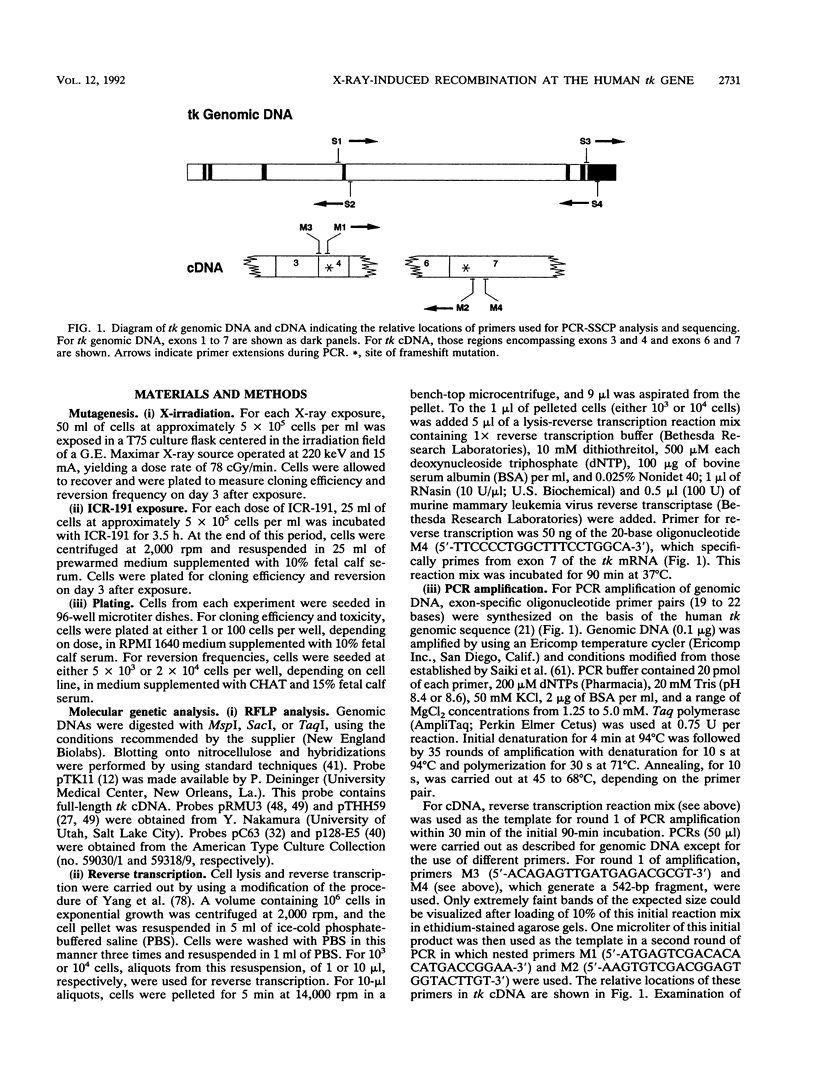
Abstract
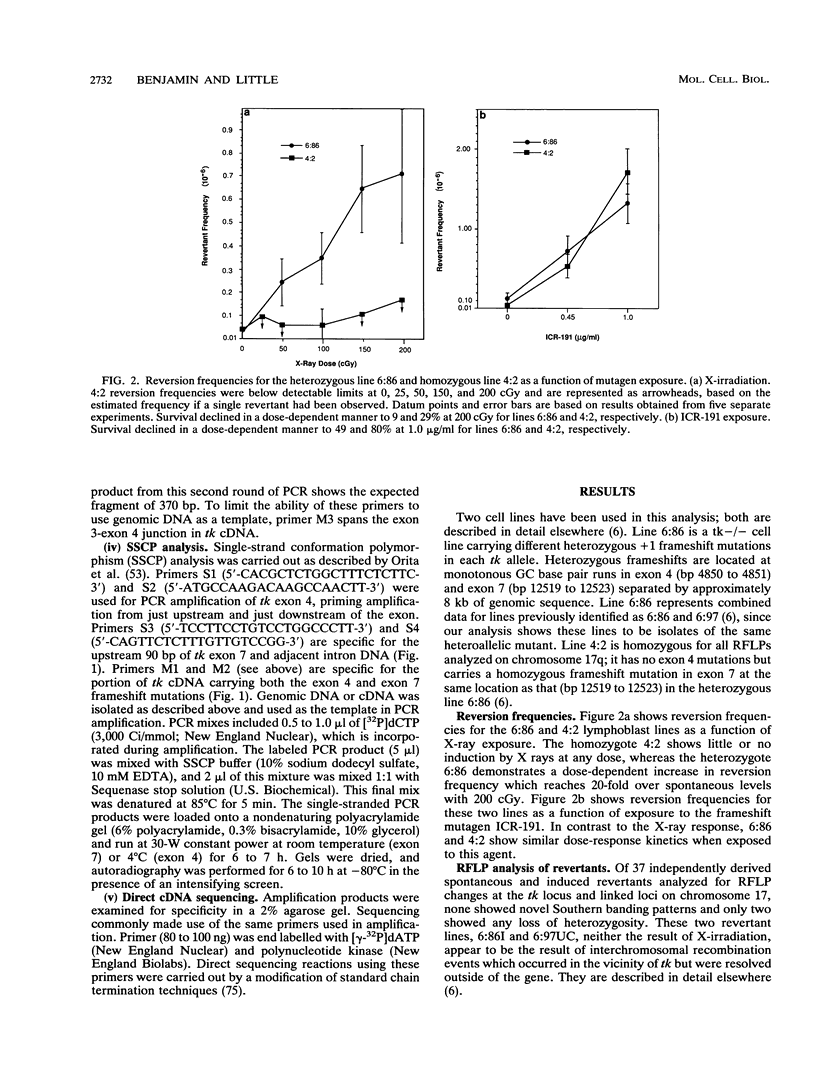
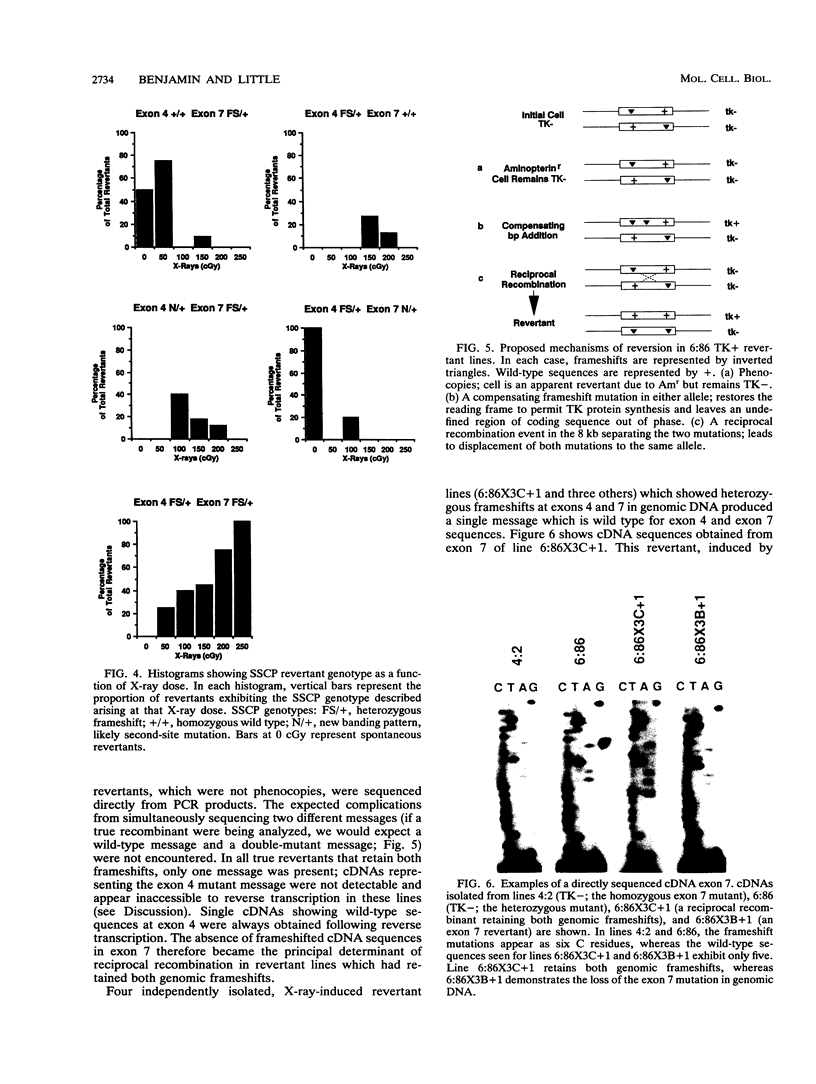
We have developed a human lymphoblast cell line for the study of interchromosomal homologous recombination at the endogenous thymidine kinase (tk) gene on chromosome 17 (M. B. Benjamin, H. Potter, D. W. Yandell, and J. B. Little, Proc. Natl. Acad. Sci. USA 88:6652-6656, 1991). This cell line (designated 6:86) carries unique heterozygous frameshift mutations in exons 4 and 7 of its endogenous tk alleles and can revert to TK+ by frame-restoring mutations, gene conversion, or reciprocal recombination. Line 6:86 reverts spontaneously to TK+ at a frequency of 10(-7) to 10(-8), and exposures to X-irradiation or the frameshift mutagen ICR-191 induce increased reversion frequencies in a dose-dependent manner. Another cell line (designated 4:2) carries a homozygous exon 7 frameshift and is not expected to revert through mechanisms other than frame-restoring mutation. Line 4:2 reverts to TK+ at a lower spontaneous frequency than does 6:86 but can be induced with similar kinetics by ICR-191. In contrast to line 6:86, however, X rays did not induce detectable reversion of line 4:2. We have characterized a number of 6:86-derived revertants by means of restriction fragment length polymorphism analysis at tk and linked loci, single-strand conformation polymorphisms, and direct transcript sequencing. For X rays, most revertants retain both original mutations in the genomic DNA, and a subset of these frameshift-retaining revertants produce frameshift-free message, indicating that reversion is the result of reciprocal recombination within the tk gene. Frame-restoring point mutations, restoration of original sequences, and phenocopy reversion by acquisition of aminopterin resistance were also found among X-ray-induced revertants, whereas the ICR-191-induced revertants examined show only loss of the exon 7 frameshift.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adair G. M., Nairn R. S., Wilson J. H., Scheerer J. B., Brotherman K. A. Targeted gene replacement at the endogenous APRT locus in CHO cells. Somat Cell Mol Genet. 1990 Sep;16(5):437–441. doi: 10.1007/BF01233193. [DOI] [PubMed] [Google Scholar]
- Ames B. N., Whitfield H. J., Jr Frameshift mutagenesis in Salmonella. Cold Spring Harb Symp Quant Biol. 1966;31:221–225. doi: 10.1101/sqb.1966.031.01.030. [DOI] [PubMed] [Google Scholar]
- Baker M. D. High-frequency homologous recombination between duplicate chromosomal immunoglobulin mu heavy-chain constant regions. Mol Cell Biol. 1989 Dec;9(12):5500–5507. doi: 10.1128/mcb.9.12.5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauchinger M., Kulka U., Schmid E. Cytogenetic effects of 3,4-dichloroaniline in human lymphocytes and V79 Chinese hamster cells. Mutat Res. 1989 Jul;226(3):197–202. doi: 10.1016/0165-7992(89)90020-1. [DOI] [PubMed] [Google Scholar]
- Bedford J. S., Cornforth M. N. Relationship between the recovery from sublethal X-ray damage and the rejoining of chromosome breaks in normal human fibroblasts. Radiat Res. 1987 Sep;111(3):406–423. [PubMed] [Google Scholar]
- Belmaaza A., Wallenburg J. C., Brouillette S., Gusew N., Chartrand P. Genetic exchange between endogenous and exogenous LINE-1 repetitive elements in mouse cells. Nucleic Acids Res. 1990 Nov 11;18(21):6385–6391. doi: 10.1093/nar/18.21.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin M. B., Potter H., Yandell D. W., Little J. B. A system for assaying homologous recombination at the endogenous human thymidine kinase gene. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6652–6656. doi: 10.1073/pnas.88.15.6652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger H., Brammar W. J., Yanofsky C. Spontaneous and ICR191-A-induced frameshift mutations in the A gene of Escherichia coli tryptophan synthetase. J Bacteriol. 1968 Nov;96(5):1672–1679. doi: 10.1128/jb.96.5.1672-1679.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya N. P., Maher V. M., McCormick J. J. Effect of nucleotide excision repair in human cells on intrachromosomal homologous recombination induced by UV and 1-nitrosopyrene. Mol Cell Biol. 1990 Aug;10(8):3945–3951. doi: 10.1128/mcb.10.8.3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya N. P., Maher V. M., McCormick J. J. Intrachromosomal homologous recombination in human cells which differ in nucleotide excision-repair capacity. Mutat Res. 1990 Feb;234(1):31–41. doi: 10.1016/0165-1161(90)90028-m. [DOI] [PubMed] [Google Scholar]
- Bollag R. J., Waldman A. S., Liskay R. M. Homologous recombination in mammalian cells. Annu Rev Genet. 1989;23:199–225. doi: 10.1146/annurev.ge.23.120189.001215. [DOI] [PubMed] [Google Scholar]
- Bradshaw H. D., Jr, Deininger P. L. Human thymidine kinase gene: molecular cloning and nucleotide sequence of a cDNA expressible in mammalian cells. Mol Cell Biol. 1984 Nov;4(11):2316–2320. doi: 10.1128/mcb.4.11.2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buetow K. H., Murray J. C., Israel J. L., London W. T., Smith M., Kew M., Blanquet V., Brechot C., Redeker A., Govindarajah S. Loss of heterozygosity suggests tumor suppressor gene responsible for primary hepatocellular carcinoma. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8852–8856. doi: 10.1073/pnas.86.22.8852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calos M. P., Miller J. H. Genetic and sequence analysis of frameshift mutations induced by ICR-191. J Mol Biol. 1981 Nov 25;153(1):39–64. doi: 10.1016/0022-2836(81)90525-8. [DOI] [PubMed] [Google Scholar]
- Cheng J., Fogel-Petrovic M., Maquat L. E. Translation to near the distal end of the penultimate exon is required for normal levels of spliced triosephosphate isomerase mRNA. Mol Cell Biol. 1990 Oct;10(10):5215–5225. doi: 10.1128/mcb.10.10.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daar I. O., Maquat L. E. Premature translation termination mediates triosephosphate isomerase mRNA degradation. Mol Cell Biol. 1988 Feb;8(2):802–813. doi: 10.1128/mcb.8.2.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao D. D., Schroeder W. T., Chao L. Y., Kikuchi H., Strong L. C., Riccardi V. M., Pathak S., Nichols W. W., Lewis W. H., Saunders G. F. Genetic mechanisms of tumor-specific loss of 11p DNA sequences in Wilms tumor. Am J Hum Genet. 1987 Aug;41(2):202–217. [PMC free article] [PubMed] [Google Scholar]
- Dikomey E., Franzke J. Three classes of DNA strand breaks induced by X-irradiation and internal beta-rays. Int J Radiat Biol Relat Stud Phys Chem Med. 1986 Nov;50(5):893–908. doi: 10.1080/09553008614551311. [DOI] [PubMed] [Google Scholar]
- Evans H. H., Ricanati M., Horng M. F., Mencl J. Relationship between topoisomerase II and radiosensitivity in mouse L5178Y lymphoma strains. Mutat Res. 1989 Jan;217(1):53–63. doi: 10.1016/0921-8777(89)90036-0. [DOI] [PubMed] [Google Scholar]
- Fasullo M. T., Davis R. W. Recombinational substrates designed to study recombination between unique and repetitive sequences in vivo. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6215–6219. doi: 10.1073/pnas.84.17.6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher G., Stephenson D. A., West J. D. Investigation of the potential for mitotic recombination in the mouse. Mutat Res. 1986 Dec;164(6):381–388. doi: 10.1016/0165-1161(86)90031-2. [DOI] [PubMed] [Google Scholar]
- Flemington E., Bradshaw H. D., Jr, Traina-Dorge V., Slagel V., Deininger P. L. Sequence, structure and promoter characterization of the human thymidine kinase gene. Gene. 1987;52(2-3):267–277. doi: 10.1016/0378-1119(87)90053-9. [DOI] [PubMed] [Google Scholar]
- Folger K. R., Thomas K., Capecchi M. R. Nonreciprocal exchanges of information between DNA duplexes coinjected into mammalian cell nuclei. Mol Cell Biol. 1985 Jan;5(1):59–69. doi: 10.1128/mcb.5.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstein R. M., Frankel W. N., Hsieh C. L., Durdik J. M., Rath S., Coffin J. M., Nisonoff A., Selsing E. Isotype switching of an immunoglobulin heavy chain transgene occurs by DNA recombination between different chromosomes. Cell. 1990 Nov 2;63(3):537–548. doi: 10.1016/0092-8674(90)90450-s. [DOI] [PubMed] [Google Scholar]
- Groden J., Nakamura Y., German J. Molecular evidence that homologous recombination occurs in proliferating human somatic cells. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4315–4319. doi: 10.1073/pnas.87.11.4315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosovsky A. J., de Boer J. G., de Jong P. J., Drobetsky E. A., Glickman B. W. Base substitutions, frameshifts, and small deletions constitute ionizing radiation-induced point mutations in mammalian cells. Proc Natl Acad Sci U S A. 1988 Jan;85(1):185–188. doi: 10.1073/pnas.85.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn P., Nevaldine B., Morgan W. F. X-ray induction of methotrexate resistance due to dhfr gene amplification. Somat Cell Mol Genet. 1990 Sep;16(5):413–423. doi: 10.1007/BF01233191. [DOI] [PubMed] [Google Scholar]
- Haines J. L., Ozelius L. J., McFarlane H., Menon A., Tzall S., Martiniuk F., Hirschhorn R., Gusella J. F. A genetic linkage map of chromosome 17. Genomics. 1990 Sep;8(1):1–6. doi: 10.1016/0888-7543(90)90218-j. [DOI] [PubMed] [Google Scholar]
- Hellgren D., Lambert B. Mechanisms for recombination between stably integrated vector sequences in CHO cells. Mutat Res. 1989 Dec;215(2):197–204. doi: 10.1016/0027-5107(89)90184-x. [DOI] [PubMed] [Google Scholar]
- Hellgren D., Luthman H., Lambert B. Induced recombination between duplicated neo genes stably integrated in the genome of CHO cells. Mutat Res. 1989 Jan;210(1):197–206. doi: 10.1016/0027-5107(89)90059-6. [DOI] [PubMed] [Google Scholar]
- Kinniburgh A. J., Maquat L. E., Schedl T., Rachmilewitz E., Ross J. mRNA-deficient beta o-thalassemia results from a single nucleotide deletion. Nucleic Acids Res. 1982 Sep 25;10(18):5421–5427. doi: 10.1093/nar/10.18.5421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondoleon S., van Tuinen P., Ledbetter D. H., Vissing H., Litt M. An anonymous single-copy clone, pC63, from chromosome 17q23-qter identifies a frequent RFLP [HGM9 No. D17S21]. Nucleic Acids Res. 1987 Nov 11;15(21):9096–9096. doi: 10.1093/nar/15.21.9096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koufos A., Hansen M. F., Copeland N. G., Jenkins N. A., Lampkin B. C., Cavenee W. K. Loss of heterozygosity in three embryonal tumours suggests a common pathogenetic mechanism. Nature. 1985 Jul 25;316(6026):330–334. doi: 10.1038/316330a0. [DOI] [PubMed] [Google Scholar]
- Kucherlapati R. S., Eves E. M., Song K. Y., Morse B. S., Smithies O. Homologous recombination between plasmids in mammalian cells can be enhanced by treatment of input DNA. Proc Natl Acad Sci U S A. 1984 May;81(10):3153–3157. doi: 10.1073/pnas.81.10.3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucherlapati R. S. Homologous recombination in mammalian somatic cells. Prog Nucleic Acid Res Mol Biol. 1989;36:301–310. doi: 10.1016/s0079-6603(08)60178-6. [DOI] [PubMed] [Google Scholar]
- Langlois R. G., Bigbee W. L., Jensen R. H., German J. Evidence for increased in vivo mutation and somatic recombination in Bloom's syndrome. Proc Natl Acad Sci U S A. 1989 Jan;86(2):670–674. doi: 10.1073/pnas.86.2.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J. A., Virolainen M., Defendi V. Human lymphoblastoid lines from lymph node and spleen. Cancer. 1968 Sep;22(3):517–524. doi: 10.1002/1097-0142(196809)22:3<517::aid-cncr2820220305>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Lin F. L., Sperle K., Sternberg N. Intermolecular recombination between DNAs introduced into mouse L cells is mediated by a nonconservative pathway that leads to crossover products. Mol Cell Biol. 1990 Jan;10(1):103–112. doi: 10.1128/mcb.10.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litt M., Kondoleon S., Vissing H., van Tuinen P., Ledbetter D. H. Cosmid 128 defines three RFLPs on chromosome 17q23-qter. [HGM9 no. D17S77]. Nucleic Acids Res. 1988 Jul 11;16(13):6251–6251. doi: 10.1093/nar/16.13.6251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannens M., Slater R. M., Heyting C., Bliek J., de Kraker J., Coad N., de Pagter-Holthuizen P., Pearson P. L. Molecular nature of genetic changes resulting in loss of heterozygosity of chromosome 11 in Wilms' tumours. Hum Genet. 1988 Dec;81(1):41–48. doi: 10.1007/BF00283727. [DOI] [PubMed] [Google Scholar]
- Mayer P. J., Lange C. S., Bradley M. O., Nichols W. W. Age-dependent decline in rejoining of X-ray-induced DNA double-strand breaks in normal human lymphocytes. Mutat Res. 1989 Mar;219(2):95–100. doi: 10.1016/0921-8734(89)90019-2. [DOI] [PubMed] [Google Scholar]
- Mirzayans R., Waters R., Paterson M. C. Induction and repair of DNA strand breaks and 1-beta-D-arabinofuranosylcytosine-detectable sites in 40-75 kVp X-irradiated compared to 60Co gamma-irradiated human cell lines. Radiat Res. 1988 Apr;114(1):168–185. [PubMed] [Google Scholar]
- Moore C. W., Hampsey D. M., Ernst J. F., Sherman F. Differential mismatch repair can explain the disproportionalities between physical distances and recombination frequencies of cyc1 mutations in yeast. Genetics. 1988 May;119(1):21–34. doi: 10.1093/genetics/119.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley A. A., Grist S. A., Turner D. R., Kutlaca A., Bennett G. Molecular nature of in vivo mutations in human cells at the autosomal HLA-A locus. Cancer Res. 1990 Aug 1;50(15):4584–4587. [PubMed] [Google Scholar]
- Mudgett J. S., Taylor W. D. Recombination between irradiated shuttle vector DNA and chromosomal DNA in African green monkey kidney cells. Mol Cell Biol. 1990 Jan;10(1):37–46. doi: 10.1128/mcb.10.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers R., Nakamura Y., Ballard L., Leppert M., O'Connell P., Lathrop G. M., Lalouel J. M., White R. Isolation and mapping of a polymorphic DNA sequence pRMU3 on chromosome 17q [D17S24]. Nucleic Acids Res. 1988 Jan 25;16(2):784–784. doi: 10.1093/nar/16.2.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y., Lathrop M., O'Connell P., Leppert M., Barker D., Wright E., Skolnick M., Kondoleon S., Litt M., Lalouel J. M. A mapped set of DNA markers for human chromosome 17. Genomics. 1988 May;2(4):302–309. doi: 10.1016/0888-7543(88)90018-3. [DOI] [PubMed] [Google Scholar]
- Nickoloff J. A., Reynolds R. J. Transcription stimulates homologous recombination in mammalian cells. Mol Cell Biol. 1990 Sep;10(9):4837–4845. doi: 10.1128/mcb.10.9.4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickoloff J. A., Singer J. D., Hoekstra M. F., Heffron F. Double-strand breaks stimulate alternative mechanisms of recombination repair. J Mol Biol. 1989 Jun 5;207(3):527–541. doi: 10.1016/0022-2836(89)90462-2. [DOI] [PubMed] [Google Scholar]
- Oeschger N. S., Hartman P. E. ICR-induced frameshift mutations in the histidine operon of Salmonella. J Bacteriol. 1970 Feb;101(2):490–504. doi: 10.1128/jb.101.2.490-504.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orita M., Suzuki Y., Sekiya T., Hayashi K. Rapid and sensitive detection of point mutations and DNA polymorphisms using the polymerase chain reaction. Genomics. 1989 Nov;5(4):874–879. doi: 10.1016/0888-7543(89)90129-8. [DOI] [PubMed] [Google Scholar]
- Orkin S. H., Goldman D. S., Sallan S. E. Development of homozygosity for chromosome 11p markers in Wilms' tumour. Nature. 1984 May 10;309(5964):172–174. doi: 10.1038/309172a0. [DOI] [PubMed] [Google Scholar]
- Panthier J. J., Guénet J. L., Condamine H., Jacob F. Evidence for mitotic recombination in Wei/+ heterozygous mice. Genetics. 1990 May;125(1):175–182. doi: 10.1093/genetics/125.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastink A., Vreeken C., Schalet A. P., Eeken J. C. DNA sequence analysis of X-ray-induced deletions at the white locus of Drosophila melanogaster. Mutat Res. 1988 Jan;207(1):23–28. doi: 10.1016/0165-7992(88)90006-1. [DOI] [PubMed] [Google Scholar]
- Radford I. R., Hodgson G. S. A comparison of the induction of DNA double-strand breakage and lethal lesions by X irradiation in ataxia telangiectasia and normal fibroblasts. Radiat Res. 1990 Dec;124(3):334–335. [PubMed] [Google Scholar]
- Ray A., Siddiqi I., Kolodkin A. L., Stahl F. W. Intra-chromosomal gene conversion induced by a DNA double-strand break in Saccharomyces cerevisiae. J Mol Biol. 1988 May 20;201(2):247–260. doi: 10.1016/0022-2836(88)90136-2. [DOI] [PubMed] [Google Scholar]
- Roman H., Ruzinski M. M. Mechanisms of gene conversion in Saccharomyces cerevisiae. Genetics. 1990 Jan;124(1):7–25. doi: 10.1093/genetics/124.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth D. B., Wilson J. H. Relative rates of homologous and nonhomologous recombination in transfected DNA. Proc Natl Acad Sci U S A. 1985 May;82(10):3355–3359. doi: 10.1073/pnas.82.10.3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Schimke R. T. Gene amplification in cultured animal cells. Cell. 1984 Jul;37(3):705–713. doi: 10.1016/0092-8674(84)90406-9. [DOI] [PubMed] [Google Scholar]
- Simon J. R., Moore P. D. Induction of homologous recombination in Saccharomyces cerevisiae. Mol Gen Genet. 1988 Sep;214(1):37–41. doi: 10.1007/BF00340176. [DOI] [PubMed] [Google Scholar]
- Smith A. J., Kalogerakis B. Replacement recombinant events targeted at immunoglobulin heavy chain DNA sequences in mouse myeloma cells. J Mol Biol. 1990 Jun 5;213(3):415–435. doi: 10.1016/s0022-2836(05)80205-0. [DOI] [PubMed] [Google Scholar]
- Stamberg J., Hirschfield L. Mitotic recombination can explain the apparent polyclonal origin of some tumors. Cancer Genet Cytogenet. 1987 Jul;27(1):5–8. doi: 10.1016/0165-4608(87)90252-4. [DOI] [PubMed] [Google Scholar]
- Stankowski L. F., Jr, Tindall K. R., Hsie A. W. Quantitative and molecular analyses of ethyl methanesulfonate- and ICR 191-induced mutation in AS52 cells. Mutat Res. 1986 Apr;160(2):133–147. doi: 10.1016/0027-5107(86)90037-0. [DOI] [PubMed] [Google Scholar]
- Sun H., Treco D., Schultes N. P., Szostak J. W. Double-strand breaks at an initiation site for meiotic gene conversion. Nature. 1989 Mar 2;338(6210):87–90. doi: 10.1038/338087a0. [DOI] [PubMed] [Google Scholar]
- Sweigert S. E., Carroll D. Repair and recombination of X-irradiated plasmids in Xenopus laevis oocytes. Mol Cell Biol. 1990 Nov;10(11):5849–5856. doi: 10.1128/mcb.10.11.5849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimura T., Maher V. M., Godwin A. R., Liskay R. M., McCormick J. J. Frequency of intrachromosomal homologous recombination induced by UV radiation in normally repairing and excision repair-deficient human cells. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1566–1570. doi: 10.1073/pnas.87.4.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner D. R., Grist S. A., Janatipour M., Morley A. A. Mutations in human lymphocytes commonly involve gene duplication and resemble those seen in cancer cels. Proc Natl Acad Sci U S A. 1988 May;85(9):3189–3192. doi: 10.1073/pnas.85.9.3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahls W. P., Moore P. D. Relative frequencies of homologous recombination between plasmids introduced into DNA repair-deficient and other mammalian somatic cell lines. Somat Cell Mol Genet. 1990 Jul;16(4):321–329. doi: 10.1007/BF01232460. [DOI] [PubMed] [Google Scholar]
- Wahls W. P., Wallace L. J., Moore P. D. Hypervariable minisatellite DNA is a hotspot for homologous recombination in human cells. Cell. 1990 Jan 12;60(1):95–103. doi: 10.1016/0092-8674(90)90719-u. [DOI] [PubMed] [Google Scholar]
- Wang Y. Y., Maher V. M., Liskay R. M., McCormick J. J. Carcinogens can induce homologous recombination between duplicated chromosomal sequences in mouse L cells. Mol Cell Biol. 1988 Jan;8(1):196–202. doi: 10.1128/mcb.8.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warters R. L., Lyons B. W., Chiu S. M., Oleinick N. L. Induction of DNA strand breaks in transcriptionally active DNA sequences of mouse cells by low doses of ionizing radiation. Mutat Res. 1987 Sep;180(1):21–29. doi: 10.1016/0027-5107(87)90063-7. [DOI] [PubMed] [Google Scholar]
- Yandell D. W., Dryja T. P. Detection of DNA sequence polymorphisms by enzymatic amplification and direct genomic sequencing. Am J Hum Genet. 1989 Oct;45(4):547–555. [PMC free article] [PubMed] [Google Scholar]
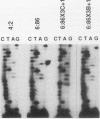
- Yandell D. W., Dryja T. P., Little J. B. Molecular genetic analysis of recessive mutations at a heterozygous autosomal locus in human cells. Mutat Res. 1990 Mar;229(1):89–102. doi: 10.1016/0027-5107(90)90011-r. [DOI] [PubMed] [Google Scholar]
- Yandell D. W., Dryja T. P., Little J. B. Somatic mutations at a heterozygous autosomal locus in human cells occur more frequently by allele loss than by intragenic structural alterations. Somat Cell Mol Genet. 1986 May;12(3):255–263. doi: 10.1007/BF01570784. [DOI] [PubMed] [Google Scholar]
- Yang J. L., Maher V. M., McCormick J. J. Amplification and direct nucleotide sequencing of cDNA from the lysate of low numbers of diploid human cells. Gene. 1989 Nov 30;83(2):347–354. doi: 10.1016/0378-1119(89)90121-2. [DOI] [PubMed] [Google Scholar]