Abstract
Background:
Radioiodine (RAI) remains the mainstay of therapy for RAI-avid (RAIA) distant metastatic thyroid carcinoma. We previously demonstrated that RAI-refractory distant metastatic thyroid cancers commonly harbor BRAF mutations. However, the molecular profile of RAIA metastatic thyroid cancer is unknown. Here we describe the mutational profile of thyroid tumors from follicular cell-derived cancer (FCDTC) patients presenting with RAIA distant metastases. In addition, we aimed to correlate clinical outcomes of RAI therapy with clinicopathological factors and tumor mutational status.
Methods:
We retrospectively identified 43 patients with FCDTC who had RAI uptake in the lungs and/or bones on their initial 131I postablation scan. Primary tumors were genotyped for known mutations in thyroid cancer genes. Structural response to RAI was assessed 6–18 months after each administered RAI activity and at the end of follow-up.
Results:
RAS, BRAF, RET/PTC, and PIK3CA mutations were found in 42, 23, 10, and 2% of tumors, respectively, and the remaining 23% were wild type. None of these patients achieved cure after repeat RAI therapies, and most patients (54%) experienced disease progression despite repeated RAI administration. There was an increased prevalence of RAS mutations in these RAIA tumors. RAS-mutant cancers were more likely to concentrate iodine on diagnostic whole body scans. Despite this, structural response to RAI was not influenced by tumor genotype.
Conclusions:
RAIA metastatic FCDTC are overrepresented with RAS mutations, whereas RAI refractory metastatic thyroid cancers are enriched with BRAF mutations. Despite a seemingly preserved ability to concentrate iodine, RAI therapy is ineffective in achieving cure in most patients with RAIA metastatic FCDTC, even in RAS-mutant disease. These poor outcomes may be improved based on recent evidence that pretreatment with MAPK kinase 1/2 inhibitors enhances responses to RAI, particularly in patients with RAS-mutant tumors.
At the time of diagnosis, distant metastases are identified in 1–4% of papillary thyroid cancer patients (1–3) and 7–28% of follicular thyroid cancer patients (2, 3). Distant metastatic disease in older patients is associated with a 5-year mortality rate close to 50% (4). Whereas radioiodine (RAI) remains the mainstay of treatment for distant metastatic thyroid cancer, the clinical response is variable and is influenced by clinicopathological factors including age at diagnosis, tumor histology, size of metastatic foci, site of metastases, presence of fluorodeoxyglucose-positron emission tomography (FDG-PET) uptake, or visible RAI uptake on diagnostic 131I whole body scan before ablation (5–7).
We previously demonstrated that RAI refractory (RAIR) advanced metastatic thyroid cancers are enriched with BRAF mutations. BRAF tumors were more likely to be FDG-PET positive and were associated with decreased median survival (8, 9). Mice with a knock-in mutation of BRAF (BrafV600E) that express endogenous levels of this oncoprotein in thyroid cells develop papillary thyroid cancers with decreased expression of the sodium iodide symporter (Nis) (10), whereas mice with thyroid-specific knock-in of HrasG12V express normal levels of Nis (11). In transgenic mouse models, tumoral RAI avidity was diminished in BRAFV600E mutant tumors, an effect that was partially reversed using the MAPK kinase 1/2 (MEK1/2) inhibitor selumetinib or the BRAF kinase inhibitor PLX4720 (12). Similarly, RAI avidity was augmented in RAIR metastatic thyroid cancer patients after selumetinib pretreatment, with resultant objective biochemical response and significant tumor regression on cross-sectional imaging after RAI therapy. Interestingly, RAI therapy after selumetinib pretreatment was particularly effective in patients with NRAS-mutant tumors (13).
The molecular profile of the primary tumors in patients with RAI-avid (RAIA) distant metastatic thyroid cancers has not been previously described. Although children with well-differentiated papillary thyroid cancer often demonstrate intense RAI avidity in metastatic lesions, in our experience high-level RAI avidity in older patients with structurally identifiable distant metastases is more common in follicular-patterned lesions (follicular thyroid cancer and follicular variant papillary thyroid cancers). Therefore, we hypothesized that the primary tumors that gave rise to RAIA structural distant metastases would be enriched in RAS rather than BRAF mutations.
The goal of this retrospective study was to describe the mutational profile of primary thyroid tumors from follicular cell-derived thyroid cancer (FCDTC) patients presenting with RAIA distant metastasis to lungs and/or bones. In addition, we aimed to correlate disease-specific clinical outcomes of RAI therapy with clinicopathological factors and tumor genotype.
Subjects and Methods
Subjects
After obtaining Institutional Review Board approval, we reviewed the electronic medical records of 364 consecutive subjects with metastatic FCDTC that had RAI therapy at Memorial Sloan-Kettering Cancer Center (MSKCC) between 1990 and 2011. Subjects were included if they met all of the following conditions: 1) pathologically confirmed FCDTC; 2) total thyroidectomy preceding RAI ablation; 3) RAI uptake on postablation scan corresponding to at least 1 metastatic focus in lungs and/or bones or presence of diffuse uptake in lungs with no structural correlation; 4) on TSH-suppressive therapy for the duration of the study; 5) ≥ 1-year follow-up after RAI ablation unless 1 of the clinical endpoints (recurrence, thyroid cancer-related death) was reached earlier; 6) appropriate follow-up with unstimulated thyroglobulin (Tg) and Tg antibodies (TgAb) and cross-sectional imaging of the involved organ 6–18 months after each RAI therapy; and 7) formalin-fixed, paraffin-embedded (FFPE) tissue blocks available at MSKCC for genotyping. Six patients with elevated neck uptake at the time of the ablation (≥ 5%) were excluded. A total of 43 patients were thus selected for the study.
None of the patients included in the study had lesional dosimetry before RAI ablation or to subsequent RAI therapies. The choice of RAI activity was at the discretion of the treating physician.
Histopathological analysis
Tumors were classified according to the last World Health Organization criteria with the exception of tall cell variant (TCV-PTC) and poorly differentiated thyroid cancer (PDTC) (14). Tumors were classified as TCV if they contained ≥ 50% tall cells. The latter cell type was defined as having a height at least twice its width with an oncocytic cytoplasm. PDTCs were defined by proliferative grading features: ≥ 5 mitoses/10 high-power fields and/or tumor necrosis regardless of architectural pattern (15). This definition differs from the most recent Turin proposal that requires the presence of a solid/trabecular/insular growth pattern in addition to proliferative grading (16). The predominant type of tumor cells present in the PDTC was classified as papillary-like, follicular-like, tall cell, or oncocytic.
Mass spectrometry genotyping
DNA was extracted from 4 10-μm sections of each FFPE tissue block using the QIAamp DNA FFPE Tissue Kit (QIAGEN, Valencia, California). Mutation detection was performed as previously described (8). We used a mass spectrometry-based genotyping assay (Sequenom Mass array; Sequenom, San Diego, California), which consists of 111 assays that interrogate mutations in 16 genes, including the most common thyroid oncogenes such as BRAF, NRAS, HRAS, KRAS, PIK3CA, and AKT1. Because the mass spectrometry genotyping assays for codons 12 and 13 of HRAS were not informative, we designed primers for this region and sequenced all the tumors that were wild type for BRAF or other RAS mutations by Sanger sequencing (8).
Screening for RET/PTC and PAX8/PPARγ rearrangements
Tumors that were wild type for RAS and BRAF were evaluated for the presence of RET/PTC and PAX8/PPARγ rearrangements. RNA extraction from FFPE samples was done using RecoverAll Total Nucleic Acid Isolation Kit (Ambion, Camarillo, California) (17). Tumor cDNA was then used as a template for quantitative PCR to analyze for unbalanced expression of exons 10–11 relative to 12–13 of RET, which flank the rearrangement site in intron 11. Samples with 12–13 > 10–11 expression were screened for specific RET recombination events by multiplexed PCR with primers bracketing the fusion points of RET/PTC1, RET/PTC2, and RET/PTC3, respectively, as previously described (8, 18). Positive controls were cDNAs from TPC1 cells expressing RET/PTC1, PCCL3 cells expressing RET/PTC2, and a PTC sample expressing RET/PTC3. We screened for the PAX8/PPARγ rearrangements by RT-PCR using primers for all possible transcripts of PAX8/PPARγ (19). cDNA from follicular thyroid cancer samples harboring the rearrangement were used as positive controls. GAPDH was used as internal control. PCR products were resolved by 2% agarose gel electrophoresis.
Clinical endpoint
Objective outcome to therapy (change in Tg and/or structurally evident disease) was assessed after initial RAI ablation and again after any subsequent RAI therapy. Patients achieving an undetectable suppressed Tg level and no structural evidence of disease were classified as having no evidence of disease. Response after RAI in patients with structurally evident disease at the time of the ablation was classified using RECIST 1.1-like criteria. For patients with macronodular lung lesions, we measured the sum of the longest diameter of all lesions that were more than 1 cm on chest computed tomography. Comparisons were done with the same imaging modalities. Patients were classified as having achieved: 1) complete response (CR) if they had disappearance of all lesions; 2) partial response (PR) if they had a ≥ 30% decrease in the sum of the largest diameter of all lesions compared to baseline, and no new lesions; 3) stable disease (SD) if the sum of the largest diameter compared to baseline was between −30% and +20%, with no new lesions; and 4) progressive disease (PD) if they had a ≥ 20% increase in the sum of the diameter of all lesions compared to baseline or if they developed 1 or more new lesions. Patients with lung lesions < 1 cm or bone-only metastases were classified as follows: 1) CR if they had disappearance of all lesions; 2) PR if they had disappearance of at least 1 lung lesion and/or ≥ a 3-mm decrease in the size of at least 1 lung lesion and/or no new lung or bone metastases; 3) SD if they had < a 3-mm change in size and no change in number of the lung nodules and bone lesions, if any; and 4) PD if they developed 1 or more new lung lesions and/or had ≥ a 3- mm increase in size of any of lung lesions or developed new bone lesions. A ≥ 20% increase in suppressed Tg was defined as biochemical progression, a ≥ 20% decrease was classified as biochemical regression, and Tg values between +20% and −20% of baseline suppressed Tg before remnant ablation were defined as stable biochemical evidence of disease.
Laboratory studies
Between 1990 and 1997, various Tg assays were used with functional sensitivities of approximately 1 ng/mL. Starting in 1998, all Tg values were measured using the Dynotest-TgS immunoradiometric assay (Brahms, Inc., Berlin, Germany; functional sensitivity, 0.6 ng/mL, normalized to Certified Reference Material 457). TgAb were measured using Siemens Immulite 2500 Chemistry Analyzer (Siemens Healthcare, Erlangen, Germany).
Statistical methods
Continuous data are presented as means and standard deviation or median and range, as appropriate for each variable. Categorical comparisons were performed with Fisher's exact or χ2 test, and continuous variables were compared using Student t test or one-way ANOVA, as appropriate. Correlation analyses were performed using Pearson's linear correlation test. Analyses were performed using SPSS software (version 18.0.1; SPSS, Inc, Chicago, Illinois). A P value of ≤ 0.05 was considered statistically significant.
Results
Clinicopathological characteristics of the cohort
The clinicopathological characteristics of the 43 patients included in this analysis are summarized in Table 1. Most were males (60%), with a predominance of pulmonary metastases that in 72% of patients were < 1 cm. Forty-two percent of the primary tumors were nonfollicular-variant PTC lesions (28% classical, 9% TCV, and 5% other variants), 33% were PTDC, and 26% were follicular-patterned lesions (19% follicular variant of papillary thyroid carcinoma, 5% follicular thyroid carcinoma, 2% Hurthle cell carcinoma). Median baseline suppressed Tg level before RAI ablation was 9.6 ng/mL (range, undetectable to 54 000 ng/mL).
Table 1.
Clinical Characteristics of the Cohort
| n | ||
|---|---|---|
| Age at diagnosis, y | 43 | |
| Mean ± SD | 54 ± 20 | |
| Median | 58 | |
| Range | 13–85 | |
| Gender | ||
| Male | 60% | 26 |
| Histology | ||
| Poorly differentiated | 33% | 14 |
| Classical papillary | 28% | 12 |
| Follicular variant papillary | 19% | 8 |
| Tall cell variant papillary | 9% | 4 |
| Other papillary | 5% | 2 |
| Follicular | 5% | 2 |
| Hurthle cell | 2% | 1 |
| Structurally evident metastases at ablation | ||
| None | 7 | |
| Lungs | 57% | 20 |
| Mixed | 37% | 13 |
| Bone | 6% | 2 |
| Size of lung metastasis | 32 | |
| <1 cm | 72% | 23 |
| ≥ 1 cm | 28% | 9 |
| AJCC stage | ||
| II | 42% | 18 |
| IV | 58% | 25 |
| FDG-PET imaging | 29 | |
| Abnormal | 55% | 16 |
| Normal | 45% | 13 |
| Preparation for ablation | ||
| rhTSH | 67% | 29 |
| Thyroid hormone withdrawal | 33% | 14 |
| Suppressed Tg before ablation (ng/mL)a | 34 | |
| Mean ± SD | 2536.4 ± 9602.7.5 | |
| Median | 9.6 | |
| Range | <0.2–54 000 | |
| DxWBS before ablation | ||
| Positive | 44% | 19 |
| 131I Ablation administered activity (mCi) | ||
| Mean ± SD | 195.2 ± 77 | |
| Median | 155 | |
| Range | 76–501 | |
| RAI therapies including ablation, n | ||
| Mean ± SD | 2.2 ± 1.2 | |
| Median | 2 | |
| Range | 1–5 | |
| 131I Cumulative administered activity (mCi) | ||
| Mean ± SD | 558.4 ± 439 | |
| Median | 501 | |
| Range | 76–1805 | |
| Follow-up duration, y | ||
| Mean ± SD | 5.0 ± 4.2 | |
| Median | 3.6 | |
| Range | 1- 17 |
Abbreviation: rhTSH, recombinant human TSH; AJCC, American Joint Committee on Cancer.
Two patients had positive TgAb at RAI ablation and reverted to detectable TgAb by the end of the study.
All patients had RAI uptake in either the lungs or the bone on the postablative 131I whole body scan, but only 46% had corresponding uptake on the preablative diagnostic whole body scan (DxWBS). Seven patients had RAI uptake in distant sites after RAI ablation without corresponding lesions on cross-sectional imaging. The median administered ablative 131I activity was 155 mCi (range, 76–501 mCi). Most patients received additional RAI treatment (63%, median 2, range 1–5). The median follow-up was 3.6 years (range, 1–17).
Mutational analysis of the primary thyroid tumors
Oncogenic mutations were found in 33 of 43 primary tumors (77%) (Figure 1). This included 18 RAS (18 of 43, or 42%; 14 NRAS, 2 HRAS, and 2 KRAS), 10 (23%) BRAF, and 4 (10%) RET/PTC mutations. One sample (2%) harbored a PIK3CA mutation. Ten (23%) thyroid tumors did not have an identifiable gene mutation.
Figure 1.
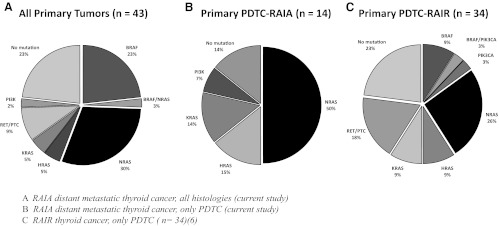
Mutational profile of primary thyroid tumors of patients with metastatic thyroid cancer divided by histology and RAI avidity.
Structural response to RAI in metastatic FCDTC
Structural response to RAI was assessed 6–18 months after RAI ablation and after each RAI activity administered (Table 2). None of the 36 patients with structurally apparent metastatic thyroid cancer achieved complete resolution of their disease after RAI ablation or after subsequent RAI therapies. Twenty-three patients (64%) had either SD or PR, and 13 (30%) had disease progression after RAI ablation. The 2 groups did not differ by age at diagnosis, sex, histology, size of primary tumor, preparation method for RAI ablation (recombinant human TSH vs thyroid hormone withdrawal), presence of FDG-PET uptake, uptake on DxWBS, and baseline Tg levels. Whereas the number of administered RAI activities per patient at the end of the study was the same between the 2 groups, the RAI activity given at ablation was higher in the patients with PD than those with SD/PR (262 ± 188 vs 179 ± 64 mCi; P = .009).
Table 2.
Structural Response of Distant Metastases to RAI
| n | ||
|---|---|---|
| Structural response of distant metastasis to RAI ablation | 43 | |
| SD | 35% | 15 |
| PD | 30% | 13 |
| PR | 19% | 8 |
| No structural disease | 16% | 7 |
| Time to structural progression after RAI ablation, mo | 16 | |
| Mean ± SD | 13.3 ± 10 | |
| Median | 10 | |
| Range | 4–43 | |
| Structural response at end of follow-up | ||
| PD | 51% | 22 |
| SD | 23% | 10 |
| No structural disease | 19% | 7 |
| PR | 9% | 4 |
| Additional therapies | 14 | |
| External beam radiation therapy (EBRT) | 9 | |
| Kinase inhibitor therapy | 1 | |
| Kinase inhibitor and EBRT | 4 | |
| Clinical outcome at end of follow-up | ||
| Alive | 72% | 31 |
| Thyroid cancer-related deaths | 28% | 12 |
Nine patients showing SD/PR after ablation (5 SD, 4 PR) had structural disease progression at the end of the follow-up period, despite additional RAI therapies. Thus, a third of the patients had disease progression after RAI ablation and more than half of the patients (51%) showed disease progression at the end of the follow-up period. Importantly, no patient with PD after ablation responded to repeat RAI therapies by disease stabilization or regression (PR). Twelve patients (28%) died due to thyroid cancer by the end of the study period (mean follow-up, 3.6 y).
Thirteen patients presented with structural disease in lungs and/or bones that was RAIA on postablation scans but had no corresponding uptake on DxWBS. Four patients (31%) had PD after ablation, and 9 (69%) had SD/PR (7 SD, 2 PR). At the end of the follow-up, all patients with initial SD after ablation continued to have SD. Two patients with initial disease regression after ablation ultimately progressed, and all 4 patients with initial disease progression continued to progress despite repeat RAI therapy. These findings suggest that repeat RAI therapy in patients with metastatic thyroid cancers that are negative on DxWBS and positive on post-therapy scan is not helpful.
Seven patients had RAI uptake in either the lungs (5 patients) or bones (2 patients) at RAI ablation without corresponding lesions on cross-sectional imaging, suggestive of micrometastatic disease. Six of them had detectable suppressed Tg levels, and most (6 of 7) had negative DxWBS at ablation. During the course of the study, none of them developed structural recurrences or a rise in Tg levels. All 7 patients had undetectable suppressed Tg levels and negative cross-sectional imaging at the end of the study. Four achieved an undetectable suppressed Tg level after ablation. Two of the 3 remaining patients received additional RAI therapies (1 or 2 RAI activities), and both achieved an undetectable suppressed Tg level at the end of the study. One patient had a detectable suppressed Tg level after RAI ablation that spontaneously became undetectable. Stimulated Tg levels were measured in 4 of these patients during follow-up: 2 were undetectable, 1 was 1.2 ng/mL, and the other was 4.7 ng/mL.
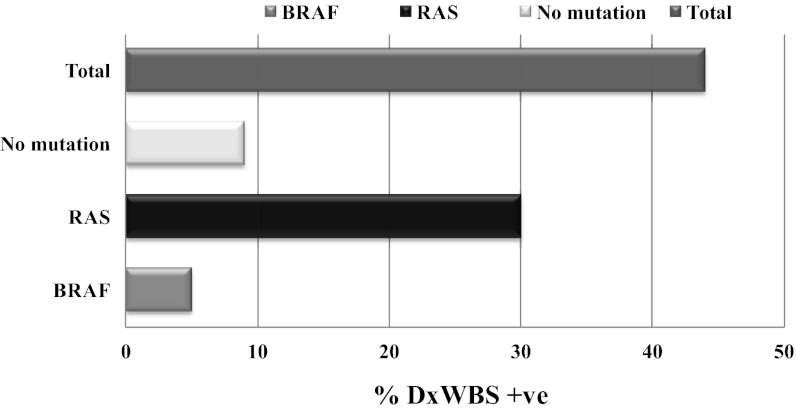
Genotype correlations with RAI uptake on DxWBS before ablation
Of the 43 patients, 19 had uptake on the DxWBS. These were significantly enriched for RAS mutations (P = .013) (Figure 2). Despite this clear difference, RAS-mutant tumors were not more likely to respond to therapeutic RAI administration than BRAF-mutant cancers or those with no known mutations, as described below.
Figure 2.
RAI uptake on DxWBS at ablation by tumor genotype in distant metastatic thyroid cancer positive on postablation scans.
Response to RAI therapy by tumor genotype
Tables 3 and 4 delineate the structural response to RAI therapy in the 36 patients with structurally evident RAIA distant metastatic thyroid cancer based on their mutational profile. Response was assessed 12–18 months after RAI ablation (Table 3) and again at the end of the follow-up period (Table 4). Of the 23 patients that had SD/PR after RAI ablation, 10 (44%) had RAS mutations (8 NRAS, 2 KRAS), 4 (17%) had BRAF, 3 (13%) had RET/PTC, and the remaining 6 were wild type. Of the 13 patients with PD after RAI ablation, 6 (47%) had RAS mutations (4 NRAS, 2 HRAS), 2 (15%) had BRAF, 1 (8%) had PIK3CA, and the remaining 4 (31%) were wild type. At the end of the follow-up, 9 of 23 (39%) had continued structural progression of their disease despite repeat RAI activities. Thus, although RAIA metastatic thyroid cancers were enriched in primary tumors harboring RAS mutations, and these were more frequently RAIA on diagnostic scans, the genotype did not correlate with structural response to RAI at ablation or at the end of the follow-up period. One noteworthy exception was the 3 patients with tumors harboring RET/PTC mutations, none of which progressed after the initial ablative RAI dose. All 7 patients presenting with micrometastatic FCDTC showed no structural progression of their thyroid cancer, irrespective of genotype (4 BRAF, 2 RAS, and 1 PIK3CA).
Table 3.
Structural Response 12–18 Months After RAI Ablation Based on Mutational Profile of Primary Tumors in Patients With Structurally Identifiable RAIA Distant Metastases
| SD/PR | PD | Total | |
|---|---|---|---|
| RAS | 10a | 6b | 16 (45%) |
| BRAF | 4 | 2 | 6 (17%) |
| RET/PTC | 3 | 0 | 3 (8%) |
| PIK3CA | 0 | 1 | 1 (2%) |
| No mutation | 6 | 4 | 10 (28%) |
| Total | 23 | 13 | 36 (100%) |
8 NRAS, 2 KRAS.
4 NRAS, 2 HRAS.
Table 4.
Structural Response at the End of the Follow-Up Based on Mutational Profile of Primary Tumors in Patients With Structurally Identifiable RAIA Distant Metastases
| SD/PR | PD | Total | |
|---|---|---|---|
| RAS | 1a | 15b | 16 (45%) |
| BRAF | 3 | 3 | 6 (17%) |
| RET/PTC | 3 | 0 | 3 (8%) |
| PIK3CA | 0 | 1 | 1 (2%) |
| No mutation | 6 | 4 | 10 (28%) |
| Total | 13 | 23 | 36 (100%) |
1 NRAS, 0 KRAS.
11 NRAS, 2 KRAS, 2 HRAS.
RAS- vs BRAF -mutated thyroid tumors
The presence of a RAS mutation in the primary thyroid tumor of patients with RAIA distant metastases was associated with older age at thyroid cancer diagnosis, PDTC histology, larger primary tumor size, the presence of RAI uptake on DxWBS, and the presence of bone metastasis. One year after RAI ablation, 13 (30%) patients had structurally evident progression of their thyroid cancer, with 7 of those patients harboring a RAS mutation in their primary thyroid tissues. No patient with RAS-mutant disease achieved a complete resolution of structurally evident distant lesions. Eight of the 36 patients with structurally evident metastatic thyroid cancer had a PR to RAI ablation, 4 of which had RAS-mutated thyroid tumors (2 NRAS, 2 KRAS). These responses were largely not durable because at the end of the follow-up only 4 of 8 continued to have PRs, 1 of which derived from an NRAS primary (2 of the others were wild type, and 1 was RET/PTC).
BRAF mutation was solely detected in papillary thyroid carcinomas (10 of 21, or 48%). Only 2 of 19 (11%) of the patients with positive DxWBS before ablation harbored BRAF-mutated tumors. Three of 10 patients with BRAF tumors presented with FDG-PET positive, macronodular distant metastasis, 2 of whom died from disease progression despite repeated RAI therapies.
Discussion
This study describes the molecular profile of primary thyroid cancer tissues in patients presenting with RAIA distant metastatic disease at the time of remnant ablation. We previously defined RAI refractoriness based on the following criteria: 1) negative diagnostic RAI scan; 2) unresponsiveness to RAI treatment of lesions positive on diagnostic or prior post-therapy scans; and 3) FDG-PET positivity (8, 13). In the current study, we investigated patients who had positive postablation scans, regardless of whether or not they responded to the ablative dose. We labeled them as RAIA simply to emphasize that characteristic. As shown in this study, most were later proven to be refractory to this therapy. We believe this is a clinically relevant patient cohort because, for most thyroid cancer specialists, uptake on a postablation scan automatically dictates a need for additional RAI therapies.
We previously established that BRAF mutations are concordant between the primary tumor and distant metastatic lesions in the same patient, and between multiple metastatic sites of patients with BRAF-mutant disease (8). RAS mutations are also thyroid tumor drivers and are likely to be present in metastases arising from primary tumors with these mutations, although to our knowledge this has not been formally proven. Even accepting this caveat, we believe it is reasonable to correlate the biological responses of the metastatic disease to the genotype of the primary tumor, particularly for BRAF, RAS, and RET/PTC (8). As hypothesized, most of the RAIA metastatic thyroid cancers with identifiable genetic events had RAS mutations (40%), whereas 23% were BRAF mutant. RAS mutations were detected in all patients with follicular thyroid cancer, in 79% of patients with PDTC, in older patients, and in those with bone metastasis (88%). BRAF mutations were solely detected in differentiated papillary thyroid carcinomas (classical and tall cell variants).
This mutational profile is quite distinct from that seen in advanced RAIR metastatic thyroid cancers, which are particularly enriched with BRAF mutations. A comparison between tumors of similar histology is also instructive. Thus, in RAIR PDTC, BRAF mutations were overrepresented in FDG-PET-positive tumors (39%) and those with extrathyroidal extension (P = .04), and were associated with decreased median survival as compared to RAS-mutated PDTC with RAIR metastases (3.3 vs 6.6 y; P = .08). In the current cohort of RAIA distant metastatic thyroid cancer, 14 patients had PDTC; 79% of those were RAS mutated, and none were BRAF mutated. Thus, RAS mutations are overrepresented in RAIA PDTC compared to RAIR PDTC (79 vs 44%) (Figure 1).
High-dose RAI is the first-line therapy after thyroidectomy for FCDTC patients presenting with RAIA distant metastasis. Prior studies estimated structural response after repeated RAI activities in patients with distant metastatic thyroid cancer based on the presence of suspicious metastasis on conventional radiographs of the distant sites and/or on the presence of 131I uptake in the lungs or bones on the last 131I post-therapy scan. Up to 43% of such patients were deemed to have no evidence of disease after repeat RAI therapies, supported by a lack of suspicious lesions on plain x-rays and negative 131I post-therapy scans. These patients were younger and had well-differentiated tumors and a lower tumor burden than nonresponders (4, 5). There were important differences between the cohorts reported in these published series and the study population reported here. In the current study, structural response to RAI was based on cross-sectional imaging: computed tomography, magnetic resonance imaging, and FDG-PET scans. By contrast to the studies from Villejuif, our patients did not routinely receive additional doses of RAI until a negative post-therapy scan was attained (4, 5). Importantly, none of our patients achieved complete resolution of structurally identifiable RAIA distant metastases. Indeed, a third of the patients continued to progress after RAI ablation despite uptake in the lesions. About half of the cohort (54%) had initial responses to the ablative dose (either SD or PRs), but 40% of these ultimately progressed despite repeated RAI administration. Furthermore, none of the patients with disease progression after RAI ablation achieved PRs after additional high-dose RAI therapies. Thus, RAI therapy is quite ineffective in treating metastatic differentiated thyroid cancer, even in the patients with detectable RAI incorporation in their metastatic sites. It is likely that patients who progressed after RAI ablation did not incorporate sufficient RAI for a tumoricidal effect, whereas those who achieved PRs had higher lesional doses, but not enough for complete eradication of the tumor. It is also possible that some of the tumors were radio-resistant. The older age of our patients and the fact that many harbored more aggressive thyroid cancer histotypes (33% PDTC) may also help explain the different results with previously published studies (4).
Patients with RAS-mutant metastatic disease were more likely to concentrate iodine at the metastatic sites on the preablative DxWBS. Despite this, we could not establish a significant correlation between tumor genotype and structural response to RAI. In all likelihood, this is because an insufficient tumoral dose of RAI is achieved, even in RAS-mutant tumors. In addition to genotype, structural response to RAI may be influenced by a variety of other clinicopathological and molecular factors, each with a small effect. Larger studies may be needed to achieve sufficient statistical power to detect genotype associations with RAI response in more homogeneously defined patient subsets (eg, age, size and site of lesions).
We previously showed in a transgenic mouse model of Braf-induced papillary thyroid carcinoma that Nis expression was augmented and RAI uptake was increased by using inhibitors of the MAPK pathway (12). In a subsequent clinical trial of patients with RAIR metastatic thyroid cancer, a 4-week pretreatment with the MAPK kinase (MEK) inhibitor selumetinib increased RAI avidity in the metastatic foci in 12 of the 20 studied patients (60%). Eight (8 of 12, or 40%) of these achieved a sufficient lesional iodine avidity (>2000 cGy) in the metastatic lesions to warrant repeat RAI therapy. Five (5 of 8, or 63%) had PRs, and 3 had SD by cross-sectional imaging at 2- and 6-month follow-up, and Tg decreased by 89% at the conclusion of the study. Interestingly, RAI therapy after selumetinib pretreatment was particularly effective in patients with NRAS-mutated tumors (5 of 5 treated patients) (13). As a corollary to this, the relative lack of efficacy of conventional RAI therapy in patients with RAIA metastatic disease suggests that a similar approach may be applicable to this subset of patients.
Surprisingly, 4 of 10 BRAF-mutant cancers in the current study were in tumors of younger patients presenting with RAIA distant metastatic thyroid cancer without corresponding lesions on cross-sectional imaging, suggestive of microscopic disease. After RAI ablation, all 4 patients continued to have no clinical evidence of disease. This suggests that the presence of BRAF mutation does not necessarily eliminate the possibility that RAI may be clinically effective, particularly in younger patients with low-volume metastatic disease.
In conclusion, RAIA distant metastatic FCDTC are overrepresented with RAS mutations, whereas RAIR advanced, FDG-PET avid, distant metastatic thyroid cancers are enriched with BRAF mutations. Despite an apparent preserved ability to concentrate iodine, RAI therapy is ineffective in achieving a durable structural response in these patients, even those with RAS-mutant disease. It is likely that responses could be augmented by increasing iodine incorporation into the metastatic sites, a goal that now seems achievable by using therapies that inhibit MAPK signaling.
Acknowledgments
This work was supported by National Institutes of Health Grant RO1 CA72597, by the J. Randolph Hearst and Lefkosky Family Foundations, and by a grant from the Society for Memorial Sloan-Kettering Cancer Center. J.M.D. was partially supported by Pontificia Universidad Católica de Chile and Becas Chile (Grant 76100021).
Disclosure Summary: No competing financial interests exist for M.M.S., J.M.D., R.A.G., S.M.L., R.K.G., and J.A.F. R.M.T. is a consultant for Genzyme.
Footnotes
- CR
- complete response
- DxWBS
- diagnostic whole body scan
- FCDTC
- follicular cell-derived thyroid cancer
- FDG-PET
- fluorodeoxyglucose-positron emission tomography
- FFPE
- formalin-fixed, paraffin-embedded
- MEK
- MAPK kinase
- Nis
- sodium iodide symporter
- PD
- progressive disease
- PDTC
- poorly differentiated thyroid cancer
- PR
- partial response
- RAI
- radioiodine
- RAIA
- RAI-avid
- RAIR
- RAI refractory
- SD
- stable disease
- TCV
- tall cell variant
- Tg
- thyroglobulin
- TgAb
- Tg antibodies.
References
- 1. Hay ID, Thompson GB, Grant CS, et al. Papillary thyroid carcinoma managed at the Mayo Clinic during six decades (1940–1999): temporal trends in initial therapy and long-term outcome in 2444 consecutively treated patients. World J Surg. 2002;26:879–885 [DOI] [PubMed] [Google Scholar]
- 2. Aschebrook-Kilfoy B, Grogan R, Ward M, Kaplan E, Devesa S. Follicular thyroid cancer incidence patterns in the United States, 1980–2009 [published online ahead of print January 29, 2013]. Thyroid. doi:10.1089/thy.2012.0356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shaha AR, Shah JP, Loree TR. Patterns of nodal and distant metastasis based on histologic varieties in differentiated carcinoma of the thyroid. Am J Surg. 1996;172:692–694 [DOI] [PubMed] [Google Scholar]
- 4. Durante C, Haddy N, Baudin E, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. 2006;91:2892–2899 [DOI] [PubMed] [Google Scholar]
- 5. Schlumberger M, Tubiana M, De Vathaire F, et al. Long-term results of treatment of 283 patients with lung and bone metastases from differentiated thyroid carcinoma. J Clin Endocrinol Metab. 1986;63:960–967 [DOI] [PubMed] [Google Scholar]
- 6. Robbins RJ, Wan Q, Grewal RK, et al. Real-time prognosis for metastatic thyroid carcinoma based on 2-[18F]fluoro-2-deoxy-D-glucose-positron emission tomography scanning. J Clin Endocrinol Metab. 2006;91:498–505 [DOI] [PubMed] [Google Scholar]
- 7. Sabra MM, Grewal RK, Tala H, Larson SM, Tuttle RM. Clinical outcomes following empiric radioiodine therapy in patients with structurally identifiable metastatic follicular cell-derived thyroid carcinoma with negative diagnostic but positive post-therapy (131)I whole-body scans. Thyroid. 2012;22:877–883 [DOI] [PubMed] [Google Scholar]
- 8. Ricarte-Filho JC, Ryder M, Chitale DA, et al. Mutational profile of advanced primary and metastatic radioactive iodine-refractory thyroid cancers reveals distinct pathogenetic roles for BRAF, PIK3CA, and AKT1. Cancer Res. 2009;69:4885–4893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Elisei R, Ugolini C, Viola D, et al. BRAF(V600E) mutation and outcome of patients with papillary thyroid carcinoma: a 15-year median follow-up study. J Clin Endocrinol Metab. 2008;93:3943–3949 [DOI] [PubMed] [Google Scholar]
- 10. Franco AT, Malaguarnera R, Refetoff S, et al. Thyrotrophin receptor signaling dependence of Braf-induced thyroid tumor initiation in mice. Proc Natl Acad Sci USA. 2011;108:1615–1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen X, Mitsutake N, LaPerle K, et al. Endogenous expression of Hras(G12V) induces developmental defects and neoplasms with copy number imbalances of the oncogene. Proc Natl Acad Sci USA. 2009;106:7979–7984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chakravarty D, Santos E, Ryder M, et al. Small-molecule MAPK inhibitors restore radioiodine incorporation in mouse thyroid cancers with conditional BRAF activation. J Clin Invest. 2011;121:4700–4711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ho AL, Grewal RK, LeBoeuf R, et al. Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. N Engl J Med. 2013;368:623–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. DeLellis R, Lloyd R, Heitz P, Eng C. 2004 Pathology and genetics of tumours of the endocrine organs. 1st ed Lyon, France: IARC Press; 2004 [Google Scholar]
- 15. Hiltzik D, Carlson DL, Tuttle RM, et al. Poorly differentiated thyroid carcinomas defined on the basis of mitosis and necrosis: a clinicopathologic study of 58 patients. Cancer. 2006;106:1286–1295 [DOI] [PubMed] [Google Scholar]
- 16. Volante M, Collini P, Nikiforov YE, et al. Poorly differentiated thyroid carcinoma: the Turin proposal for the use of uniform diagnostic criteria and an algorithmic diagnostic approach. Am J Surg Pathol. 2007;31:1256–1264 [DOI] [PubMed] [Google Scholar]
- 17. Li J, Smyth P, Cahill S, et al. Improved RNA quality and TaqMan pre-amplification method (PreAmp) to enhance expression analysis from formalin fixed paraffin embedded (FFPE) materials. BMC Biotechnol. 2008;8:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Imkamp F, von Wasielewski R, Musholt TJ, Musholt PB. Rearrangement analysis in archival thyroid tissues: punching microdissection and artificial RET/PTC 1–12 transcripts. J Surg Res. 2007;143:350–363 [DOI] [PubMed] [Google Scholar]
- 19. Nikiforova MN, Biddinger PW, Caudill CM, Kroll TG, Nikiforov YE. PAX8-PPARγ rearrangement in thyroid tumors: RT-PCR and immunohistochemical analyses. Am J Surg Pathol. 2002;26:1016–1023 [DOI] [PubMed] [Google Scholar]