Abstract
Laser speckle imaging (LSI) is a noninvasive optical imaging technique able to provide wide-field two-dimensional maps of moving particles. Raw laser speckle images are typically taken with a scientific-grade monochrome camera. We demonstrate that a digital single-lens reflex (dSLR) camera with a Bayer filter is able to provide similar sensitivity despite taking information only from a specific pixel color. Here we demonstrate the effect of changing three primary dSLR exposure settings (i.e., aperture, exposure time/shutter speed, and gain/sensitivity (ISO)) on speckle contrast. In addition, we present data from an in vivo reactive hyperemia experiment that demonstrates the qualitative similarity in blood-flow dynamics visualized with a color dSLR and a scientific-grade monochrome camera.
Laser speckle imaging (LSI) is a real-time, wide-field optical technique that is frequently used to assess blood flow in biological tissues [1–3]. Raw laser speckle images contain areas of reduced speckle contrast due to the movement of scatterers (e.g., red blood cells) that occurs during the camera exposure time. Efficient algorithms exist to quantify blood flow, either in the form of speckle contrast or speckle flow index (SFI) images [4–7].
Currently, LSI typically is performed in a laboratory setting, with the most frequent application being preclinical imaging of cerebral blood flow in rodents [8]. Recently, LSI was applied to clinical imaging during neurosurgery [9] and laser surgery of vascular birthmarks [10]. Here we demonstrate the potential of a point-of-care LSI system based on a digital single-lens reflex (dSLR) camera.
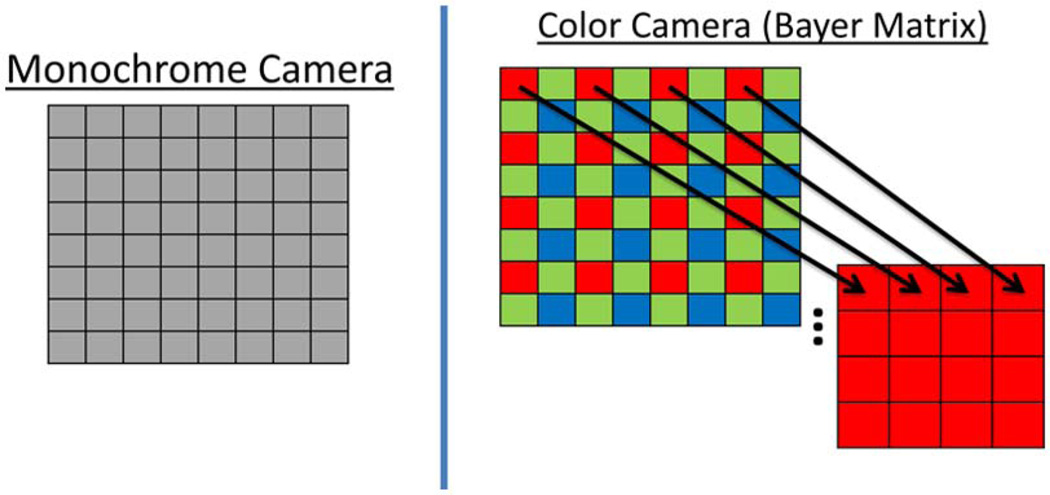
A key difference between a monochrome camera and a color camera is the spectral sensitivity of each pixel. As shown in Fig. 1, a monochrome camera has a full array of pixels that typically are sensitive to the entire visible/near-IR spectrum, whereas a color camera contains a Bayer matrix array of pixel sensors that each possess a specific color filter, allowing only a specific range of wavelengths to pass through (i.e., red, green, and blue). For our application of LSI with a 633 nm He–Ne laser, we only utilized the red-filtered pixels, effectively reducing the pixel resolution to 25%.
Fig. 1.
(Color online) Pixels of monochrome cameras are sensitive to light covering both visible and near-IR wavelengths. Color cameras have a Bayer matrix of pixels that only allow a certain wavelength range to pass through (namely red, green, and blue) to each pixel. For LSI, we used only the red pixels from the raw Bayer image of the color camera.
In this Letter, we present data from a sensitivity study in which we varied three primary camera settings: aperture, exposure time/shutter speed, and gain/ISO. In addition, data from an in vivo reactive hyperemia experiment of the inner palm of a human subject demonstrate the potential of LSI with a color dSLR.
In the experiments, we used a monochrome and a color camera. The monochrome camera was a 12 bit thermoelectrically cooled CCD camera (Retiga 2000R, QImaging, Burnaby, BC, Canada) equipped with a closefocus zoom lens (18–108 mm, f /2.5–close, Edmund Optics #52-274, Barrington, NJ, USA). The pixel resolution was 1600 × 1200 pixels. This camera is used routinely in our laboratory for LSI during laser surgery of vascular birthmarks [10]. The color camera was a 14 bit color complementary metal–oxide–semiconductor (CMOS) camera (Canon 5D Mark II, Canon, Tokyo, Japan) with the bundled kit zoom lens (24–105 mm, f /4–22, Canon, Tokyo, Japan). The pixel resolution of the complete Bayer matrix was 5616 × 3744 pixels; however, after removing the blue and green pixels from the raw Bayer image, the effective resolution was 2808 × 1872 pixels. For all experiments, a continuous-wave He–Ne laser (30 mW, Edmund Industrial Optics, Barrington, New Jersey, USA) was used as the excitation source.
Image acquisition from the monochrome camera was achieved with the QCapture software supplied by the manufacturer. Images from the color camera were captured directly onto the memory card and transferred to PC for post processing with purpose-written MATLAB software. Canon’s proprietary raw Bayer data (.CRW files) from the dSLR was converted to an open-source format using freeware available online (dcraw) [11] and subsequently decimated in MATLAB to extract the red pixels from the raw Bayer matrix. Both monochrome and color camera raw images were converted to speckle contrast images using a 7 × 7 pixel sliding window algorithm with the speckle contrast formula K = σ /〈I〉 [1], where K is speckle contrast and σ and 〈I〉 are the standard deviation and mean of the 49 pixel intensity values in each window, respectively. Ten images were collected per condition and their respective speckle contrast images averaged together to obtain an averaged speckle contrast image. A region of interest (ROI) was selected within the averaged speckle contrast image and a mean speckle contrast value calculated.
We first imaged a silicone phantom with optical properties similar to those of human skin [12]. Fluid wells within the phantom were filled with varying quantities (0%, 0.2%, and 0.4% by weight) of methylcellulose in a 1% Intralipid solution, to change the Brownian motion of the Intralipid particles.
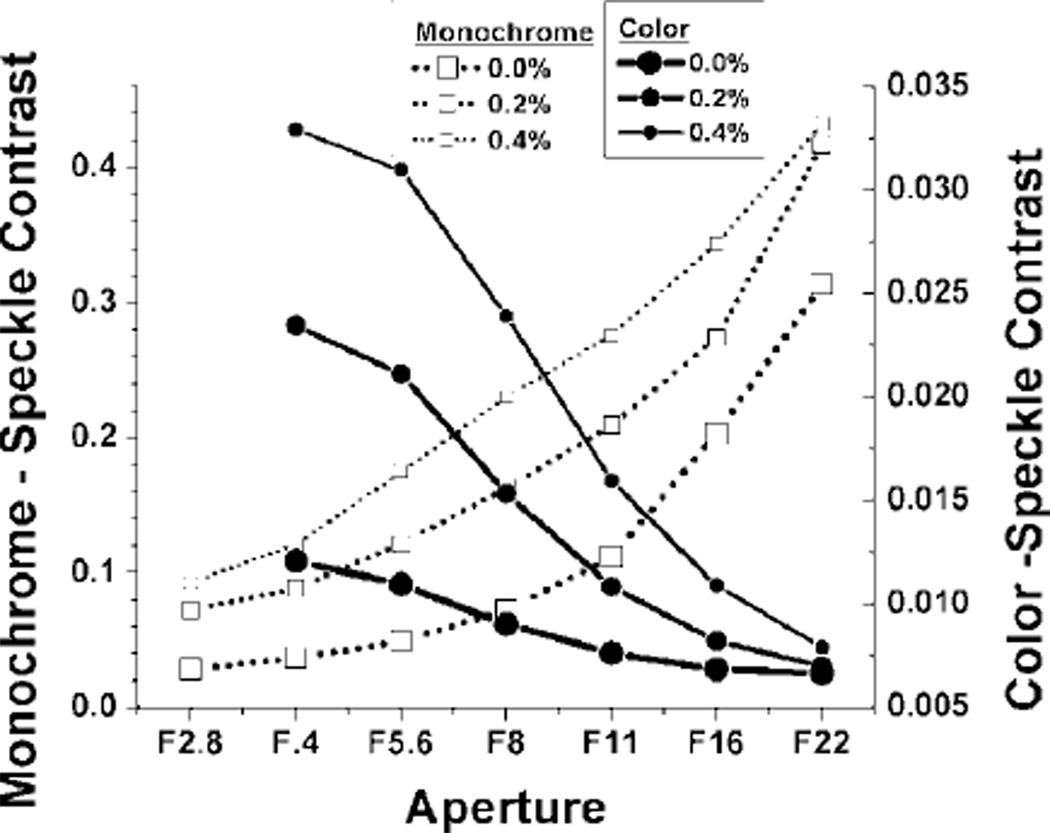
We first studied the effect of aperture on speckle contrast. Exposure time/shutter speed was set at 10 ms for both cameras. Gain was set to unity (minimum) for the monochrome camera and ISO set to 400 for the color camera. For each concentration of methylcellulose, sets of 10 raw speckle images were taken at different apertures (monochrome f /2.8–f /22, color f /4–f /22). An ROI was selected within each well for speckle contrast analysis. It is apparent that both the monochrome and the color camera were able to distinguish between different viscosities at a wide range of apertures, specifically f /4–f /16 (Fig. 2).
Fig. 2.
Plot of average speckle contrast value versus aperture for monochrome and color camera imaging of suspensions of methylcellulose and Intralipid.
The reason for the apparent difference in trends between the monochrome and colors cameras is due to the noise floors associated with each camera. The scientific-grade monochrome camera had a noise floor of approximately five intensity counts, which yielded a higher speckle contrast with decreasing light throughput. On the other hand, the color dSLR camera had a considerably higher noise floor of approximately 1000 counts, which instead yielded a lower speckle contrast with decreasing light throughput.
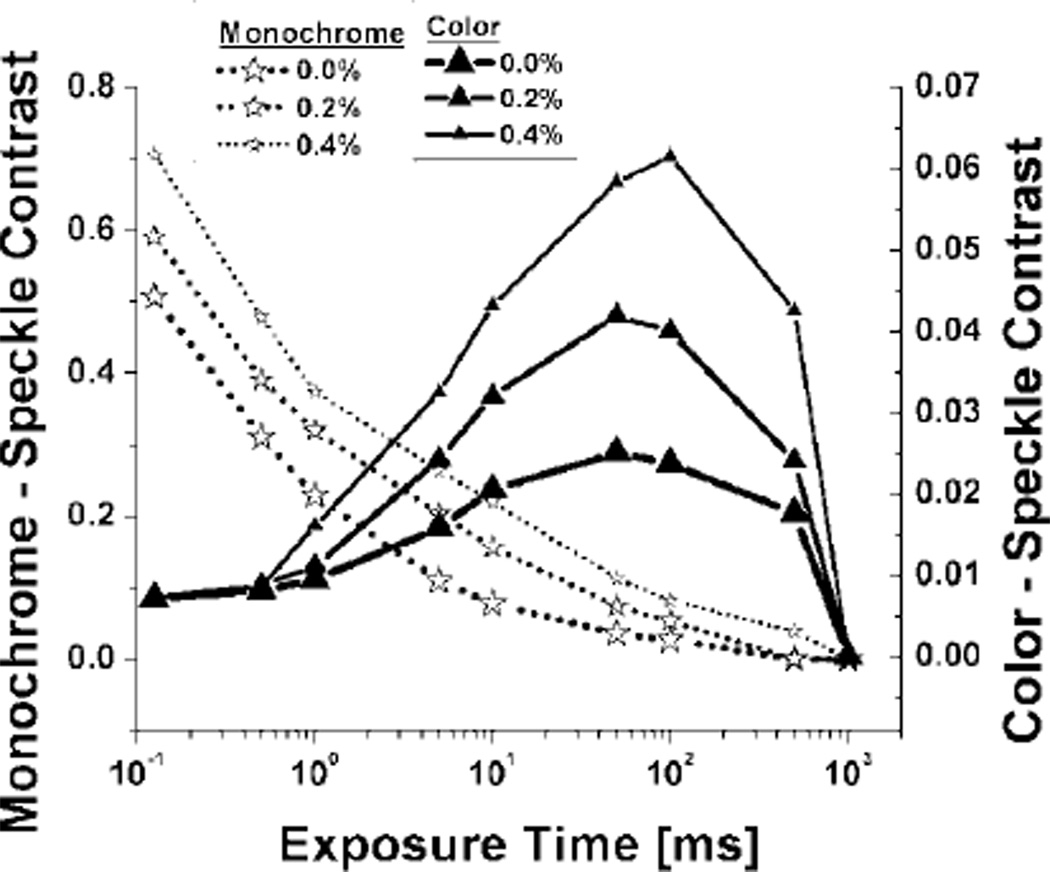
We next studied the effect of exposure time/shutter speed on speckle contrast. The aperture was set to f /8 and gain at unity for the monochrome camera, and ISO at 400 for the color camera. The exposure time/shutter speed was adjusted from 0.125 to 1000 ms, to span the entire pixel intensity dynamic range. As seen in Fig. 3, the monochrome camera exhibited sensitivity to viscosity changes even at very short exposure times (0.125 ms), with sensitivity maintained up to ~500 ms exposure time. The color camera exhibited sensitivity to viscosity changes in an exposure time/shutter speed range of approximately 1 to 500 ms. Similar to the effect of increasing aperture (decreasing f /) in Fig. 2, speckle contrast with the color camera increased with increasing laser light until the image dynamic range reached pixel saturation conditions at shutter speeds greater than approximately 100 ms.
Fig. 3.
Semilog plot of average speckle contrast value versus exposure time/shutter speed for monochrome and color camera imaging of suspensions of methylcellulose and Intralipid.
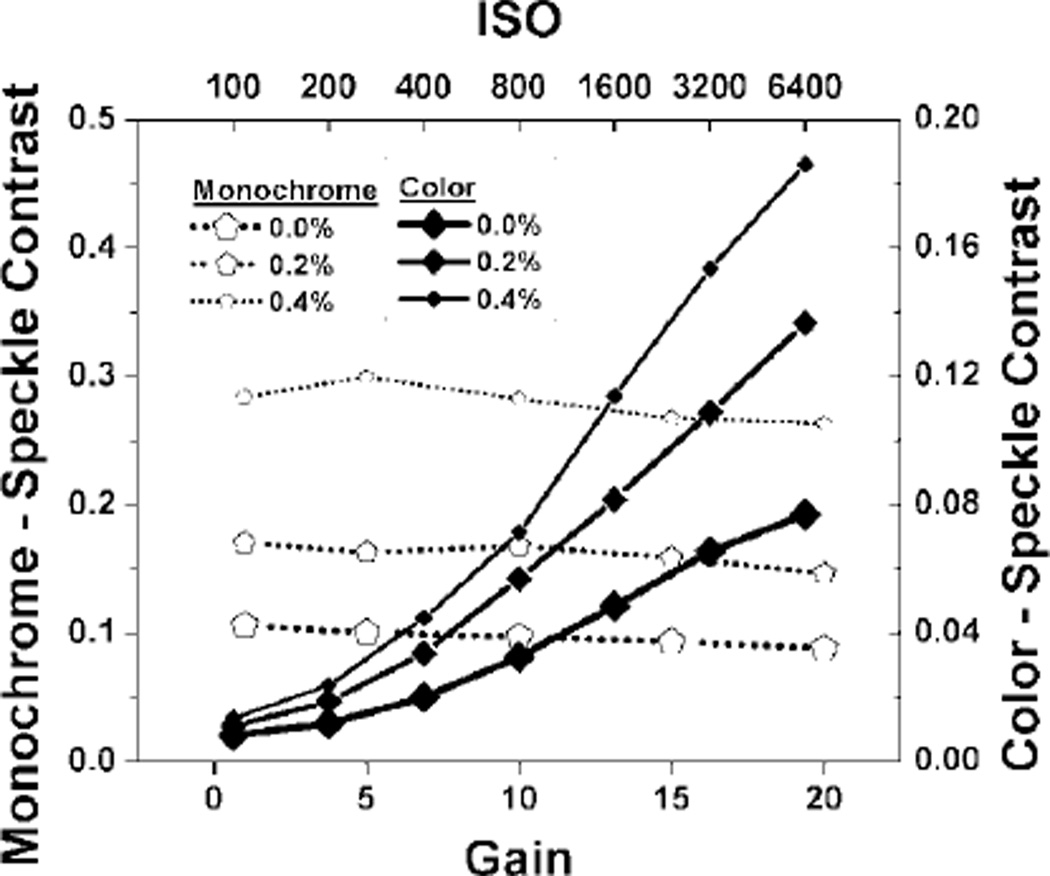
We then studied the effect of sensor sensitivity on speckle contrast. Exposure time/shutter speed was held constant at 10 ms and the aperture at f /8. With the monochrome camera, the gain setting had minimal effect on speckle contrast, suggesting that the setting acts strictly as a digital gain (Fig. 4). On the other hand, increasing ISO of the color camera increased speckle contrast values similarly to increasing aperture and shutter speed (up to 100 ms) before the dynamic range reached pixel saturation.
Fig. 4.
Plot of average speckle contrast value versus gain/ISO for monochrome and color camera imaging, respectively, of suspensions of methylcellulose and Intralipid.
To demonstrate the feasibility of using a color camera for in vivo measurements of blood flow dynamics, a reactive hyperemia experiment on the arm was performed and images taken of the palm (UCI IRB Protocol #2004-3626). A pressure cuff was placed over the brachial artery of a human subject. Baseline images were acquired simultaneous with both cameras during a period of 30 s.
The pressure was increased to 220 mm Hg for four min. Raw speckle images were taken during the occlusion period and for approimately two min after release of the pressure cuff. The raw images were converted to speckle contrast images and ultimately SFI images using the equation SFI = (2TK2)−1 [6], where T is the exposure time/shutter speed. The settings for both cameras were held constant at f /8 aperture and 10 ms exposure time/shutter speed. Gain was set to unity and ISO to 400 for the monochrome and color cameras, respectively. The field of view is approximately 4.1 × 5.5 cm and 4.0 × 6.0 cm for the monochrome and color cameras, respectively.
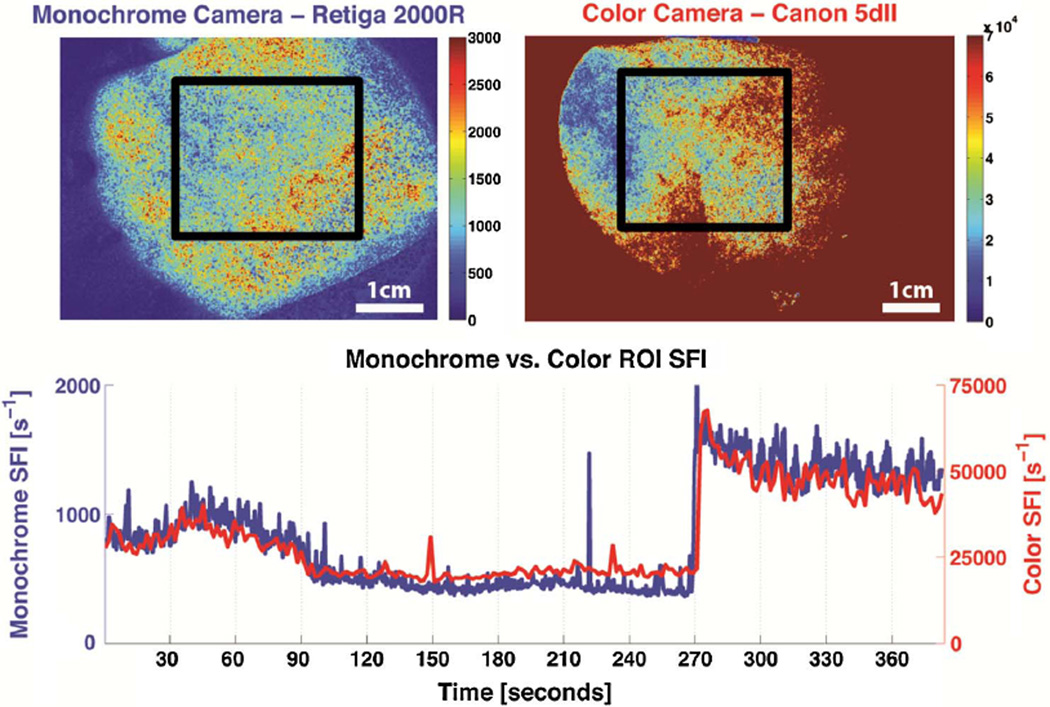
The video in Fig. 5 (Media 1) illustrates changes in SFI maps between the monochrome and color cameras throughout the entire reactive hyperemia experiment. An ROI is selected within the palm for each data set, and the average SFI value is plotted over time. The qualitative similarity of the color and monochrome cameras’ SFI values is readily evident, supporting the hypothesis that a color camera is capable of in vivo LSI of blood flow dynamics.
Fig. 5.
(Color online) Single frame excerpt of video taken from simultaneous imaging of a human palm during a reactive hyperemia experiment using both monochrome and color cameras (Media 1). The upper left and upper right images are SFI maps using raw images taken concurrently from a monochrome and a color camera, respectively. The bottom graph is a time trace of the average SFI value of an ROI (bounded by black lines) taken within the palm.
In conclusion, we presented data that collectively demonstrate the sensitivity of speckle contrast values to three key exposure settings (aperture, exposure time/shutter speed, and gain/ISO). Both monochrome and color cameras were associated with a wide range of settings that enabled sensitivity to motion of the underlying particles that interact with the laser. Also, we showed that a color camera could provide similar maps of blood flow dynamics to those provided by a scientific-grade monochrome camera. Collectively, these data demonstrate the potential of developing a point-of-care LSI instrument with consumer-grade electronics.
The project described was supported in part by the Arnold and Mabel Beckman Foundation, a summer graduate fellowship from the Edwards Lifesciences Center for Advanced Cardiovascular Technology, and award number P41EB015890 from the National Institute of Biomedical Imaging and Bioengineering.
References
- 1.Fercher AF, Briers JD. Opt. Commun. 1981;37:326. [Google Scholar]
- 2.Boas DA, Dunn AK. J. Biomed. Opt. 2010;15:011109. doi: 10.1117/1.3285504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirkpatrick SJ, Duncan DD, Wang RK, Hinds MT. J. Opt. Soc. Am. A. 2007;24:3728. doi: 10.1364/josaa.24.003728. [DOI] [PubMed] [Google Scholar]
- 4.Yang O, Cuccia D, Choi B. J. Biomed. Opt. 2011;16:016009. doi: 10.1117/1.3528610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tom WJ, Ponticorvo A, Dunn AK. IEEE Trans. Med. Imaging. 2008;27:1728. doi: 10.1109/TMI.2008.925081. [DOI] [PubMed] [Google Scholar]
- 6.Ramirez-San-Juan JC, Ramos-Garcia R, Guizar-Iturbide I, Martinez-Niconoff G, Choi B. Opt. Express. 2008;16:3197. doi: 10.1364/oe.16.003197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qiu J, Li P, Luo W, Wang J, Zhang H, Luo Q. J. Biomed. Opt. 2010;15:016003. doi: 10.1117/1.3290804. [DOI] [PubMed] [Google Scholar]
- 8.Dunn AK. Ann. Biomed. Eng. 2012;40:367. doi: 10.1007/s10439-011-0469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parthasarathy AB, Weber EL, Richards LM, Fox DJ, Dunn AK. J. Biomed. Opt. 2010;15:066030. doi: 10.1117/1.3526368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang YC, Tran N, Shumaker PR, Kelly K, Ross EV, Nelson JS, Choi B. Lasers Surg. Med. 2009;41:563. doi: 10.1002/lsm.20840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coffin D. Decoding raw digital photos in Linux. 2012 http://www.cybercom.net/~dcoffin/dcraw/ [Google Scholar]
- 12.Frederick Ayers AG, Kuo Danny, Durkin AJ, Cuccia D. Proc. SPIE. 2008;6870:687007. [Google Scholar]