or without the same concentrations of 1, 2, or vehicle. Some experiments also contained the isotype control IgG2 (10 μg/mL).
Measurement of cAMP production
Extracellular levels of cAMP were measured by a cAMP assay (KGE002B; R&D Systems, Minneapolis, Minnesota) using a polyclonal antibody that competitively binds cAMP in the standards or sample supernatants. Results are expressed as fold change (increase or inhibition) in cAMP production relative to that in appropriate controls.
Measurement of Akt phosphorylation
Phosphorylation of Akt protein was assessed using a commercial ELISA kit [DUOSet IC Human Phospho-Akt (S473) Pan Specific ELISA; R&D Systems]. This cell-based kit quantifies activated (serine 473 phosphorylated) phospho-Akt protein (pAkt) relative to total Akt protein. Results are expressed as fold change (increase or inhibition) in the pAkt-to-Akt ratio relative to that in parallel control or comparison cultures.
pAkt was also measured using Western blotting. Confluent cultures were serum-starved for 24 hours and then were either untreated or pretreated for 30 minutes with 1 (30 μM). Cells were then cultured for 30 minutes in serum-free medium, with or without 1, containing M22 (100 ng/mL) or control. Cell protein was extracted using a complete lysis-M, EDTA-free protocol (Roche, Indianapolis, Indiana)a to extract total cytoplasmic and nuclear protein. Extracts were subjected to electrophoresis on 4% to 12% Bis-Tris gel, electrotransferred to polyvinylidene fluoride membrane, and blotted with primary antibody against total Akt, Ser473pAkt, or glyceraldehyde 3-phosphate dehydrogenase (9272, 9271, and 2118, respectively; Cell Signaling Technology, Danvers, Massachusetts) at 1:1000 dilution. The appropriate secondary IgG-horseradish peroxidase–linked conjugate (7074; Cell Signaling Technology) at 1:2000 dilution was applied, followed by enhanced chemiluminescence detection. Findings are expressed both visually by a representative immunoblot and via densitometric analysis of bands produced (pAkt/total Akt).
Measurement of HA production
HA production was assayed per the manufacturer's instructions using a commercial HA ELISA Kit (K-1200; Echelon Biosciences Inc, Salt Lake City, Utah) by measuring the amount of HA released into the culture medium during the 48-hour incubation. Results are expressed as fold change (increase or inhibition) compared with that in parallel controls.
Statistical analyses
The paired t test was used to evaluate differences in means for continuous variables, with values presented as the mean ± SEM. Differences between values was considered significant at a value of P < .05. The Mann-Whitney rank sum test was used to assess differences between groups.
Results
Inhibition by TSHR antagonists of cAMP production stimulated by M22
We found significant stimulation of cAMP production in GO orbital fibroblasts (n = 6) treated for 30 minutes with M22 (increase of 43.0% ± 2.25% [mean ± SEM], P = .001) (Figure 1A). Similar results were observed at 10 minutes (data not shown). We also examined the dose-dependent effects of M22 or bTSH on cAMP production and observed significant stimulation by M22 at 1.0, 5.0, 10, or 100 ng/mL and by bTSH at 0.1, 1.0, or 10 U/L (Supplemental Figure published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org).
Figure 1.
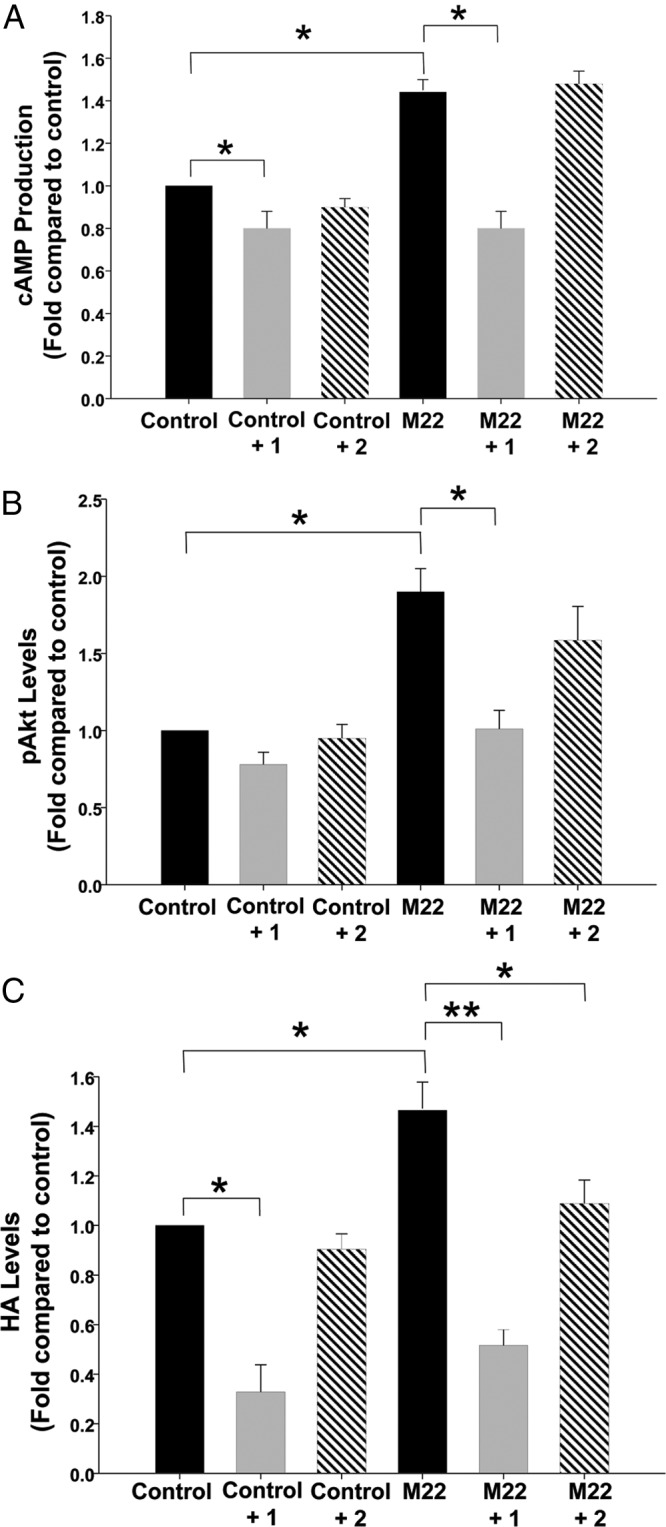
Effect of treatment with M22 (100 μg/mL) with or without 1 or 2 (10 μM each) on cAMP production (A), phosphorylation of Akt (B), or HA production (C) in undifferentiated GO orbital fibroblast cultures (n = 6). Results are expressed as mean ± SEM fold change relative to control cultures or to parallel M22-treated cultures. *P < .05; **P < .001.
Addition of 1 to the GO cultures significantly decreased constitutive (ligand-independent) cAMP production and inhibited M22-stimulated cAMP production to levels below control levels and to the same level that it inhibited constitutive cAMP production (Figure 1A). In contrast, as expected, the neutral antagonist 2 did not decrease constitutive cAMP production in control cultures and did not inhibit M22 stimulation of cAMP production in these cultures.
Inhibition by TSHR antagonists of Akt phosphorylation stimulated by M22
We found that phosphorylation of Akt (as assessed by ELISA) was significantly increased when GO orbital cultures were exposed for 30 minutes to M22 (increase of 91.0% ± 5.7%, P = .002) (Figure 1B). Similar results were observed at 10 minutes.
Addition of 1 to the GO cultures significantly inhibited Akt phosphorylation induced by M22 to control levels at 30 minutes as assessed by ELISA (Figure 1B). Similar results were observed at 10 minutes. A trend toward lower levels of Akt phosphorylation was seen when 1 was added to control cultures, but this effect was not statistically significant. As expected, the neutral antagonist 2 did not decrease constitutive Akt phosphorylation in control cultures. In contrast, the expected inhibition by 2 of M22-stimulated pAkt production did not reach significance but showed a trend in that direction. This lack of significance may be explained both by the relatively small magnitude of the decrease and by the wide variability in levels of HA produced between the various patient strains.
Western blotting experiments (n = 3) with densitometric quantitation confirmed these results, showing an increase in Akt phosphorylation (Ser473pAkt/total Akt) in cultures treated with M22 (fold change of 1.5 ± 0.9, P = .003) with inhibition of this by 1 to control levels (P = .023) (Figure 2).
Figure 2.
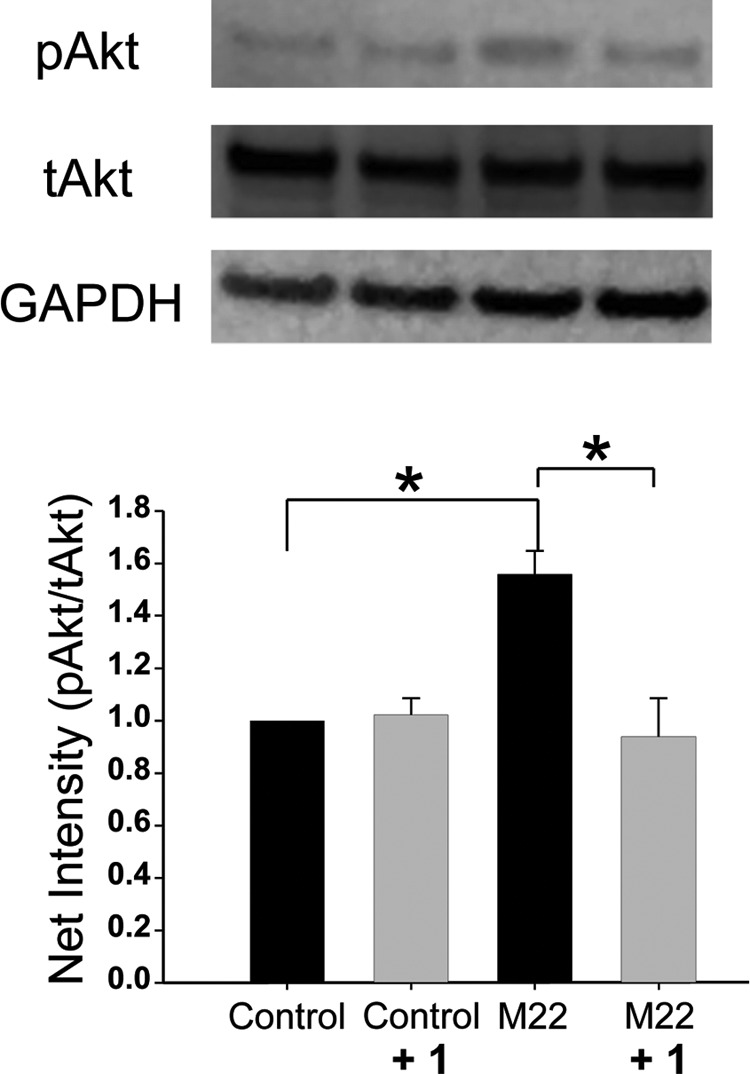
Top, representative Western blots showing total Akt (tAkt), Ser473 phosphorylated Akt (pAkt), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) protein bands in confluent undifferentiated GO orbital fibroblast cultures exposed to 1 (30 μM), M22 (100 μg/mL), both treatments, or the control. Bottom, densitometric quantitation of net intensity of bands (pAkt/tAkt) in Western blots (n = 3) is shown.
Inhibition by 1 of HA production stimulated by M22
Treatment of GO orbital fibroblasts with M22 resulted in increased production of HA (increase of 48.0% ± 2.6%, P = .001) relative to that with control media (Figure 1C). Treatment of M22-stimulated cultures with 1 (10 μM) resulted in significant inhibition of HA production to levels below control levels. In addition, 1 reduced constitutive (agonist-independent) HA production (inhibition of 61.7% ± 8.5%) compared with that for control media (P = .008). In contrast, the neutral antagonist 2 (10 μM) did not decrease constitutive HA production in control cultures. However, as for 1, 2 significantly inhibited M22-stimulated HA production to control levels in these cells.
We also examined the impact of various concentrations of 1 and 2 on ligand-independent and M22-stimulated HA production in GO orbital cells. We found that 1 significantly inhibited constitutive HA production at 100 nM and higher doses (between 24.9% ± 10.9% and 61.7% ± 8.5%) compared with that for control media (Figure 3A). In contrast, 2 (1.0–30 μM) did not inhibit constitutive HA production at any dose. We also demonstrated that 1 significantly inhibited M22-stimulated HA production to levels below control levels at 100 nM and higher doses and that 2 significantly inhibited M22-stimulated HA production at 10 μM and higher doses (Figure 3B).
Figure 3.
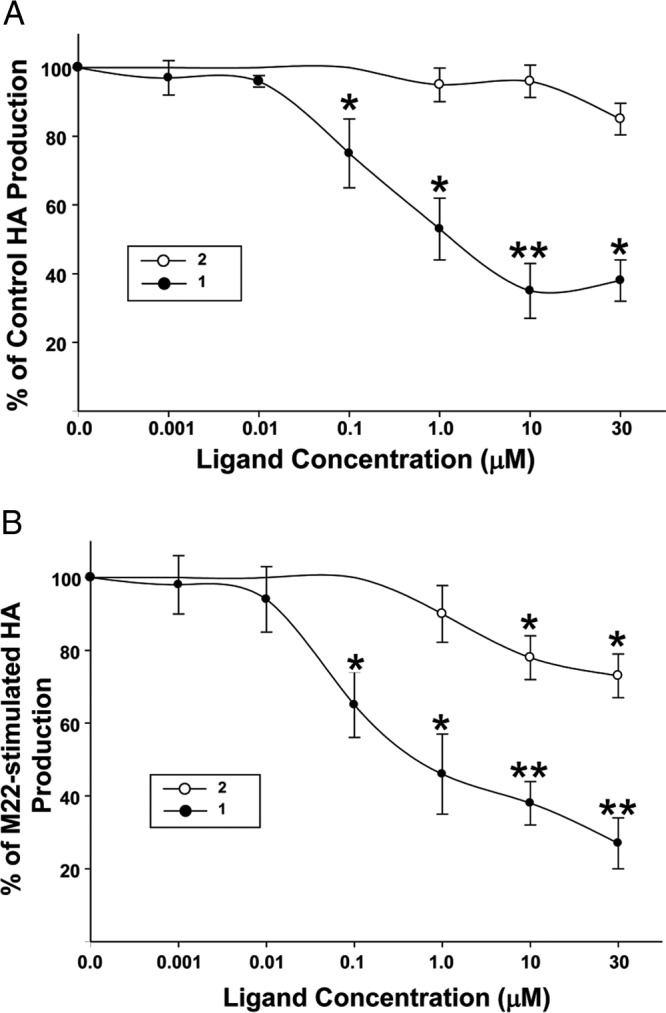
Impact of the concentration of 1 or 2 on HA production in ligand-independent (A) and M22-stimulated (B) cultures of undifferentiated GO orbital fibroblasts (n = 6). Cultures were treated with 1 (●) or 2 (○) at the indicated concentrations (A) or with M22 (100 μg/mL) plus 1 or 2 at the indicated concentrations (B). Culture media were adjusted to contain the same DMSO concentration as was present in the parallel cultures treated with the various doses of 1 or 2. ELISA results are expressed as percent decrease (mean ± SEM) in HA production relative to that in parallel untreated cultures (A) or percent decrease (mean ± SEM) in HA production relative to that in parallel M22-treated cultures (B). *P < .05; **P < .001, for inhibition of HA production relative to that of parallel untreated cultures or to that of parallel M22-treated cultures without 1 or 2 (*P < .05).
Furthermore, we examined these data with respect to the impact of dose of 1 on percent inhibition of M22-stimulated HA production and determined that the concentration of 1 required to half-maximally inhibit (IC50) M22-stimulated HA production was 830 nM (Figure 3B). Inhibition of HA production to below control levels was evident when cells were treated with higher concentrations of 1, representing inhibition of constitutive receptor activity as well.
Stimulation of cAMP production, Akt phosphorylation, and HA production by other TSHR agonists and their inhibition by 1
To determine whether cAMP production, Akt phosphorylation, or HA production can be stimulated by other TSHR ligands and whether the stimulation can be inhibited by 1, we performed another set of experiments (n = 7) using bTSH (10 U/L) or the TSAb MS-1 (10 μg/mL) as an agonist. We demonstrated that bTSH stimulated cAMP, pAkt, and HA production in GO orbital fibroblasts (increases of 102% ± 3.0%, P = .005; 144% ± 13.2%, P = .02; and 31.0% ± 4.8%, P = .04, respectively) as did MS-1 (increases of 67.0% ± 5.6%, P = .002; 229% ± 23.0%, P = .046; and 28.0% ± 1.25%, P < .001, respectively) (Figure 4, A–C). In addition, 1 (30 μM) inhibited cAMP production induced by bTSH to near control levels and inhibited HA production to below control levels, with a trend toward a decrease in TSH-stimulated Akt phosphorylation that did not reach significance. Similarly, 1 inhibited MS-1-stimulated cAMP and HA production to levels below control levels and pAkt to a level near the control levels.
Figure 4.
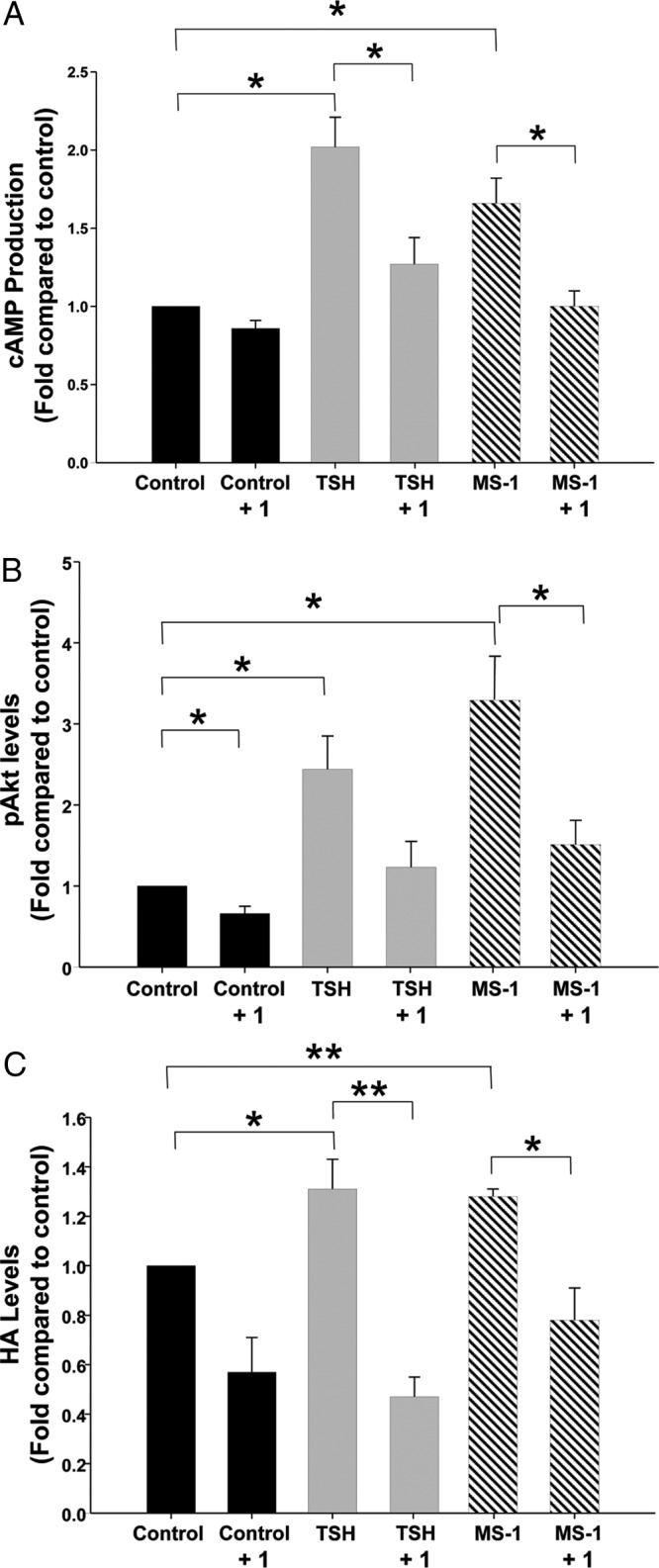
Effect of treatment with bTSH (10 U/L) or MS-1 (10 μg/mL) with or without 1 (30 μM) on cAMP production (A), phosphorylation of Akt (B), or HA production (C) in undifferentiated GO orbital fibroblast cultures (n = 7). Results are expressed as mean ± SEM fold change relative to that of parallel untreated cultures or to that of parallel MS-1 or TSH-treated cultures. *P < .05.
Discussion
Studies from several laboratories support important roles for HA accumulation (18) and enlargement of adipose tissues within the orbit in the development of GO (1, 2). We and others have shown that the TSAb M22 (4, 5), stimulatory IgG obtained from the sera of patients with Graves hyperthyroidism (5), or cells transfected with a stimulatory mutant TSHR (6) increase HA production, gene expression of hyaluronan synthases, or adipogenesis (3) in human orbital cells. The current study demonstrates for the first time that MS-1, another monoclonal TSAb, increases HA production and both cAMP and pAkt generation in GO orbital fibroblasts, supporting our earlier findings regarding M22 and TSH as activators of Gαs and perhaps Gαq effectors and as stimulators of HA production in GO orbital cells (3, 4). Our findings are similar to those of Morshed et al (16) who used rat thyrocytes (FRTL-5) to characterize various signaling cascades activated by TSH and TSAbs.
The small molecule TSHR antagonist 1 has been shown to inhibit both constitutive and GD-IgG– or TSH-stimulated cAMP production in model cells with stable overexpression of human TSHR (HEK-EM293) and in human thyroid cell cultures (13). Similarly, the current studies showed significant inhibition of MS-1– or M22-stimulated HA production and Akt phosphorylation in undifferentiated orbital fibroblasts by 1 to control levels or lower and confirmed our earlier demonstration of cAMP inhibition by this compound (10). van Zeijl and colleagues (11) recently reported that another low-molecular-weight TSHR antagonist, Org-274179-0, completely blocks cAMP production induced by human recombinant TSH, GD-IgG, or M22 in differentiated GO orbital fibroblasts at nanomolar concentrations. We recently demonstrated similar inhibition by 1 at 30 μM of M22-stimulated cAMP production (10). In the current studies, we showed inhibition of M22-induced cAMP production, Akt phosphorylation, and HA production using 1 at a concentration of 10 μM (Figures 1, 2, and 4). Although lower doses of 1 were not studied in terms of cAMP or pAkt production, we did determine the impact of concentrations of 1 less than 10 μM with respect to inhibition of M22-induced HA production. We found that high doses of 1 inhibited M22-stimulated HA production to below control levels, representing inhibition of constitutive receptor activity as well. The compound Org 274179-0 has been shown in experiments examining the cAMP response to have an IC50 in the low nanomolar range (11 nM) (19). Our data show that the IC50 of 1 for inhibition of HA production is 830 nM, indicating that the potency of 1 is higher for HA production than for cAMP production (2.0 μM) (20).
A difference between our study and the study of van Zeijl and colleagues is that they used GO orbital fibroblasts that had undergone adipocyte differentiation, whereas our cells were not differentiated. Because orbital fibroblasts increase their TSHR expression as they differentiate into adipocytes (21, 22), it is likely that the orbital cells used by van Zeijl and colleagues had a greater density of TSHR than did our cells. In addition, van Zeijl et al (5, 23) have shown that differentiated orbital fibroblasts respond with more robust production of HA when stimulated by M22 or GD-IgG than do undifferentiated cells. These findings suggest that differentiated orbital fibroblasts may model mid-stage to late-stage GO, whereas undifferentiated fibroblasts might reflect an earlier stage of the disease. Further studies will determine whether small molecule antagonists of TSHR also inhibit adipogenesis in orbital fibroblasts, a process primarily dependent on PI3K/Akt activation (3).
These studies support the concept that TSAbs play a central role in GO pathogenesis and clearly link TSHR ligation on orbital fibroblasts to accumulation of HA within the orbit (2). Inhibition of this pathologically important cellular function to control levels or lower by 1 represents the strongest evidence to date, suggesting that similar drug-like small molecule antagonists of TSHR with increased potency may be beneficial in the future as a treatment for GO (8, 9). The demonstration of this effect in undifferentiated orbital fibroblasts, a model of early-stage disease, suggests that these compounds may be particularly useful in early GO to prevent extensive connective tissue remodeling or for the prevention of GO in patients with Graves hyperthyroidism.
Acknowledgments
This work was supported in part by the National Institute of Diabetes and Digestive and Kidney Diseases (Grant DK77814).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- bTSH
- bovine TSH
- DMSO
- dimethyl sulfoxide
- FBS
- fetal bovine serum
- GD
- Graves disease
- GD-IgG
- immunoglobulin G from the sera of patients with Graves disease
- GO
- Graves ophthalmopathy
- HA
- hyaluronan
- PI3K
- phosphoinositol 3-kinase
- TSAb
- thyroid-stimulating antibody
- TSHR
- thyrotropin receptor.
References
- 1. Bahn RS. Graves' ophthalmopathy. N Engl J Med. 2010;362:726–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wiersinga WM. Autoimmunity in Graves' ophthalmopathy: the result of an unfortunate marriage between TSH receptors and IGF-1 receptors? J Clin Endocrinol Metab. 2011;96:2386–2394 [DOI] [PubMed] [Google Scholar]
- 3. Kumar S, Nadeem S, Stan MN, Coenen M, Bahn RS. A stimulatory thyrotropin receptor antibody enhances adipogenesis via phosphoinositide 3-kinase activation in orbital preadipocytes from patients with Graves' ophthalmopathy. J Mol Endocrinol. 2011;46:155–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kumar S, Iyer S, Bauer H, Coenen M, Bahn RS. A stimulatory thyrotropin receptor antibody enhances hyaluronic acid synthesis in Graves' orbital fibroblasts: inhibition by an IGF-I receptor blocking antibody. J Clin Endocrinol Metab. 2012;97:1681–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van Zeijl CJ, Fliers E, van Koppen CJ, et al. Thyrotropin receptor-stimulating Graves' disease immunoglobulins induce hyaluronan synthesis by differentiated orbital fibroblasts from patients with Graves' ophthalmopathy not only via cyclic adenosine monophosphate signaling pathways. Thyroid. 2011;21:169–176 [DOI] [PubMed] [Google Scholar]
- 6. Zhang L, Bowen T, Grennan-Jones F, et al. Thyrotropin receptor activation increases hyaluronan production in preadipocyte fibroblasts: contributory role in hyaluronan accumulation in thyroid dysfunction. J Biol Chem. 2009;284:26447–26455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Neumann S, Kleinau G, Costanzi S, et al. A low-molecular-weight antagonist for the human thyrotropin receptor with therapeutic potential for hyperthyroidism. Endocrinology. 2008;149:5945–5950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bahn RS. Autoimmunity and Graves' disease. Clin Pharmacol Ther. 2012;91:577–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gershengorn MC, Neumann S. Update in TSH receptor agonists and antagonists. J Clin Endocrinol Metab. 2012;97:4287–4292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Neumann S, Pope A, Geras-Raaka E, et al. A drug-like antagonist inhibits thyrotropin receptor-mediated stimulation of cAMP production in Graves' orbital fibroblasts. Thyroid. 2012;22:839–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van Zeijl CJ, van Koppen CJ, Surovtseva OV, et al. Complete Inhibition of rhTSH-, Graves' disease IgG-, and M22-induced cAMP production in differentiated orbital fibroblasts by a low-molecular-weight TSHR antagonist. J Clin Endocrinol Metab. 2012;97:E781–E785 [DOI] [PubMed] [Google Scholar]
- 12. Allen MD, Neumann S, Gershengorn MC. Small-molecule thyrotropin receptor agonist activates naturally occurring thyrotropin-insensitive mutants and reveals their distinct cyclic adenosine monophosphate signal persistence. Thyroid. 2011;21:907–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Neumann S, Eliseeva E, McCoy JG, et al. A new small-molecule antagonist inhibits Graves' disease antibody activation of the TSH receptor. J Clin Endocrinol Metab. 2011;96:548–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bahn RS, Gorman CA, Johnson CM, Smith TJ. Presence of antibodies in the sera of patients with Graves' disease recognizing a 23 kilodalton fibroblast protein. J Clin Endocrinol Metab. 1989;69:622–628 [DOI] [PubMed] [Google Scholar]
- 15. Sanders J, Evans M, Premawardhana LD, et al. Human monoclonal thyroid stimulating autoantibody. Lancet. 2003;362:126–128 [DOI] [PubMed] [Google Scholar]
- 16. Morshed SA, Latif R, Davies TF. Characterization of thyrotropin receptor antibody-induced signaling cascades. Endocrinology. 2009;150:519–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boutin A, Allen MD, Geras-Raaka E, Huang W, Neumann S, Gershengorn MC. Thyrotropin receptor stimulates internalization-independent persistent phosphoinositide signaling. Mol Pharmacol. 2011;80:240–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Smith TJ. Pathogenesis of Graves' orbitopathy: a 2010 update. J Endocrinol Invest. 2010;33:414–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. van Koppen CJ, de Gooyer ME, Karstens WJ, et al. Mechanism of action of a nanomolar potent, allosteric antagonist of the thyroid-stimulating hormone receptor. Br J Pharmacol. 2012;165:2314–2324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Neumann S, Huang W, Eliseeva E, Titus S, Thomas CJ, Gershengorn MC. A small molecule inverse agonist for the human thyroid-stimulating hormone receptor. Endocrinology. 2010;151:3454–3459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Valyasevi RW, Erickson DZ, Harteneck DA, et al. Differentiation of human orbital preadipocyte fibroblasts induces expression of functional thyrotropin receptor. J Clin Endocrinol Metab. 1999;84:2557–2562 [DOI] [PubMed] [Google Scholar]
- 22. Valyasevi RW, Harteneck DA, Dutton CM, Bahn RS. Stimulation of adipogenesis, peroxisome proliferator-activated receptor-γ (PPARγ), and thyrotropin receptor by PPARγ agonist in human orbital preadipocyte fibroblasts. J Clin Endocrinol Metab. 2002;87:2352–2358 [DOI] [PubMed] [Google Scholar]
- 23. van Zeijl CJ, Fliers E, van Koppen CJ, et al. Effects of thyrotropin and thyrotropin-receptor-stimulating Graves' disease immunoglobulin G on cyclic adenosine monophosphate and hyaluronan production in nondifferentiated orbital fibroblasts of Graves' ophthalmopathy patients. Thyroid. 2010;20:535–544 [DOI] [PubMed] [Google Scholar]


