Abstract
S-phase kinase-associated protein 2 (Skp2) functions as the receptor component of the Skp–Cullin–F-box complex and is implicated in the degradation of several cell cycle regulators, such as p21Cip1, p27Kip1, p57Kip2, and cyclin E. Numerous studies in human and experimental tumors have demonstrated low p27Kip1 levels and elevated Skp2 expression. However, a direct association between the inverse correlation of Skp2 and p27Kip1 with tumorigenesis has not been demonstrated. Herein, we provide evidence that skin tumorigenesis is inhibited in Skp2−/− mice. An analysis of mouse keratinocytes indicates that increased p27Kip1 levels in Skp2−/− epidermis cause reduced cell proliferation that is alleviated in the epidermis from Skp2−/−/p27−/− compound mice. In contrast, we establish that a p27Kip1 deficiency does not overturn the reduced skin tumorigenesis experienced by Skp2−/− mice. In addition, Skp2−/− epidermis exhibits an accumulation of p53-cofactor CBP/p300 that is associated with elevated apoptosis in hair follicles and decreased skin tumorigenesis. We conclude that p27Kip1 accumulation is responsible for the hypoplasia observed in normal tissues of Skp2−/− mice but does not have a preponderant function in reducing skin tumorigenesis.
The proteasome pathway involves ubiquitin modification and degradation of substrates by the proteasome complex. The S-phase kinase-associated protein (Skp)–Cullin–F-box complex is a ubiquitin ligase that typically contains four subunits, termed Skp1, Cullin, Ring-finger protein, and a member of the large family of F-box adaptor proteins involved in specific substrate recognition.1 Skp2 is an F-box protein that targets several cell cycle regulators for ubiquitination and subsequent degradation.2,3 The specific substrates of Skp2 include p21Cip1, p27Kip1, p57Kip2, p130, Tob1, FOXO1, and c-Myc.4–10 Because most of these substrates are tumor suppressor proteins, Skp2 has been classified as an oncogene. Moreover, Skp2 also suppresses the p53-dependent apoptosis pathway by antagonizing the interaction between CBP/p300 and p53.11 Although multiple substrates for Skp2 have been established, the best described is p27Kip1.12,13 Accordingly, Skp2 knockout (KO) mice exhibit high p27Kip1 levels; these mice grow slower than littermate controls and have smaller organs, with hypoplastic tissues.14 The ablation of p27Kip1 abolishes all of the phenotypes observed in the Skp2−/− mouse, which suggests that p27Kip1 is the main target of Skp2.15
Skp2 induces cell proliferation in various experimental assays, has transforming activity, is found overexpressed in diverse human cancers, and is, therefore, classified as an oncogene.16–18 Presumably, Skp2 expression or improper temporal expression confers a growth advantage by increasing p27Kip1 degradation. Moreover, experimental and human tumors have shown a strong correlation between increased levels of the Skp2 oncoprotein and diminished p27Kip1 levels, which suggests that reduced p27Kip1 levels have a preponderant function in tumorigenesis.19,20 Thus, decreased p27Kip1 protein levels are commonly observed in many human cancers, including epithelial cancers and brain tumors.21 Consequently, high Skp2 and low p27Kip1 levels are indicators for shorter disease-free survival or unfavorable melanoma, breast, prostate, and lung cancer prognoses.18,22,23 However, whether the inverse correlation between Skp2 and p27Kip1 protein levels and the association between Skp2 levels and tumor grade are directly responsible for Skp2 oncogenic activity has never been addressed.
Our laboratory has previously demonstrated that Myc-induced keratinocyte proliferation was abolished by the loss of Skp2 and presumably by the increased level of p27Kip1.24 However, we also observed that Skp2 ablation did not affect Myc-driven oral tumorigenesis. These finding suggested that Skp2 and p27Kip1 are critical for Myc-driven proliferation, although Myc-mediated tumorigenesis in the oral epithelium is independent of the Skp2-p27Kip1 axis.24 In this study, we seek to determine the effect of an Skp2 deficiency on p27Kip1 levels and the rates of keratinocyte proliferation and skin tumorigenesis. Herein, we show that an Skp2 deficiency diminishes ras-mediated skin tumorigenesis that correlates with p27Kip1 accumulation. Furthermore, we determined that a p27Kip1 deficiency reverses Skp2−/− epidermal hypoplasia, but surprisingly, this deficiency does not overturn the reduced skin tumorigenesis that is experienced by the Skp2−/− mice. These data provide direct genetic evidence that p27Kip1 accumulation is responsible for the reduced keratinocyte proliferation and epidermal hypoplasia, but not for the reduced number of tumors observed in the Skp2−/− mice. Our data also suggest that Skp2-mediated apoptosis in the bulge region of hair follicles (HFs) plays a preponderant role by blocking an early stage of mouse skin tumorigenesis.
Materials and Methods
Generation of Transgenic Mice
Skp2−/− animals were developed as previously described by Nakayama et al.14 Mice heterozygous for Skp2 (Skp2+/−) on a C57BL/6 background were bred to generate mice that were homozygous, heterozygous, or nullizygous for Skp2. The p27Kip1 heterozygous mice were purchased from The Jackson Laboratory (Bar Harbor, ME; strain B6.129S4-Cdkn1btm1Mlf) and crossed back to the genetic background SENCAR for two generations. Skp2−/−/p27−/− compound mice, p27−/−, Skp2−/−, and control wild-type (WT) mice were generated by crossing p27Kip1 heterozygous mice with Skp2 heterozygous mice. The p53−/− mice on the C57BL/6 background were a gift from Dr. Robert Smart at North Carolina State University, Raleigh (TSG-p53; Taconic, Hudson, NY). Skp2−/−/p53+/− mice and control littermates were generated by breeding Skp2+/− with p53+/− mice.25
Mouse Experiments
For the two-stage carcinogenesis experiment, 3-week-old Skp2−/− mice and WT siblings were initiated with a topical application of 200 nmol 7,12-dimethylbenz(a)anthracene (DMBA) in 200 μL of acetone on their dorsal surface. Two weeks later, the mice were dosed topically twice weekly with 4 μg of 12-O-tetradecanoylphorbol-13-acetate (TPA) in 200 μL of acetone for 30 weeks. The tumors were counted weekly and recorded to determine multiplicity, latency, and incidence.
In the Skp2−/−/p27−/− two-stage carcinogenesis experiments, newborn mice were initiated at day 1 after birth with an application of 50 μg of DMBA in 50 μL of acetone on their dorsal surface. At day 21, the mice were dosed twice weekly with 2.5 μg of TPA in 200 μL of acetone for 25 weeks. The skin tumors were counted weekly until the end of the experiment at 30 weeks. Malignant progression to squamous cell carcinomas (SCCs) was determined by macroscopic observation and further confirmed by the histopathological analysis of paraffin-embedded H&E-stained cross sections.
Western Blot Analyses and Kinase Assays
For immunoblotting, the epidermal tissue was scraped off with a razor blade, placed into homogenization buffer [150 mmol/L NaCl, 1.0% polyoxyethylene nonylphenol, 0.5% deoxycholic acid, 0.1% SDS, and 50 mmol/L Tris (pH 8.0)], and homogenized using a manual homogenizer.26 For immunoblot analysis of skin tumors, the papillomas were snap frozen in liquid nitrogen and crushed with a pestle and mortar. The homogenates were sonicated and centrifuged at 11,000 × g at 4°C. The supernatants were boiled in 2 μL of Laemmli sample buffer for Western blot analysis or stored at −80°C. The protein concentration was measured with a Bio-Rad protein assay system (Bio-Rad Laboratories, Richmond, CA). The protein lysates (25 μg from each sample) were electrophoresed through 12% acrylamide gels and electrophoretically transferred onto nitrocellulose membranes. After being blocked with 5% nonfat powdered milk in Dulbecco’s PBS, the membranes were incubated with 1 μg/mL of specific antibodies. The following antibodies were used: polyclonal antibodies against CDK4 (C22), CDK2 (M2), p27Kip1 (M197), p21Cip1 (H164), Puma (G3), p300 (N15), actin (H6), cyclin A (C19), and cyclin E (C19) (Santa Cruz Biotechnology, Santa Cruz, CA), p53 (1C12), and acetylated p53 (Cys379) (Cell Signaling Technology Inc., Boston, MA).
To assess the CDK2 kinase activity, proteins were extracted and immunoprecipitated in NP40 lysis buffer [Tris (pH 7.5), 150 mmol/L NaCl, 0.5% NP40, 50 mmol/L NaF, 1 mmol/L Na3VO4, 1 mmol/L dithiothreitol, and 1 mmol/L phenylmethylsulfonyl fluoride]. To assess the CDK4 kinase activity, proteins were extracted and immunoprecipitated in Tween 20 buffer (50 mmol/L HEPES, 150 mmol/L NaCl, 1 mmol/L EDTA, 2.5 mmol/L EGTA, 10% glycerol, 0.1% Tween 20, 1 mmol/L NaF, 1 mmol/L Na3VO4, and 1 mmol/L dithiothreitol). Briefly, 250 μg of protein lysates was immunoprecipitated with 2.5 μg of antibodies against CDK2 (M-20) or CDK4 (C-22) (Santa Cruz Biotechnology) for 2 hours at 4°C and then incubated with 35 μL of protein A–agarose beads. The beads were twice washed in an immunoprecipitation buffer and a kinase buffer [50 mmol/L HEPES (pH 7), 10 mmol/L MgCl2, and 5 mmol/L MnCl2]. Subsequently, 30 μL of kinase buffer, 1 μg of pRb peptide or histone H1 (EMD Millipore, Billerica, MA) substrate, 5 μCi of [γ-32P]ATP (6000 Ci/mmol), 1 mmol/L dithiothreitol, and 5 μmol/L ATP were added to the bead pellet and incubated for 30 minutes at 30°C. SDS sample buffer was added. Each sample was boiled for 3 minutes to stop the reaction and electrophoresed through a polyacrylamide gel. The Western blot images and kinase assay bands were quantified using UN-SCAN-IT gel software version 6.1 for Windows (Silk Scientific, Inc., Orem, UT).
Tunnel Assay
Apoptotic cells were determined using TUNEL assays with the FragEL DNA Fragmentation Detection kit (Colorimetric-TdT enzyme; Calbiochem, EMB Biosciences Inc.), following the manufacturer’s instructions. To quantify the normal and apoptotic cells, the cells were counterstained with methyl green. Apoptotic keratinocytes in the interfollicular and follicular epidermis were quantified in sections (1 cm thick). To determine the incidence of apoptosis in the HFs, HFs that contained at least one apoptotic cell in the bulge were counted as positive. In all cases, 12 fields were counted per section in a total of 10 paraffin-embedded sections, which represented five mice per genotype.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism Software version 4 (GraphPad Software, San Diego, CA).
Results
Skp2 Deficiency Reduces Skin Tumorigenesis
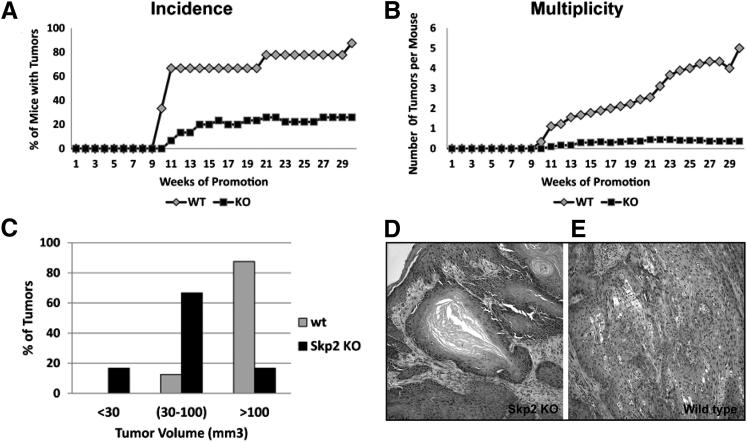
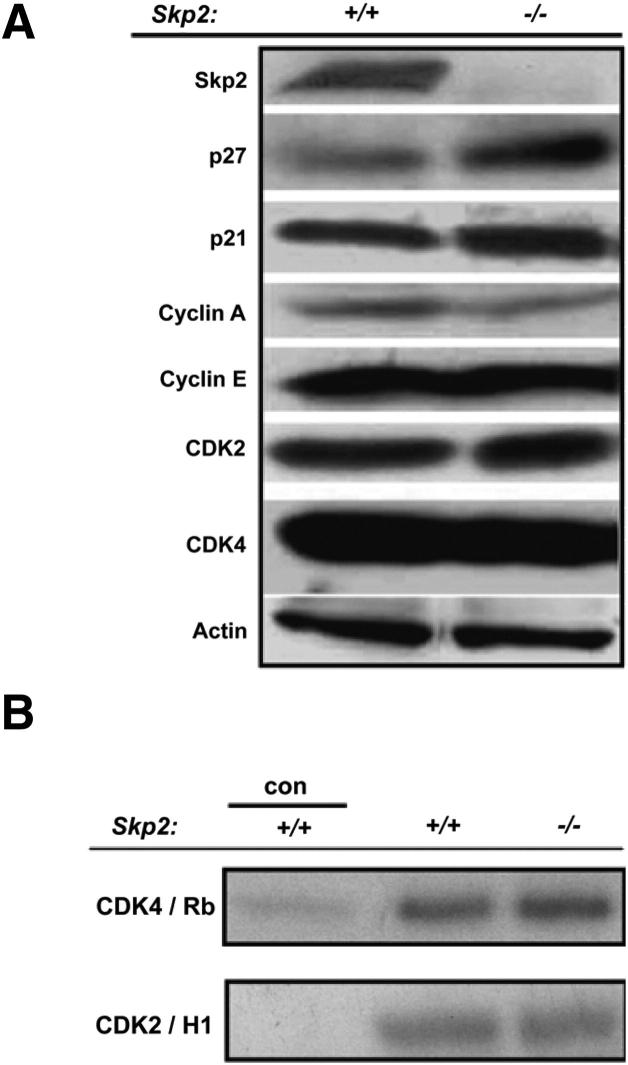
To investigate the function of Skp2 in skin carcinogenesis, we assessed the response of Skp2−/− mice to the two-stage carcinogenesis protocol. This is a well-suited model for understanding the multistage nature of tumor progression, in which tumor initiation is accomplished through a single topical application of a carcinogen, typically DMBA. This treatment produces a somatic mutation in the Ha-ras oncogene, and tumor promotion occurs when the initiated cells are expanded through multiple applications of a tumor promoter, usually TPA, leading to skin papilloma development. Therefore, Skp2−/− and WT littermates were subjected to the DMBA/TPA regimen for up to 30 weeks, and the incidence and multiplicity of papillomas were scored weekly. The incidence of papilloma formation reached a plateau at approximately 11 weeks, in which 70% to 80% of the WT mice developed skin tumors. In contrast, a strong reduction was observed in the Skp2−/− mice, which reached a plateau of approximately 20% at 14 weeks of promotion (Figure 1A). Skp2 deficiency also caused a robust decline in the number of tumors per mouse (multiplicity). Thirty weeks after the first TPA application, the Skp2−/− group exhibited an average of 0.37 papillomas per mouse, whereas the WT mice developed five papillomas per mouse (P < 0.001, U-test) (Figure 1B). The size of the tumors also varied among the genotypes. The WT papillomas were the largest; 85% of tumors exceeded 100 mm3. Conversely, the Skp2−/− papillomas were smaller; 16% of these tumors failed to reach a volume of 30 mm3, and 65% of the tumors did not surpass 100 mm3 (Figure 1C). Histopathological analysis of the skin tumors collected at 30 weeks of promotion indicated that all Skp2−/− tumors were well-differentiated papillomas with no atypia in the basal layers. In brief, 25% of the WT tumors were classified as moderately differentiated squamous cell carcinomas (approximately 50% of differentiating cells), and 75% of these tumors were well-differentiated papillomas (Figure 1, D and E). Comparable to the Skp2−/− mouse epidermis,24 a biochemical analysis of papillomas presented a fourfold increase in p27Kip1 protein levels, whereas other putative Skp-Cullin-F-boxSkp2 substrates, such as cyclin E, exhibited no difference between the Skp2−/− and WT papillomas. A mild increase, although not significant, was observed in the level of p21Cip1 in Skp2−/− papillomas (Figure 2A). Similar to mouse epidermis,24 CDK2 kinase activity in Skp2−/− papillomas was comparable to that of WT mice. Moreover, we did not observe significant differences in the CDK4 kinase activity of WT and Skp2−/− tumors (Figure 2B). Collectively, these observations suggest that a lack of Skp2 expression in mouse epidermis leads to a relevant increase in p27Kip1 stability in skin papillomas but has either a minimal or a null effect on CDK2 and CDK4 kinase activities. Therefore, we conclude that a lack of Skp2 expression severely affects the onset of skin tumorigenesis and the proliferative/antiproliferative balance, which leads to a reduced tumor size. However, these effects are unlikely to be mediated by the inhibition of CDK2/CDK4 activities.
Figure 1.
The effect of an Skp2 deficiency on induced tumorigenesis in mouse skin. A: Percentage of mice with at least one papilloma (incidence). B: Average number of papillomas per mouse (multiplicity) within 30 weeks of a biweekly administration of TPA. C: Skin papillomas were classified according their volume and expressed as a percentage of the total number of tumors for each genotype. Representative paraffin sections of papillomas at 30 weeks of promotion from Skp2 KO (D) and WT (E) sibling mice. Original magnification, ×10. The Skp2-null mice exhibit only moderate dysplasia in a well-differentiated tumor, whereas sections of the WT papillomas exhibit a marked dysplasia with anaplastic areas.
Figure 2.
Biochemical analysis of cell cycle regulators in papillomas of Skp2-null and WT mice. A: Immunoblot analysis of WT (Skp2+/+) and Skp2−/− papilloma lysates developed with antibodies against Skp2, p27Kip1, p21Cip1, cyclin A, cyclin E, CDK2, CDK4, and actin (a loading control). B: CDK4 and CDK2 kinase activities of papillomas from Skp2−/− and Skp2+/+ mice. Fresh tumor lysates were immunoprecipitated with specific antibodies against CDKs, and in vitro kinase assays were performed with pRb or histone H1 peptides as substrates. Con and Skp2+/+ control lysates were immunoprecipitated with normal rabbit IgG.
Inhibition of Skin Tumorigenesis in Skp2-Null Mice Is Independent of p27 Accumulation
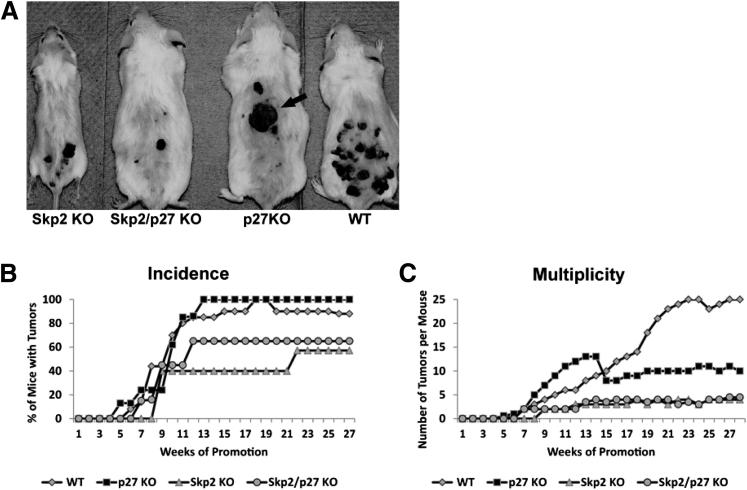
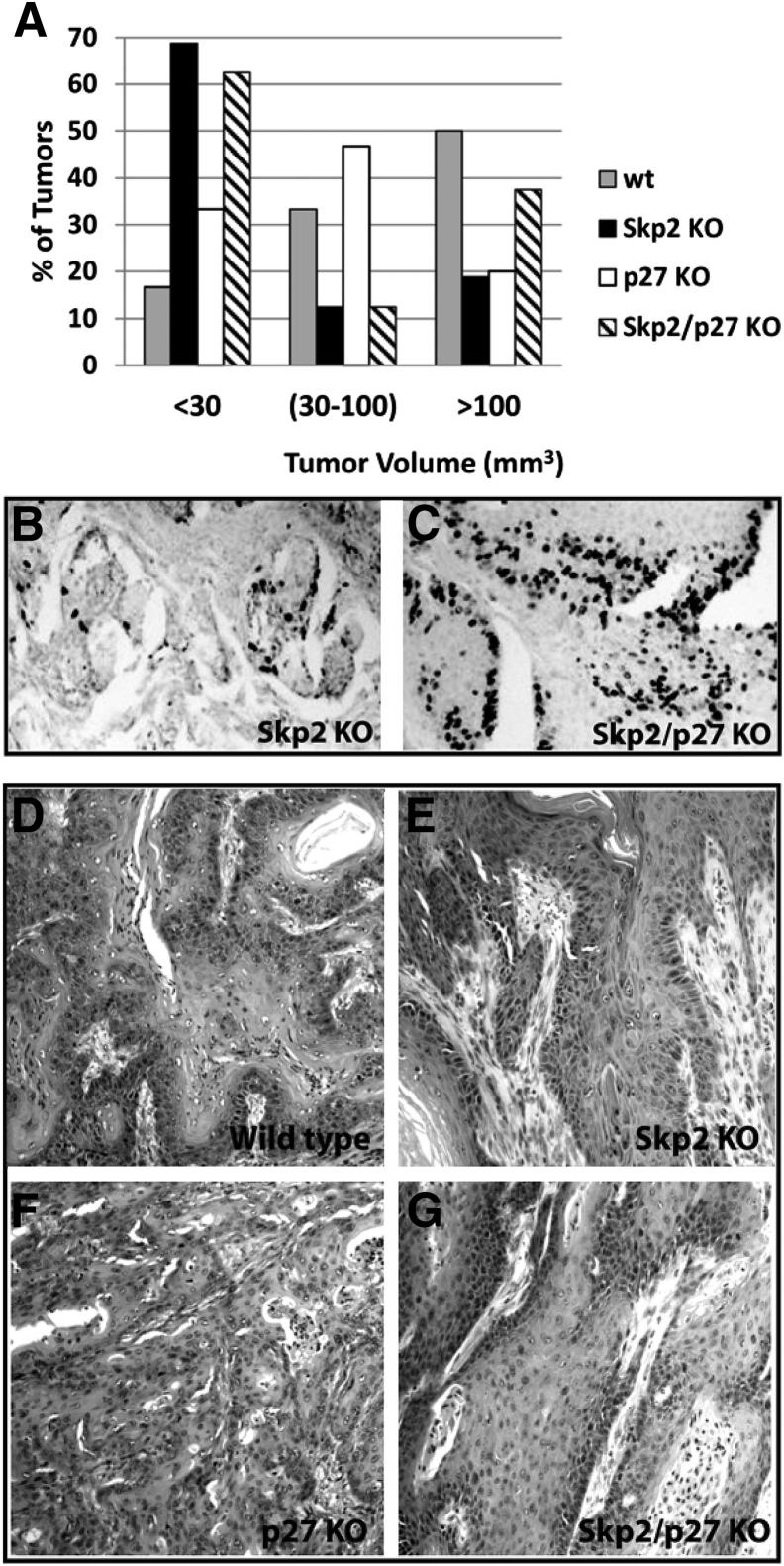
To evaluate the hypothesis that elevated p27Kip1 protein levels are associated with reduced tumorigenesis in Skp2−/− mice, we developed Skp2−/−/p27−/− compound mice and challenged this mouse model to the classic DMBA/TPA regimen. The C57BL/6 mouse genetic background, used in the previous experiment, is refractory to the initiation-promotion protocol when TPA is used as a promoter.27 Therefore, the Skp2−/−/p27−/− compound mice were generated on an FVB/Sencar hybrid background that exhibits higher sensitivity to the two-stage carcinogenesis protocol.27 As previously reported by Nakayama et al,15 the body size of p27−/− mice was larger than that of WT siblings, and Skp2−/− mice were smaller than the WT controls, whereas the body size of the Skp2−/−/p27−/− double mutant was similar to that of the WT controls (Figure 3A). More important, thymus measurements for each of the four genotypes analyzed confirmed that a p27Kip1 deficiency partially compensates for the absence of Skp2 in the Skp2−/− affected organs (data not shown). These data confirm that a p27Kip1 deficiency reverses the gross phenotype observed in the single KO mice.15 Four groups of mice (Skp2−/−, p27−/−, Skp2−/−/p27−/−, and WT mice) were treated with DMBA at 3 weeks of age, followed by biweekly applications of TPA for up to 25 weeks, and the incidence and multiplicity of papillomas were scored in each group for 30 weeks. The incidences of papilloma formation that reached a plateau at approximately 12 to 13 weeks in the p27−/− and WT mice were 100% and 90%, respectively (Figure 3B). As expected, the Skp2−/− group exhibited a reduction in tumor incidence, with approximately 60% of mice developing papillomas by week 22. Unexpectedly, the absence of p27Kip1 in the Skp2-null background did not overturn the reduced papilloma incidences that were observed in the Skp2−/− mice. In fact, the Skp2−/−/p27−/− group reached a plateau of 65% at 12 to 30 weeks (Figure 3B). Moreover, no significant differences were observed in the number of papillomas per mouse (multiplicity) between the Skp2−/− and Skp2−/−/p27−/− mice, which developed an average of 4.5 papillomas per mouse, whereas the WT siblings developed approximately 25 tumors per mouse (Figure 3C). The p27Kip1 deficiency did not affect tumor latency or incidence; however, a significant reduction in tumor multiplicity was observed in the p27−/− mice (approximately 10 tumors per mouse) compared with the WT siblings (Figure 3C). Although it is unclear why the p27Kip1 deficiency leads to fewer papillomas per mouse, we hypothesize that reduced p27Kip1 levels could compose the assembly of CDK4/D-type cyclin complexes, with a direct effect in the proliferative balance of the initiated keratinocytes. These results diverge from those previously reported by Philipp et al,28 in which the mean number of papillomas did not differ between the p27−/− and control littermates. A further evaluation of mouse skin tumors confirmed that the Skp2−/− mice develop fewer papillomas and that those tumors were smaller compared with those of the WT mice (Figure 4A). The WT mice developed significantly larger papillomas, in which 50% of the tumors were >100 mm3, and only 16% of the tumors were <30 mm3. Conversely, the papillomas from the Skp2−/− mice were smaller than those from the other groups: 68% of the tumors were <30 mm3. More important, although the incidence and multiplicity values were similar between the Skp2−/− and Skp2−/−/p27−/− mice, 40% of papillomas from the latter group were >100 mm3 (Figure 4A). Therefore, it is tempting to hypothesize that, in some cases, p27Kip1 deficiency can partially compensate for the absence of Skp2 during the tumor-growing phase, which could cause a consistent increase in the tumor size, but not during the initiation stage with the subsequent reduction in the number of tumors. More important, we determined that approximately 30% of the Skp2−/−/p27−/− papillomas exhibited a threefold increase in the number of proliferative keratinocytes [5-bromo-2′-deoxyuridine (BrdU)–positive cells] compared with the Skp2−/− tumors (Figure 4, B and C).
Figure 3.
The kinetics of papilloma formation in Skp2−/−/p27−/− compound mice. A: Representative female Skp2−/− (Skp2 KO), Skp2−/−/p27−/− (Skp2/p27 KO), p27−/− (p27 KO), and WT littermates at 25 weeks of age. The p27−/− mice exhibited an increased malignant transformation to SCCs (arrow). B: The percentage of WT, p27−/− (p27 KO), Skp2−/− (Skp2 KO), and Skp2−/−/p27−/− (Skp2/p27 KO) mice with at least one papilloma (incidence). C: Average number of papillomas per mouse (multiplicity) within 30 weeks of a biweekly administration of TPA.
Figure 4.
Histopathological analysis and tumor size of the skin papillomas. A: Skin papillomas were classified according to their volume and depicted as a percentage of the total number of tumors for the WT, Skp2−/− (Skp2 KO), p27−/− (p27 KO), and Skp2−/−/p27−/− (Skp2/p27 KO) mice. Immunostaining with anti-BrdU antibody of representative sections of Skp2−/− (B) and Skp2−/−/p27−/− (C) papillomas. Representative paraffin sections of papillomas at 30 weeks of promotion from WT (D), Skp2−/− (E), p27−/− (F), and Skp2−/−/p27−/− (G) mice. Original magnification, ×10. Skp2−/− and Skp2−/−/p27−/− mice exhibit only moderate dysplasia in well-differentiated tumors. A section of a WT papilloma exhibits moderated dysplasia and some anaplastic areas, whereas severe dysplasia and anaplastic areas were observed in the p27−/− tumors.
A histopathological analysis of the skin tumors indicated that 100% of the Skp2−/− and Skp2−/−/p27−/− tumors were well-differentiated papillomas with no atypia in the basal layers (Table 1 and Figure 4, E and G). In contrast, 28% of the WT and 40% of the p27−/− tumors were classified according to a modified Broders’ classification system29 as squamous cell carcinoma II, which consisted of moderately differentiated cells, with 50% of differentiated cells (Table 1 and Figure 4, D and F). More important, 40% of the p27−/− tumors were SCC, grade 3 (ie, a poorly differentiated tumor with little keratinization) (Table 1 and Figures 3A and 4F). Therefore, similar to the report by Kudo et al,19 our studies confirmed that the p27−/− mice exhibited an increased rate of malignant progression to SCCs.
Table 1.
Histopathological Analysis of Skin Tumors
| Mice | No. of tumors/group | No. (%) of tumors classified |
||
|---|---|---|---|---|
| Papilloma∗ | SCC II† | SCC III‡ | ||
| WT | 18 | 13 (72) | 5 (28) | 0 (0) |
| Skp2−/− | 22 | 22 (100) | 0 (0) | 0 (0) |
| p27−/− | 10 | 2 (20) | 4 (40) | 4 (40) |
| Skp2−/−/p27−/− | 10 | 10 (100) | 0 (0) | 0 (0) |
No atypia in the basal layers, basal cell hyperplasia, mild acanthosis and hyperkeratosis, and no invasion of epidermal cells into the dermis.
Moderately differentiated SCC, with 50% of differentiated cells, expansion of the basal and spinous layers, and loss of polarity.
Poorly differentiated SCC, with little keratinization, and cords of epidermal cells contiguous to the basal layer that are invading the dermis.
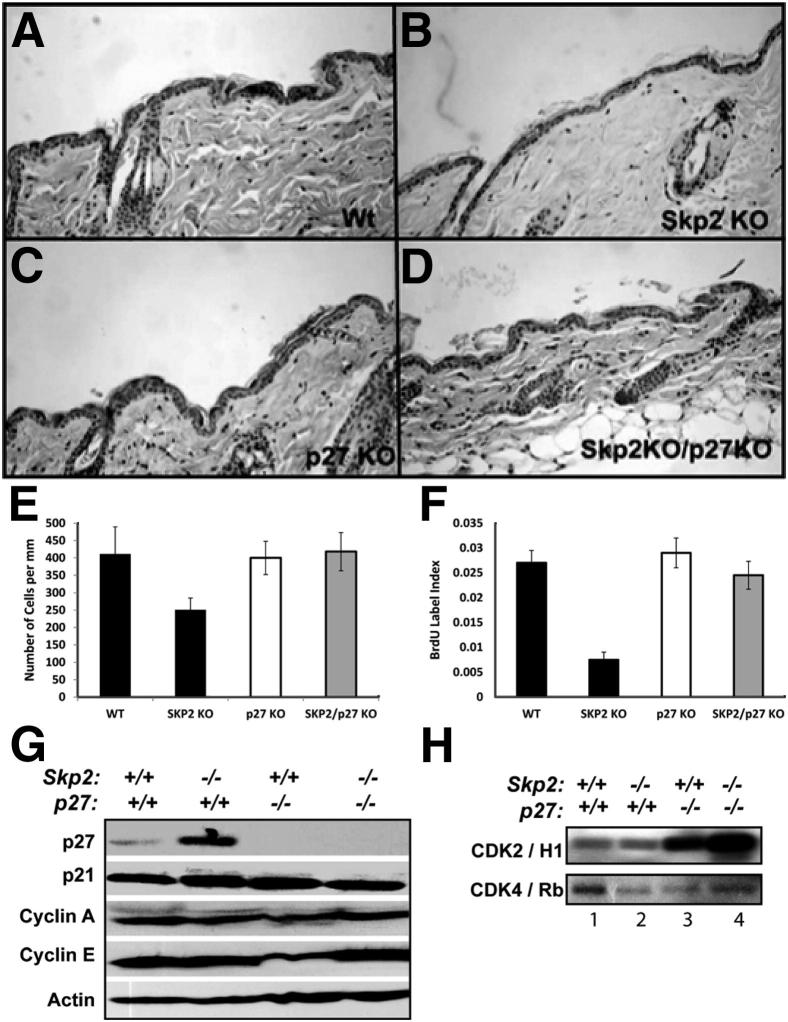
We have previously reported that Skp2−/− mice developed a hypoplastic epidermis.24 Therefore, we examined whether simultaneous ablation of p27Kip1 and Skp2 reversed the epidermal hypoplasia observed in the Skp2−/− mice.24 Consistent with our previous report,24 a histological analysis of mouse epidermis indicated that a lack of Skp2 expression resulted in a threefold decrease in the number of proliferative keratinocytes (BrdU label index), along with a twofold reduction in the number of nucleated cells, which contributed to the hypoplasia observed in the Skp2−/− epidermis24 (Figure 5, A–F). Ablation of p27Kip1 in the Skp2-null background overturned the reduced keratinocyte proliferation and hypoplasia observed in the Skp2−/− mice (Figure 5, A–F). Biochemical analysis of epidermal scrapes from the Skp2−/− and control mice indicated elevated p27Kip1 protein levels in the Skp2−/− mice, with mild or no modifications in other putative Skp2 substrates, such as p21Cip1, cyclin A, or cyclin E (Figure 5G). Moreover, the Skp2−/− epidermis exhibited a threefold reduction in the CDK4 kinase activity when compared with that of the WT controls (Figure 5H). More important, a lack of p27Kip1 expression in the Skp2-null epidermis demonstrated a reversion of CDK4 activity, thereby achieving a level similar to the WT epidermis (Figure 5H). However, the increased accumulation of p27Kip1 in the Skp2−/− epidermis had a mild or no effect, reducing the CDK2 kinase activity compared with that of the WT mice (Figure 5H). As expected, p27Kip1 ablation greatly increased the CDK2 kinase activity in mouse keratinocytes, as previously described by Macias et al30 (Figure 5H). Interestingly, simultaneous ablation of Skp2 and p27Kip1 greatly increased the CDK2 kinase activity that was observed in the p27−/− epidermis (Figure 5H).
Figure 5.
Histopathological and biochemical analyses of mouse epidermis from Skp2−/−/p27−/− compound mice. Representative paraffin sections of skin from WT (A), Skp2−/− (B), p27−/− (C), and Skp2−/−/p27−/− (D) mice were stained with H&E. Quantification of nucleated cells (E) and BrdU stained (F) in the epidermis of WT, Skp2−/− (Skp2 KO), p27−/− (p27 KO), and Skp2−/−/p27−/− (Skp2KO/p27KO) mice. G: Epidermal lysates were separated by SDS-PAGE and transferred onto a nitrocellulose membrane. Primary antibodies against p27Kip1, p21Cip1, cyclin A, and cyclin E were used for immunoblot analysis. Actin was used as a loading control. H: CDK2 and CDK4 kinase activities from the epidermis of the four genotypes were analyzed. Fresh epidermal lysates were immunoprecipitated with specific antibodies against CDKs, and in vitro kinase assays were performed with pRb or histone H1 peptides as substrates.
Collectively, these observations suggest that a p27Kip1 deficiency reverses the epidermal phenotype that was observed in Skp2−/− mice by increasing the kinase activity of both CDK4 and CDK2 in mouse keratinocytes. These data are consistent with previous reports indicating that all cellular and histopathological abnormalities observed in Skp2−/− mice are abolished in Skp2−/−/p27−/− mice.15 Therefore, we provide genetic evidence that p27Kip1 accumulation is responsible for the reduced keratinocyte proliferation and epidermal hypoplasia that was observed in the Skp2−/− mice. Moreover, the inhibition of tumorigenesis that was observed in the Skp2−/− epidermis occurred in a p27Kip1-independent manner, which suggests that an ablation of Skp2 initiates additional events that have an important function during mouse skin tumor initiation.
Skp2 Ablation Is Associated with p53-Dependent Apoptosis in the Epidermis and Skin Tumorigenesis
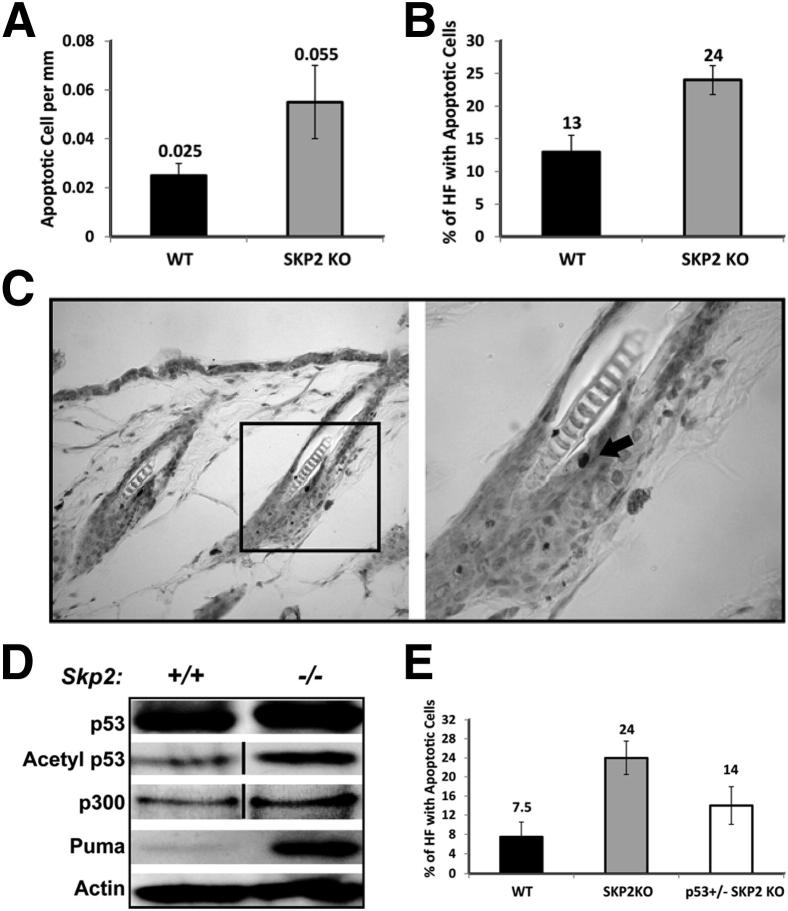
Down-regulation of Skp2 decreases cell growth and induces apoptosis.11,31,32 Therefore, we investigated whether the ablation of Skp2 induces keratinocyte apoptosis in mouse epidermis and skin papillomas. Notably, stem cells localized in the bulge region of HFs retain carcinogen-DNA adducts after DMBA, which supports the theory that these cells are responsible for skin tumor initiation.33 Therefore, we first quantified the number of apoptotic cells within the interfollicular epidermis and HFs from the Skp2−/− and WT control mice. We observed a 2.2-fold increase in the rate of apoptotic cells in the Skp2−/− mice compared with WT control mice (P = 0.0014, t-test) (Figure 6A). In addition, the incidence of apoptotic HFs (the percentage of HFs containing apoptotic cells) was elevated 1.8-fold in Skp2−/− epidermis compared with that of the WT control mice (Figure 6B). More important, most of the apoptotic cells were localized in the bulge region of the HF (Figure 6C), which suggests that apoptosis may be responsible for reducing the number of skin tumors observed in the Skp2−/− mice. The fact that Skp2−/− papillomas are smaller than those of the WT control mice suggests that apoptosis may have an important role during the tumor promotion stage. However, no significant differences in the number of apoptotic cells were observed between the Skp2−/− and WT papillomas (data not shown). Consistent with these data, another report demonstrated that Skp2 expression suppresses p53-dependent apoptosis by inhibiting the p53-p300/CBP interaction.11 Analysis of mouse epidermis indicated a twofold increase in p300/CBP protein levels and acetylated-p53 in Skp2−/− mice compared with that of the WT siblings (Figure 6D). Accordingly, Puma, which is an apoptosis mediator and p53-target gene, increased fourfold in the Skp2−/− epidermis (Figure 6D). Therefore, we hypothesized that an Skp2 deficiency leads to increased p53-dependent apoptosis in mouse epidermis. To test this hypothesis, we developed Skp2−/−/p53+/− compound mice. Evaluation of HF apoptosis showed a 1.8-fold reduction in the percentage of HFs that contained apoptotic cells in Skp2−/−/p53+/− mice compared with Skp2−/− mice (P = 0.045, t-test) (Figure 6E). Nevertheless, the reduced apoptotic level observed in the Skp2−/−/p53+/− mice did not reach the levels that were detected in the WT siblings. These results suggest that an Skp2 deficiency triggers p53-dependent keratinocyte apoptosis. However, we cannot exclude the possibility that p53-independent mechanisms could also play a relevant function in epidermal apoptosis.
Figure 6.
Histopathological and biochemical analyses of mouse epidermis from Skp2−/−/p53+/− mice. A: Apoptosis in interfollicular epidermis and HF. B: The percentage of HFs with at least one apoptotic cell in the bulge area was analyzed by the TUNEL assay. C: Apoptotic keratinocyte (arrow) in the HF of Skp2−/− mice. The boxed area in the left panel corresponds to a higher-magnification (original magnification, ×20) image in the right panel. D: Epidermal lysates were separated by SDS-PAGE and transferred onto a nitrocellulose membrane. Primary antibodies against p53, acetylated p53, p300, and Puma were used for immunoblot analysis. Actin was used as a loading control. E: The percentage of HFs with at least one apoptotic cell in the bulge area in the epidermis of WT, Skp2−/− (Skp2 KO), and p53+/−/Skp2−/− (p53+/− SKP2 KO) mice. A vertical line was depicted in blots of D to denote rearrangements of the lines.
Discussion
As previously noted, the data accumulated recently have supported the hypothesis that Skp2 has an oncogenic function, mainly by inducing p27Kip1 degradation. However, this concept has not been confirmed experimentally. Thus, we have studied the specific function of the Skp2/p27Kip1 axis in a well-known mouse model of chemical carcinogenesis.
We have previously reported that an Skp2 ablation caused hypoplastic epidermis and reduced keratinocyte proliferation associated with increased p27Kip1 levels and decreased CDK4 activity.24 Herein, we examined the skin phenotype and skin tumorigenesis of Skp2-null mice in the presence or absence of the Skp2 main substrate, p27Kip1. In agreement with previous reports demonstrating that a p27Kip1 deficiency restores most of the Skp2−/− phenotype,15 we determined that a p27Kip1 ablation reinstated epidermal homeostasis and keratinocyte proliferation of Skp2−/− epidermis. Biochemical analysis of epidermal scrapes indicated that a p27Kip1 ablation causes increased CDK4 and CDK2 kinase activities in Skp2−/−/p27−/− keratinocytes. We conclude that p27Kip1 functions as a cell cycle regulator downstream of Skp2 in mouse keratinocytes. However, we detected an important difference in mouse hepatocytes14 and keratinocytes24 on ablating Skp2. Although p27Kip1 accumulation inhibits CDK2 and CDC2 activities in hepatocytes, only CDK4 activity was affected in keratinocytes. Therefore, accumulation of p27Kip1 appears to have an important function in the inhibition of G1/S phase progression in mouse keratinocytes and S/G2 progression in hepatocytes. Although the molecular mechanisms responsible for these variances are not understood, these differences can explain why accumulation of p27Kip1 in mouse hepatocytes causes polyploidy and multiple centrosomes,14,15 whereas those events have not been observed in keratinocytes. Although the nature of p27Kip1-mediated CDK4 kinase inhibition has yet to be fully established, recent investigations have shown that p27Kip1 can bind to and inhibit CDK4 activation by preventing the activating phosphorylation actions of cyclin H–CDK7, the CDK-activating kinase.34
To determine the effect of Skp2 ablation in skin tumorigenesis, we used the two-stage carcinogenesis protocol, which is a well-suited model for understanding the multistage nature of tumor progression. This model allows for the study of the effect of Skp2 ablation in tumor initiation (an irreversible genetic alteration in a target cell), tumor promotion (the process by which an initiated tissue develops focal proliferation leading to tumor development), and malignant progression (the final transition to invasive behavior).35 We observed a significant reduction in the number of skin papillomas in Skp2-null mice compared with WT siblings. Similar to Skp2−/− epidermis,24 a biochemical analysis of Skp2−/− papillomas showed increased p27Kip1 levels but no other putative Skp2 substrates, such as p21Cip1, cyclin E, and cyclin D1. Therefore, we hypothesized that increased p27Kip1 levels have a major role in inhibiting skin tumorigenesis. To test this hypothesis, we studied the kinetics of skin tumor development in Skp2−/−/p27−/− compound mice. Contrary to the restoration of normal phenotype and proliferation observed in mouse epidermis, a p27Kip1 deficiency did not reinstate the number of tumors in Skp2−/−/p27−/− mice. We observed a relevant reduction in the number of skin papillomas per mouse (multiplicity) and tumor incidence in both Skp2−/− and Skp2−/−/p27−/− mice. The number of skin tumors is affected by the number of initiated cells and/or other early events, such as the clonal expansion of initiated cells. Therefore, our results provide genetic evidence that initiation and/or clonal expansion of initiated cells strongly depends on Skp2 expression but not on its main substrate, p27Kip1.
Substantial evidence has suggested that cells from the bulge area of HFs have characteristics of stem cells, such as slow-cycling, label-retaining properties and high proliferative capacity.36 In this regard, Morris et al33 demonstrated that HF stem cells also retain carcinogen-DNA adducts, which supports the concept that these are target cells during chemical initiation. More important, we observed an elevated number of apoptotic cells in the interfollicular epidermis and HFs of Skp2−/− mice. Moreover, Skp2−/− mice exhibited an increased incidence of apoptotic cells in the bulge area of HFs. These results suggest that a lack of Skp2 expression leads to programmed cell death of the HF stem cells, which affects tumor initiation (or an early event during skin tumorigenesis). Consistent with our data, Skp2 inhibition by siRNA decreases cell growth and increases apoptosis.31,37
Biochemical analysis of Skp2−/− mouse epidermis showed increased levels of p300/CBP protein and increased p53 acetylation, which is essential for p53 activation.38 Consistent with these results, we found an increased level of Puma, which is a p53 target and pro-apoptotic regulator, in Skp2−/− epidermis. Kitagawa et al11 have reported that Skp2 antagonizes the interaction between p300/CBP and p53, which leads to diminished p53 acetylation and further suppresses the transactivation ability of p53. Therefore, our data suggest that an Skp2 deficiency favors p300/CBP-mediated p53 acetylation, which leads to elevated p53-mediated apoptosis in HF stem cells and a reduction in tumorigenesis. To test this hypothesis, we developed Skp2−/−/p53+/− mice and determined the incidence of apoptosis in HFs. Supporting our hypothesis, a reduced p53 dosage caused a twofold decrease in the percentage of HFs that contained apoptotic cells on an Skp2-null background. However, the fact that we did not observe a full reversion of HF apoptosis led us to hypothesize that a lack of Skp2 expression also induced p53-independent apoptosis in HFs. However, we cannot exclude the possibility that the partial decrease in HF apoptosis is because we used a mouse model that expressed half of the normal p53 level in mouse epidermis. Notably, Skp2−/−/p53−/− mice were born at a low rate, and this observation impeded the analysis of the epidermis phenotype at the same ages as the control mice (7 weeks). Furthermore, Skp2 inhibits p53 activities in additional pathways. In particular, studies by Song et al39 have identified RassF1a as a specific target of Skp2. RassF1a inhibits Mdm2 through stimulation of Mdm2 self-ubiquitination, leading to p53 activation.10 In addition, Rassf1a also induces apoptosis through the activation and translocation of p73, which increases the transcription of the pro-apoptotic protein, Puma.40 Therefore, Skp2 can inhibit p53 by preventing CBP/p300 acetylation activation and by increasing levels of Mdm2 by inhibiting Rassf1a, thereby yielding two independent pathways to inhibit apoptosis. More important, our studies also demonstrate that an Skp2 deficiency causes an accumulation of RassF1a in mouse epidermis (C. Sistrunk and M. Rodriguez-Puebla, unpublished data) and a reduction of Mdm2 protein levels. These findings suggest that the RassF1a pathway can also accelerate p53-dependent apoptosis in mouse epidermis. Whether a synergistic effect exists between p300/CBP-mediated p53 activation and RassF1a pathways warrants further investigation.
To determine the effect of disrupting the Skp2/p27Kip1 axis in tumor promotion and malignant progression, we performed histopathological analyses of skin tumors from Skp2−/−/p27−/− and control sibling mice. We determined a clear reduction in the tumor size of Skp2−/− papillomas that was partially restored in Skp2−/−/p27−/− papillomas. Therefore, we infer that an Skp2 deficiency inhibits early-stage tumorigenesis. However, the few Skp2−/−/p27−/− tumors that avoid this early blockage grow more rapidly compared with Skp2−/− papillomas. Therefore, we hypothesize that p27Kip1 ablation favors fast keratinocyte proliferation during the tumor promotion stage of a fraction of the Skp2−/−/p27−/− papillomas, which causes a partial rescue in tumor volume. Consistent with this hypothesis, we observed elevated keratinocyte proliferation in approximately 30% of the Skp2−/−/p27−/− papillomas compared with Skp2−/− tumors. We also evaluated the relevance of the apoptotic profile displayed in the papillomas from the four genotypes. However, we did not observe significant differences among them (data not shown). Therefore, our results suggest that initiation, and not the promotion stage of skin carcinogenesis, was affected by the apoptosis that was induced by an Skp2 deficiency.
Histopathological analysis of the skin tumors indicated that, although the tumorigenesis blockages in animals devoid of Skp2 are independent of p27Kip1 accumulation, p27−/− animals developed more aggressive tumors that were classified as SCCs. The fact that lack of p27Kip1 expression causes elevated CDK4 and CDK2 kinase activities suggests that the CDK inhibitor role of p27Kip1 plays important functions during malignant progression. Supporting this theory, CDK4 overexpression in K5CDK4 transgenic mice also induces malignant progression mediated by the sequestration of p27Kip1 and p21Cip1 by D-type cyclin/CDK4 complexes.41 Moreover, a lack of CDK2 expression reduces the malignant progression to SCCs in K5CDK4/CDK2−/− mice.42 It is not clear why p27−/− mice showed a reduction in the number of papillomas per mouse compared with the WT siblings. However, we hypothesize that an ablation of p27Kip1 compromises the assembly of active CDK4/D-type cyclin complexes that, in this case, rely on the presence of p21Cip1.43
More important, an ablation of Skp2 appears to block malignant progression in Skp2−/−/p27−/− mice because all of the Skp2−/− and Skp2−/−/p27−/− tumors were classified as well-differentiated papillomas. Notably, an analysis of the skin tumors from Skp2−/−/p27−/−, Skp2−/−, p27−/−, and WT mice did not show a significant difference in the number of apoptotic cells (data not shown). Therefore, one may conclude that Skp2-regulated inhibitory pathways other than apoptosis impede malignant progression. Supporting this concept, aberrant oncogene signals initiate a senescence response in Skp2-null cells through a p19ARF-p53–independent pathway,44 and chemical inhibition of Skp2 activity causes cell death through activation of autophagy.45
In summary, our data demonstrate that Skp2 inhibition causes blockage of an early event during the multistage process of nonmelanoma skin tumorigenesis through a p27Kip1-independent pathway. We also suggest that Skp2-mediated inhibition of p53-dependent apoptosis has an important function in the elevated apoptosis observed in Skp2−/− HF stem cells, which correlates with fewer papillomas. In addition, a partial rescue of keratinocyte proliferation has been observed in Skp2−/−/p27−/− papillomas, which suggests that cell proliferation during the promotion stage is partially dependent on the p27Kip1 level. Whether the variations in the effects of Skp2 and p27Kip1 levels at specific stages of tumorigenesis are unique for epidermal tumors warrants further investigation. Finally, although a strong inverse correlation between Skp2 and p27Kip1 levels has been demonstrated in numerous experimental and human tumors, Skp2 is also a p27Kip1-independent indicator of poor prognosis in other human tumors, such as biliary tract carcinoma,46 soft tissue sarcomas,47 and urinary tract transitional cell carcinoma.48 Therefore, our results support the hypothesis that molecular targets of Skp2, other than p27Kip1, may also be important factors in cancer pathogenesis. Moreover, a combination of Skp2 and targets, other than p27Kip1, in addition to the individual molecular targets, might be useful prognostic factors of cancer.
Acknowledgments
We thank Dr. Paula Miliani de Marval for her critical review of this article, Dr. Keiichi Nakayama (Kyushu University, Kyushu, Japan) for kindly providing the Skp2-null mice, Juan Carlos Santiago for his technical support, and the Laboratory Animal Resources and the Histology Core (College of Veterinary Medicine, North Carolina State University) for helping with the processing and staining of skin and tumor samples.
Footnotes
Supported by National Cancer Institute/NIH grant RO1 CA116328 (M.L.R.-P.).
C.S. and S.H.K. contributed equally to this work.
References
- 1.Weissman A.M. Themes and variations on ubiquitylation. Nat Rev Mol Cell Biol. 2001;2:169–178. doi: 10.1038/35056563. [DOI] [PubMed] [Google Scholar]
- 2.Gregory M.A., Hann S.R. c-Myc proteolysis by the ubiquitin-proteasome pathway: stabilization of c-Myc in Burkitt’s lymphoma cells. Mol Cell Biol. 2000;20:2423–2435. doi: 10.1128/mcb.20.7.2423-2435.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakayama K.I., Nakayama K. Ubiquitin ligases: cell-cycle control and cancer. Nat Rev Cancer. 2006;6:369–381. doi: 10.1038/nrc1881. [DOI] [PubMed] [Google Scholar]
- 4.Bahram F., von der Lehr N., Cetinkaya C., Larsson L.G. c-Myc hot spot mutations in lymphomas result in inefficient ubiquitination and decreased proteasome-mediated turnover. Blood. 2000;95:2104–2110. [PubMed] [Google Scholar]
- 5.Yu Z.K., Gervais J.L., Zhang H. Human CUL-1 associates with the SKP1/SKP2 complex and regulates p21(CIP1/WAF1) and cyclin D proteins. Proc Natl Acad Sci U S A. 1998;95:11324–11329. doi: 10.1073/pnas.95.19.11324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamura T., Hara T., Kotoshiba S., Yada M., Ishida N., Imaki H., Hatakeyama S., Nakayama K., Nakayama K.I. Degradation of p57Kip2 mediated by SCFSkp2-dependent ubiquitylation. Proc Natl Acad Sci U S A. 2003;100:10231–10236. doi: 10.1073/pnas.1831009100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tedesco D., Lukas J., Reed S.I. The pRb-related protein p130 is regulated by phosphorylation-dependent proteolysis via the protein-ubiquitin ligase SCF(Skp2) Genes Dev. 2002;16:2946–2957. doi: 10.1101/gad.1011202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hiramatsu Y., Kitagawa K., Suzuki T., Uchida C., Hattori T., Kikuchi H., Oda T., Hatakeyama S., Nakayama K.I., Yamamoto T., Konno H., Kitagawa M. Degradation of Tob1 mediated by SCFSkp2-dependent ubiquitination. Cancer Res. 2006;66:8477–8483. doi: 10.1158/0008-5472.CAN-06-1603. [DOI] [PubMed] [Google Scholar]
- 9.Huang H., Regan K.M., Wang F., Wang D., Smith D.I., van Deursen J.M., Tindall D.J. Skp2 inhibits FOXO1 in tumor suppression through ubiquitin-mediated degradation. Proc Natl Acad Sci U S A. 2005;102:1649–1654. doi: 10.1073/pnas.0406789102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song M.S., Song S.J., Kim S.J., Nakayama K., Nakayama K.I., Lim D.S. Skp2 regulates the antiproliferative function of the tumor suppressor RASSF1A via ubiquitin-mediated degradation at the G1-S transition. Oncogene. 2008;27:3176–3185. doi: 10.1038/sj.onc.1210971. [DOI] [PubMed] [Google Scholar]
- 11.Kitagawa M., Lee S.H., McCormick F. Skp2 suppresses p53-dependent apoptosis by inhibiting p300. Mol Cell. 2008;29:217–231. doi: 10.1016/j.molcel.2007.11.036. [DOI] [PubMed] [Google Scholar]
- 12.Bai C., Sen P., Hofmann K., Ma L., Goebl M., Harper J.W., Elledge S.J. SKP1 connects cell cycle regulators to the ubiquitin proteolysis machinery through a novel motif, the F-box. Cell. 1996;86:263–274. doi: 10.1016/s0092-8674(00)80098-7. [DOI] [PubMed] [Google Scholar]
- 13.Carrano A.C., Eytan E., Hershko A., Pagano M. SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat Cell Biol. 1999;1:193–199. doi: 10.1038/12013. [DOI] [PubMed] [Google Scholar]
- 14.Nakayama K., Nagahama H., Minamishima Y.A., Matsumoto M., Nakamichi I., Kitagawa K., Shirane M., Tsunematsu R., Tsukiyama T., Ishida N., Kitagawa M., Nakayama K., Hatakeyama S. Targeted disruption of Skp2 results in accumulation of cyclin E and p27(Kip1), polyploidy and centrosome overeduplication. EMBO J. 2000;19:2069–2081. doi: 10.1093/emboj/19.9.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakayama K., Nagahama H., Minamishima Y.A., Miyake S., Ishida N., Hatakeyama S., Kitagawa M., Iemura S., Natsume T., Nakayama K.I. Skp2-mediated degradation of p27 regulates progression into mitosis. Dev Cell. 2004;6:661–672. doi: 10.1016/s1534-5807(04)00131-5. [DOI] [PubMed] [Google Scholar]
- 16.Nakayama K.I., Nakayama K. Regulation of the cell cycle by SCF-type ubiquitin ligases. Semin Cell Dev Biol. 2005;16:323–333. doi: 10.1016/j.semcdb.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 17.Latres E., Chiarle R., Schulman B.A., Pavletich N.P., Pellicer A., Inghirami G., Pagano M. Role of the F-box protein Skp2 in lymphomagenesis. Proc Natl Acad Sci U S A. 2001;98:2515–2520. doi: 10.1073/pnas.041475098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carrano A.C., Pagano M. Role of the F-box protein Skp2 in adhesion-dependent cell cycle progression. J Cell Biol. 2001;153:1381–1390. doi: 10.1083/jcb.153.7.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kudo Y., Kitajima S., Sato S., Miyauchi M., Ogawa I., Takata T. High expression of S-phase kinase-interacting protein 2, human F-box protein, correlates with poor prognosis in oral squamous cell carcinomas. Cancer Res. 2001;61:7044–7047. [PubMed] [Google Scholar]
- 20.Shim E.H., Johnson L., Noh H.L., Kim Y.J., Sun H., Zeiss C., Zhang H. Expression of the F-box protein SKP2 induces hyperplasia, dysplasia, and low-grade carcinoma in the mouse prostate. Cancer Res. 2003;63:1583–1588. [PubMed] [Google Scholar]
- 21.Slingerland J., Pagano M. Regulation of the cdk inhibitor p27 and its deregulation in cancer. J Cell Physiol. 2000;183:10–17. doi: 10.1002/(SICI)1097-4652(200004)183:1<10::AID-JCP2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 22.Osoegawa A., Yoshino I., Tanaka S., Sugio K., Kameyama T., Yamaguchi M., Maehara Y. Regulation of p27 by S-phase kinase-associated protein 2 is associated with aggressiveness in non-small-cell lung cancer. J Clin Oncol. 2004;22:4165–4173. doi: 10.1200/JCO.2004.01.035. [DOI] [PubMed] [Google Scholar]
- 23.Li Q., Murphy M., Ross J., Sheehan C., Carlson J.A. Skp2 and p27kip1 expression in melanocytic nevi and melanoma: an inverse relationship. J Cutan Pathol. 2004;31:633–642. doi: 10.1111/j.0303-6987.2004.00243.x. [DOI] [PubMed] [Google Scholar]
- 24.Sistrunk C., Macias E., Nakayama K., Kim Y., Rodriguez-Puebla M.L. Skp2 is necessary for myc-induced keratinocyte proliferation but dispensable for myc oncogenic activity in the oral epithelium. Am J Pathol. 2011;178:2470–2477. doi: 10.1016/j.ajpath.2011.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donehower L.A., Harvey M., Slagle B.L., McArthur M.J., Montgomery C.A., Jr., Butel J.S., Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez-Puebla M.L., Robles A.I., Johnson D.G., LaCava M., Conti C.J. Synchronized proliferation induced by 12-O-tetradecanoylphorbol-13-acetate treatment of mouse skin: an in vivo model for cell cycle regulation. Cell Growth Differ. 1998;9:31–39. [PubMed] [Google Scholar]
- 27.Naito M., DiGiovanni J. Genetic background and development of skin tumors. In: Conti C.J., Slaga T.J., Klein-Szanto A.J., editors. Carcinogenesis A Comprehensive Survey. Raven Press; New York: 1989. pp. 187–212. [PubMed] [Google Scholar]
- 28.Philipp J., Vo K., Gurley K.E., Seidel K., Kemp C.J. Tumor suppression by p27Kip1 and p21Cip1 during chemically induced skin carcinogenesis. Oncogene. 1999;18:4689–4698. doi: 10.1038/sj.onc.1202840. [DOI] [PubMed] [Google Scholar]
- 29.Klein-Szanto A. European Late Effects Project; Schattauer: 1997. Melanotic and non-melanotic tumours of the rodent skin. Pathology of Neoplasia and Preneoplasia in Rodents. pp 1–18. [Google Scholar]
- 30.Macias E., de Marval P.L., Senderowicz A., Cullen J., Rodriguez-Puebla M.L. Expression of CDK4 or CDK2 in mouse oral cavity is retained in adult pituitary with distinct effects on tumorigenesis. Cancer Res. 2008;68:162–171. doi: 10.1158/0008-5472.CAN-07-2461. [DOI] [PubMed] [Google Scholar]
- 31.Harada K., Supriatno, Kawashima Y., Itashiki Y., Yoshida H., Sato M. Down-regulation of S-phase kinase associated protein 2 (Skp2) induces apoptosis in oral cancer cells. Oral Oncol. 2005;41:623–630. doi: 10.1016/j.oraloncology.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 32.Jiang F., Caraway N.P., Li R., Katz R.L. RNA silencing of S-phase kinase-interacting protein 2 inhibits proliferation and centrosome amplification in lung cancer cells. Oncogene. 2005;24:3409–3418. doi: 10.1038/sj.onc.1208459. [DOI] [PubMed] [Google Scholar]
- 33.Morris R.J., Fischer S.M., Slaga T.J. Evidence that a slowly cycling subpopulation of adult murine epidermal cells retains carcinogen. Cancer Res. 1986;46:3061–3066. [PubMed] [Google Scholar]
- 34.Ray A., James M.K., Larochelle S., Fisher R.P., Blain S.W. p27Kip1 inhibits cyclin D-cyclin-dependent kinase 4 by two independent modes. Mol Cell Biol. 2009;29:986–999. doi: 10.1128/MCB.00898-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Slaga T. Mechanism involved in two-stage carcinogenesis in mouse skin. In: Slaga T., editor. Mechanism of Tumor Promotion. CRC Press; Boca Raton: 1984. pp. 1–16. [Google Scholar]
- 36.Morris R.J. Keratinocyte stem cells: targets for cutaneous carcinogens. J Clin Invest. 2000;106:3–8. doi: 10.1172/JCI10508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee S.H., McCormick F. Downregulation of Skp2 and p27/Kip1 synergistically induces apoptosis in T98G glioblastoma cells. J Mol Med. 2005;83:296–307. doi: 10.1007/s00109-004-0611-7. [DOI] [PubMed] [Google Scholar]
- 38.Grossman S.R. p300/CBP/p53 interaction and regulation of the p53 response. Eur J Biochem. 2001;268:2773–2778. doi: 10.1046/j.1432-1327.2001.02226.x. [DOI] [PubMed] [Google Scholar]
- 39.Song M.S., Song S.J., Kim S.Y., Oh H.J., Lim D.S. The tumour suppressor RASSF1A promotes MDM2 self-ubiquitination by disrupting the MDM2-DAXX-HAUSP complex. EMBO J. 2008;27:1863–1874. doi: 10.1038/emboj.2008.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matallanas D., Romano D., Yee K., Meissl K., Kucerova L., Piazzolla D., Baccarini M., Vass J.K., Kolch W., O’Neill E. RASSF1A elicits apoptosis through an MST2 pathway directing proapoptotic transcription by the p73 tumor suppressor protein. Mol Cell. 2007;27:962–975. doi: 10.1016/j.molcel.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miliani de Marval P.L., Macias E., Conti C.J., Rodriguez-Puebla M.L. Enhanced malignant tumorigenesis in Cdk4 transgenic mice. Oncogene. 2004;23:1863–1873. doi: 10.1038/sj.onc.1207309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Macias E., Kim Y., Miliani de Marval P.L., Klein-Szanto A., Rodriguez-Puebla M.L. Cdk2 deficiency decreases ras/CDK4-dependent malignant progression, but not myc-induced tumorigenesis. Cancer Res. 2007;67:9713–9720. doi: 10.1158/0008-5472.CAN-07-2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheng M., Olivier P., Diehl J.A., Fero M., Roussel M.F., Roberts J.M., Sherr C.J. The p21(Cip1) and p27(Kip1) CDK “inhibitors” are essential activators of cyclin D-dependent kinases in murine fibroblasts. EMBO J. 1999;18:1571–1583. doi: 10.1093/emboj/18.6.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin H.K., Chen Z., Wang G., Nardella C., Lee S.W., Chan C.H., Yang W.L., Wang J., Egia A., Nakayama K.I., Cordon-Cardo C., Teruya-Feldstein J., Pandolfi P.P. Skp2 targeting suppresses tumorigenesis by Arf-p53-independent cellular senescence. Nature. 2010;464:374–379. doi: 10.1038/nature08815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen Q., Xie W., Kuhn D.J., Voorhees P.M., Lopez-Girona A., Mendy D., Corral L.G., Krenitsky V.P., Xu W., Moutouh–de Parseval L., Webb D.R., Mercurio F., Nakayama K.I., Nakayama K., Orlowski R.Z. Targeting the p27 E3 ligase SCF(Skp2) results in p27- and Skp2-mediated cell-cycle arrest and activation of autophagy. Blood. 2008;111:4690–4699. doi: 10.1182/blood-2007-09-112904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sanada T., Yokoi S., Arii S., Yasui K., Imoto I., Inazawa J. Skp2 overexpression is a p27Kip1-independent predictor of poor prognosis in patients with biliary tract cancers. Cancer Sci. 2004;95:969–976. doi: 10.1111/j.1349-7006.2004.tb03185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oliveira A.M., Okuno S.H., Nascimento A.G., Lloyd R.V. Skp2 protein expression in soft tissue sarcomas. J Clin Oncol. 2003;21:722–727. doi: 10.1200/JCO.2003.05.112. [DOI] [PubMed] [Google Scholar]
- 48.Langner C., von Wasielewski R., Ratschek M., Rehak P., Zigeuner R. Expression of p27 and its ubiquitin ligase subunit Skp2 in upper urinary tract transitional cell carcinoma. Urology. 2004;64:611–616. doi: 10.1016/j.urology.2004.04.072. [DOI] [PubMed] [Google Scholar]