Abstract
The simple method for amplifying RNA targets (SMART) was used to detect K103N, a common HIV-1 reverse transcriptase drug-resistance mutation. Novel amplifiable SMART probes served as reporter molecules for RNA sequences that are captured and separated on a microfluidic platform under zero-flow conditions. Assays were performed both off chip and in a microchip reservoir using a modified version of real-time nucleic acid sequence-based amplification, without the noncyclic phase, and 65°C preheat. A total of 6000 copies/mL of the synthetic sequences were detected within 180 minutes of amplification. Although the sensitivity of research platforms is higher, SMART has the potential to offer comparable sensitivity and speed to commercially available viral load and HIV detection kits. Furthermore, SMART uses an inexpensive, practical, and more accurate isothermal exponential amplification technique. The use of molecular beacons resulted in relatively fast real-time detection (<180 minutes); however, they were also shown to hinder the amplification process when compared with end point detection. Finally, SMART probes were used for modeling of K103N concentrations within an unknown sample. Only 1% of the SMART probes was detected within the wild-type population (6 × 108 copies/mL). These results establish the groundwork for point-of-care drug resistance and viral load monitoring in clinical samples, which can revolutionize HIV patient care globally.
HIV is a daunting global pandemic.1 The development of resistance to antiretroviral medications is the main cause for treatment failure of HIV-infected individuals.2 K103N, a point mutation of lysine to asparagine at position 103 of the HIV reverse transcriptase (RT), is a common mutation that causes high-level resistance to first-generation nonnucleoside reverse transcriptase inhibitors, such as nevirapine and efavirenz.3 HIV-infected patients receiving nonnucleoside reverse transcriptase inhibitor–based treatment who harbor K103N, either before or after exposure to antiretroviral therapy, will most likely fail therapy and will require alternative antiretroviral treatment.3 Therefore, the detection of K103N, and other drug-resistance mutations, is of great importance, especially in resource-limited settings (RLSs), in which nonnucleoside reverse transcriptase inhibitor–based regimens serve as first-line treatment and subsequent options are limited.4
A sensitive method to detect drug resistance in a small rural clinic with no advanced laboratory infrastructure or the use of a field-deployable device to quickly detect resistance, although extremely beneficial, is lacking. The success of any field-deployable device will depend on variables such as the cost per test, the need for specialized equipment, staff expertise, refrigeration of reagents during shipping, and shelf life. It would be beneficial if the device would also use minimal sample volume and provide excellent assay sensitivity. Presently available assays, some with significant strengths, do not meet all these considerations.5,6
Various field-deployable assays to detect HIV and/or measure its RNA levels [viral load (VL)] are under development,7 although, to our knowledge, none incorporate drug-resistance testing. For example, progress has been made on a device for HIV detection via immunoassay intended for RLS,8 and a successful antibody-based HIV-1/2 test device has recently been granted Food and Drug Administration approval for at-home use.9 Many commercial HIV-1 detections and VL kits are available, including the COBAS TaqMan assay (Roche, Pleasanton, CA), a real-time HIV-1 assay (Abbott, Chicago, IL), and an Ultrasensitive p24 Assay (PerkinElmer, Waltham, MA).10 These kits use a variety of blood markers for detection and directly monitor VL by measuring HIV-1 RNA or indirectly by measuring p24 antigen, HIV antibodies, or CD4+ T-cell counts. However, none of these HIV detection devices and/or VL kits is capable of testing for drug-resistant mutations.
Two main commercial HIV genotyping assays are available to monitor drug resistance, ViroSeq (Abbott) and TRUGENE (Siemens, Washington, DC).10–14 ViroSeq is a PCR-based DNA sequencing technique requiring a full-scale sequencer with multiple primers. It requires an initial preparation of a plasma sample using a viral RNA isolation kit, followed by RNA reverse transcription, DNA PCR amplification, and sequencing amplified products using ViroSeq equipment in preparation for data analysis. Similarly, TRUGENE is an approved DNA sequencing-based technique, targeting the protease and reverse transcriptase (codons 40 to 247). Both methods require an HIV VL in the range of 2000 to 750,000 copies/mL13,15 for successful amplification and resistance detection, and both can detect K103N mutations in samples containing approximately 20% to 25% K103N variants.16
Recent research-only assays have been developed to increase the sensitivity of resistance detection, which seems to be important for clinical care.17 Such assays include next-generation sequencing, pyrosequencing,18 allele-specific PCR,15,19–21 and single-genome sequencing,22 which can detect resistance mutations at levels as low as 0.1%. Despite high sensitivity, pyrosequencing and single-genome sequencing are costly and time-consuming, requiring sequencing of multiple genomes. Allele-specific PCR is mutation specific and limited by occurrences of false negatives from nucleotide polymorphisms that can destabilize primer binding. Other novel approaches use ligation of probe molecules as an indicator of mutation detection.23 Ligation amplification depends on two single-stranded DNA probes hybridizing adjacently to a target mutation sequence. In addition to PCR amplification, rolling circle amplification is also used in conjunction with this ligation approach.24 These and other approaches have been shown to be effective and sensitive, but may have limited functionality in diverse HIV-1 genomes because of the variability of target sequences, resulting in mismatch recognition and inherent copying error of the amplicon during PCR amplification25 and loss of specificity. The previously mentioned commercial and noncommercial techniques can sensitively detect HIV resistance mutations, such as K103N; however, they still require laboratory infrastructure and expertise, which are limited in RLS. The application of a new method in RLS will require comparable sensitivity to existing methods with reduction of duration of sample-to-result time and less equipment restrictions based on the amplification technique.
Most of the previously mentioned drug-resistance detection techniques depend on PCR or another form of temperature cycling. PCR requires expertise and equipment, and can be challenging when amplifying a small population of mutant viral sequences due to non-specific amplification. As an alternative, nucleic acid sequence-based amplification (NASBA), used in HIV VL testing, can be faster than PCR for certain RNA amplification and compares favorably with PCR for HIV RNA detection.10,26,27 Because NASBA is an isothermal and exponential process, it avoids rapid degradation of enzymes and undesirable hybridization of participating nucleic acids.28 NASBA also has its limitations; it can be limited by the secondary structure of RNA, making primer design and multiplexing difficult. Clinically relevant hybridization sites on long RNA strands may require stringent oligonucleotide design, with only a small fraction of sequences hybridizing efficiently to RNA, thus reducing the efficiency of the amplification process.29 To combat these issues, NASBA usually begins with a 65°C heating step before the addition of enzymes to disrupt full-length RNA secondary structure30 and expose primer binding sites, typically 100 to 250 nucleotides apart, to avoid products with an inhibitory secondary structure.31 An additional disadvantage is the fact that NASBA initiates with a noncyclic reverse transcription phase, resulting in a relatively long initial step.32 NASBA has been used in a variety of noncommercial HIV-1 VL assays27,33,34 and in commercially available kits, such as the NASBA HIV-1 RNA QT amplification system35 or the NucliSens HIV-1 QT36 and its updated, more rapid, convenient, and reliable version, NucliSens EasyQ.37 However, the NASBA principle has not been used in detection of drug-resistant mutations.
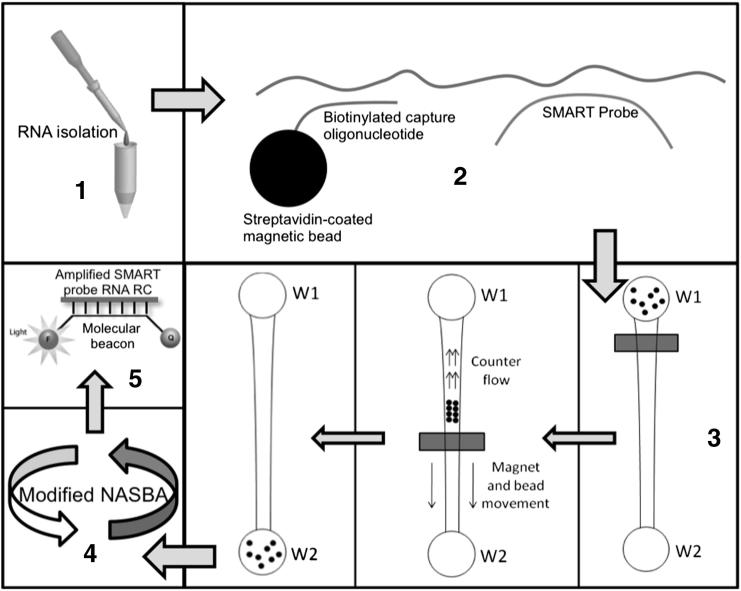
Herein, we re-introduce and implement a new approach38 for mutation detection, termed simple method for amplifying RNA targets (SMART), and use the common K103N as a model HIV-1 drug-resistant mutation. SMART is a significant deviation from currently used methods (namely, PCR and NASBA), specifically focused on cost-constrained and low laboratory infrastructure settings. Figure 1 provides an overview of the processes in SMART, which are further detailed in our previous article38 and in the supplemental materials. SMART incorporates the advantages of microfluidics and avoids the limitations of NASBA. This goal is achieved by incorporating a binding step and a microfluidic separation step, which allow the hybridization sites to be located anywhere along the RNA target. As the basis of the new approach, SMART amplification and detection do not depend on target RNA variability, but rather on the specific hybridization of a small single-stranded DNA probe termed the SMART probe.
Figure 1.
Overview of SMART. 1) RNA sample is isolated from a clinical sample and put into a TE buffer solution. 2) Streptavidin-coated beads conjugated with biotinylated capture oligonucleotides and SMART probes are added to the solution. 3) The solution is added to W1 of the SMART microchip, and a magnet is used to separate the bead-bound complex from the unbound structures. 4) SMART probes are amplified via a modified NASBA scheme. 5) Sample is quantified in real-time using molecular beacons.
This article describes the use of SMART in detecting the K103N drug-resistance mutation, both on and off chip, with synthetic HIV-1 model sequences, establishing the groundwork for drug-resistance monitoring and VL assays in clinical samples.
Materials and Methods
Engineered Oligonucleotide Sequences
To design a comprehensive set of sequences that would represent the high variability of the HIV-1 K103N region, which is present in multiple HIV-1 subtypes and recombinant forms,39 we used a curated aligned data set of 300 K103N and 6100 wild-type (WT) sequences, obtained from the nonsubtype B working group data set.40
The diverse regions adjacent to RT position 103 were examined in this multi–HIV-1 subtype data set, and the most conserved K103N mutated (5′-AGAACAAATCGGTAAC-3′) and WT (5′-AAAAAGAAAAAATCAGTAACA-3′) sequences were determined, covering approximately 75% of K103N and approximately 60% of WT sequences. One K103N sequence and one WT sequence were chosen, both containing these conserved sequences, respectively. A relevant portion of these individual sequences (approximately 30 nucleotides that include the respective conserved sequences) were used to engineer K103N and WT synthetic DNA (sDNA) fragments.
In subsequent experiments, additional sequences will be used to cover 100% of mutant and WT variants. The K103N sDNA used (Table 1) was in a Tris-EDTA buffer solution and contained two distinct sequences representing a conserved region (5′-ATGGGTGCGAGAGCGTCAATATTAAG-3′; capture oligo) of the HIV-1 gag gene, which is biologically upstream of the K103N region; and a K103N mutation region sequence (5′-CAGGGTTAAAGAAGAACAAATCGGTAACAG-3′; SMART probe). These two regions were separated by 10 adenine oligonucleotides to give ample space for hybridizations of the capture oligo and SMART probe. Table 1 similarly provides the WT sDNA sequence, SMART probes, primers, molecular beacon sequences, and nucleotide length of these model sequences. All of these custom oligonucleotides were purchased from Integrated DNA Technologies, Inc. (Coralville, IA). Table 1 also shows the K103N and WT RNA reverse complement (RC) sequences.
Table 1.
Oligonucleotides Used in the SMART Assay
| Oligo | Sequence | Length (nucleotides) |
|---|---|---|
| K103N sDNA | 5′-aaaaCAGGGTTAAAGAAGAACAAATCGGTAACAGaaaaaaaaaaATGGGTGCGAGAGCGTCAATATTAAGaaaa-3′ | 74 |
| WT sDNA | 5′-aaaaGGGTCTAAAAAAGAAAAAATCAGTAACaaaaaaaaaaaATGGGTGCGAGAGCGTCAATATTAAGaaaa-3′ | 72 |
| Biotinylated oligo | 5′-5BioTEG/CTTAATATTGACGCTCTCGCACCCAT-3′ | 26 |
| K103N SMART probe | 5′-TCAAGAGTAGACACCTGTTACCGATTTGTTCTTCTTTAACCCTGGTAATCAGATCAGAGCAATAGGTCA-3′ | 69 |
| WT SMART probe | 5′-ACTAGCCTTCACGGTGTTACTGATTTTTTCTTTTTTAACCCTGGTAATCAGATCAGAGCAATAGGTCA-3′ | 68 |
| Primer sequence 1 (K103N + WT) | 5′-taatacgactcactatagggTGACCTATTGCTCTGATCTGATTAC-3′ | 45 |
| Primer sequence 2 (K103N) | 5′-TCAAGAGTAGACACCTGTTACCGAT-3′ | 25 |
| Primer sequence 3 (WT) | 5′-ACTAGCCTTCACGGTGTTACTGATT-3′ | 25 |
| K103N RNA RC | 5′-TGACCTATTGCTCTGATCTGATTACCAGGGTTAAAGAAGAACAAATCGGTAACAGGTGTCTACTCTTGA-3′ | 69 |
| WT RNA RC | 5′-TGACCTATTGCTCTGATCTGATTACCAGGGTTAAAAAAGAAAAAATCAGTAACACCGTGAAGGCTAGT-3′ | 68 |
| Beacon 1 (K103N) | 5′-/6-FAM/cgcgTCAAGAGTAGACACCTGTTACCGATcgcg/3IABlk_FQ/-3′ | 33 |
| Beacon 2 (WT) | 5′-/Cy5/cgcgACTAGCCTTCACGGTGTTACTGATTcgcg/3IABlk_FQ/-3′ | 33 |
The single-stranded K103N sDNA sequence was designed with a mutated sequence (boldfaced) centered about the K103N codon (underlined), and the single-stranded WT sDNA sequence was designed with a nonmutated sequence (plain text) centered about the nonmutated lysine codon (underlined) (repetition of that previously described). Poly(A) sequences (lowercased) flanking the target regions extend the sDNA sequence to simulate the longer viral RNA sequence occurring in nature, and the poly(A) sequences in the middle simulate the sequences that are between the capture and target region in nature. K103N and WT SMART probes have hybridization regions (boldfaced) with their respective sDNA targets and a primer 1 region (underlined). Primer 1 was designed to hybridize with both probes (underlined) and contains a T7 promoter region (lowercased). Primer 2 (K103N) and primer 3 (WT) were designed to hybridize (boldfaced and underlined) with the K103N RNA RC and the WT RNA RC, respectively, and all potential SMART probe variations. Beacon 1 (K103N) and beacon 2 (WT) were also designed to competitively hybridize with the same regions (boldfaced and underlined) of the K103N RNA RC and the WT RNA RC, respectively. Again, this is to ensure that only one K103N beacon and one WT beacon are necessary for all variations of K103N and WT probes. The stem-loop region of each beacon is lowercased.
Theoretical Hybridizations
Theoretical hybridizations of sDNA to biotinylated oligos, sDNA to SMART probes, beacon dimers, and beacon to target RNA RC were calculated using the DINAMelt and mFold Web Server software version 3.1 (DINAMelt Server; RNA Institute, The State University of New York at Albany, Albany, NY; http://mfold.rna.albany.edu, last accessed July 1, 2011), for reaction conditions: temperature = 41°C, [Na+] = 70 mmol/L, and [Mg2+] = 12 mmol/L. Oligonucleotide hybridizations are characterized by two properties: the Gibbs free energy (ΔG), where a more negative ΔG value represents a more energetically favorable state (higher probability of hybridization), and the melting temperature of the hybrid at which 50% of the structures will be unhybridized. The probability of hybridization can be expressed as follows: P ∝ exp(− ΔG/RT) where R = a rate constant and T = hybridization temperature (constant). This equation shows that a more negative ΔG exponentially increases the probability of hybridization. Probes and targets were designed to effectively hybridize while avoiding non-specific hybridizations. The theoretical hybridization energy of probes and targets is shown in Supplemental Table S1.
Microchip Device
The microchip was constructed using a glass substrate and a polydimethylsiloxane layer containing microchannels and reservoirs. The microchip design (Supplemental Figure S1B) is a simple four-channel device (one channel per sample), in which each channel is tapered and designed for adequate separation of conjugated magnetic beads from unbound SMART probes using a magnetic bar. The microchip can process four samples simultaneously. The 2.8-μm diameter M-280 magnetic beads were obtained from Invitrogen (Carlsbad, CA).
NASBA Reaction Mix
The reagents used for NASBA and their concentrations at the final reaction volume were 40 mmol/L Tris (pH 8.0), 12.5 mmol/L MgCl2, 73.5 mmol/L CH3COOK, 5.25 mmol/L dithiothreitol, 1.05 mmol/L dNTP, 2.1 mmol/L rNTP, 0.21 μmol/L each primer, 52.5 nmol/L molecular beacon, 15.75% dimethyl sulfoxide, 1.68 μ/μL T7 RNA polymerase, 0.34 μ/μL AMV-RT, 0.005 μ/μL RNase-H, and 0.11 mg/mL bovine serum albumin. Enzymes and reagents used were purchased from Promega (Madison, WI).
Heating Devices
For on-chip amplification, an external RK-80H power supply (Matsusada Precision Inc., Kusatsu City, Shiga, Japan) provided 4 to 5 V to the Minco flexible transparent heater to achieve 41°C in well 2 of the microchip. We validated the heating temperature using a thermocouple attached to our microchip wells.
For off-chip real-time amplification and detection, we used a single cuvette peltier (Photon Technology International, Birmingham, NJ); and for off-chip amplification followed by off-chip end point gel electrophoresis, we used a C1000 thermal cycler (Bio-Rad, Hercules, CA).
Optical Equipment
For on-chip real-time detection, we used a Nikon Eclipse TE2000-U fluorescent microscope (Nikon Instruments, Inc., Melville, NY) with a blue filter set for 6-carboxyfluorescein (450- to 490-nm excitation, 515-nm long-pass emission), a red filter set for Cy5 (590- to 650-nm excitation, 663- to 738-nm band-pass emission), a 4× objective, a model 814 Photomultiplier Detection System with a gain of 700 V (Photon Technology International), and an integrated high-speed shutter (Melles Griot, Albuquerque, NM) set to open for 0.2 seconds. A custom in-house LabVIEW (National Instruments Corporation, Austin, TX) data acquisition program collected photomultiplier tube output at a rate of 12 to 15 Hz.
For off-chip real-time detection, samples were prepared in disposable Uvettes (Eppendorf AG, Hamburg, Germany), and real-time fluorescence was measured using a Quantamaster spectrofluorometer (Photon Technology International, Birmingham, NJ).
SMART Experiments
K103N and WT sDNA samples with a concentration ranging from 6 × 108 to 6000 copies/mL were used. This range of VLs is only two orders of magnitude higher than the range of detection of most commercially available HIV-1 VL assays (107 to approximately 50 copies/mL),10 thus most of these starting concentrations of sDNA are clinically relevant. Biotinylated oligos, sDNA sample, SMART probes, magnetic beads, and hybridization buffer were incubated for 30 minutes before loading onto the microchip. The polydimethylsiloxane chip was prewashed with nuclease decontamination solution (Integrated DNA Technologies) and presoaked with hybridization buffer (Tris, MgCl2, NaCl, Tween-20, and bovine serum albumin). Microfluidic separation occurred after the incubation as biotinylated oligos, sDNA sample, SMART probes, magnetic beads, and hybridization buffer were added to well 1 (W1; Figure 1) and moved through the channel by moving a neodymium magnet along the bottom of the channel at approximately 0.6 mm/second toward well 2 (W2; Figure 1). To ensure that beads would not clog channels, an optimized concentration of 0.05 μg/μL was used. On-chip or off-chip amplification was then performed for 180 minutes at 41°C, described later. Although on-chip detection can be performed by integrating a better detection system, we used off-chip detection because the fluorometer was already equipped for more accurate, higher-sensitivity fluorescence detection, allowing us to better gauge the sensitivity of our assay.
Although real-time amplification analysis was performed with molecular beacons, end point analysis of amplification products was performed with gel electrophoresis via a 2100 Bioanalyzer Small RNA Assay (Agilent Technologies, Marshfield, MA).
On-Chip Amplification
At the conclusion of the separation step, the magnetic bead complexes were <1 cm from W2 and the flow was manually stopped by the removal of solution in both W1 and W2 and the addition of 12 μL of paraffin oil to W1 to avoid evaporation of reagents. The NASBA reaction mix was then introduced for on-chip amplification (total volume, 5 μL) and the on-chip amplification apparatus was connected. On-chip amplification was used to preliminarily demonstrate the ability of the assay to be used as a point-of-care (POC) device.
Off-Chip Amplification
At the conclusion of the separation step, the magnetic bead complex was moved into W2. Then, the W2 contents were transferred into a polypropylene tube and W2 was rinsed with an additional 6 μL of hybridization buffer. The beads were then collected at the bottom of the tube, and all but 2 μL of solution was removed for off-chip NASBA amplification using a fluorometer. Fluorescence readings were taken every 5 minutes to yield real-time fluorescence data. Off-chip amplification used the same ratio of NABSA reaction mix as on-chip amplification, with a larger total solution volume of 80 μL.
Results
The SMART probe can be engineered specifically to have favorable or unfavorable binding energies (eg, ensuring it does not bind to other oligonucleotides in the mix). SMART requires only single-time diffusion and binding of small molecules (SMART probes) into the RNA structure. Hence, diffusion is fast and less dependent on the RNA structure itself than in traditional NASBA. The amplification step of SMART uses an abridged version of NASBA, starting with the short SMART probe DNA template rather than the actual long RNA template. This crucial design feature completely eliminates the noncyclic reverse transcription phase of NASBA and uses a small amplicon (100 nucleotides) compared with traditional NASBA or PCR for amplifying RNA, resulting in faster and more accurate amplification and detection. SMART can be performed on a closed microfluidic chip device that uses isothermal heating in low temperature (41°C) without thermal cycling or preheating, thus driving cost down. In this preliminary work, we used sDNA in place of clinical HIV RNA samples, to establish the feasibility of this method. Future work will integrate isolation of RNA from clinical samples from HIV-infected patients.
Validation of SMART Using On-Chip Amplification
SMART experiments were initially performed for K103N detection using capture, separation, and on-chip amplification for 180 minutes at 41°C, followed by off-chip end point gel electrophoresis. End point detection was used rather than real-time detection to validate the capture and separation steps, ensuring that the correct size oligonucleotide was amplified and visible in the gel plot. In addition, to evaluate the effect of the molecular beacons, amplification was performed with (B+) and without (B−) beacons, to investigate if there was any inhibitory effect on amplification.
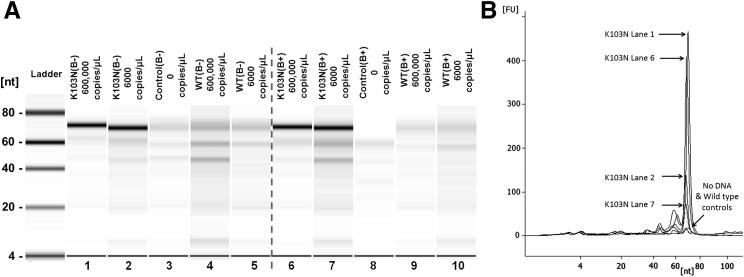
Figure 2A summarizes the outcomes of the experiment, confirming that capture, separation, and amplification reactions were occurring as expected. K103N probe RC was expected to be approximately 69 nucleotides. The dark bands in the K103N-positive lanes (1, 2, 6, and 7) correspond to this product. This K103N probe RC band also occurred in K103N-negative lanes (3, 4, 5, 8, 9, and 10), but at light intensity (low concentration), likely because of low concentrations of K103N probe carryover during separation, which was expected.38 This minimal probe carryover limits the sensitivity of our assay, and we are exploring ways to further reduce carryover for even greater sensitivity. Light bands also occurred at approximately 45 and approximately 22 nucleotides, likely attributed to the primers used. An unexpected band of light intensity (approximately 60 nucleotides) may have been generated as the polymerase prematurely aborted transcription, resulting in RNA RC products that are shorter than expected. This prematurely aborted transcription was also observed in previous work38 and does not have a negative impact on the assay sensitivity. Corresponding electropherographic data (Figure 2B) showed, as expected, that high starting concentrations of K103N sDNA yielded the highest fluorescence signal and that controls with no DNA and WT exhibited minimal fluorescence.
Figure 2.
A: SMART gel plot after on-chip amplification for 180 minutes, showing the detection of K103N sDNA at different concentrations (lanes 1, 2, 6, and 7), using a K103N probe in the absence (B−) or presence (B+) of beacons. No DNA (lanes 3 and 8) and low and high concentrations of WT single-stranded DNA (lanes 4, 5, 9, and 10) were also included as negative controls for detection with a K103N probe. B: SMART electropherogram of the gel plot shown in A, showing that the addition of molecular beacons has a significant inhibitory effect on probe amplification, especially for low starting concentrations of K103N sDNA.
Interestingly, the addition of molecular beacons had a significant effect on the amplification, particularly at the low concentration. In Figure 2B, the area under the curve for the starting concentration of 6 × 108 copies/mL declined approximately 10% (lane 1 to lane 6) with the addition of beacon while decreasing approximately 45% for 6 × 106 copies/mL (lane 2 to lane 7). This suggests that beacon is not only inhibitory to the amplification cycle, but also that inhibitory events are more pronounced at lower K103N probe concentrations. A lower starting concentration of K103N sDNA yields less hybridized K103N probe, as evident in the electropherogram when comparing 6 × 108 and 6 × 106 copies/mL, making the starting concentration of available K103N probe template for the NASBA cycle lower. Because the amplification cycle is exponential, the suggested inhibitory events are of a greater magnitude at lower K103N probe concentrations. This is directly supported by the data when comparing end point amplification.
Validation of SMART Using Off-Chip Amplification with Real-Time Detection
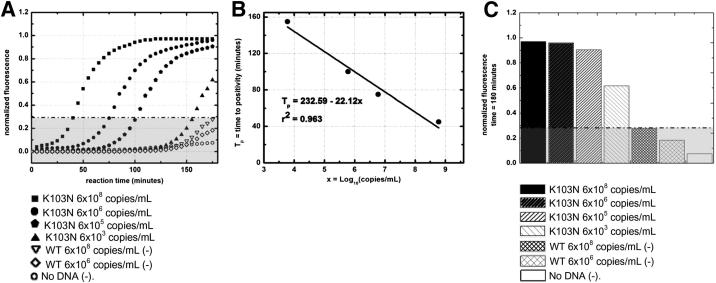
On verification of the amplified product using end point detection, by interpreting the gel plot (Figure 2A) and electropherogram (Figure 2B), SMART amplification was performed off chip for real-time K103N detection. Figure 3A shows the real-time results. First, the exponential curves plateau toward a maximum because the fluorescence represents the beacons hybridizing with the amplifying K103N RNA RC, in the presence of a limited amount (52.5 nmol/L) of molecular beacons in the reaction mix. For the high concentration (6 × 108 copies/mL), 90 minutes of amplification yielded distinguishable detection versus the controls, with the amplification curve crossing the threshold of detection [ie, time to positivity value (Tp) in 45 minutes]. For the lower concentrations (6 × 106 through 6000 copies/mL), this level of detection was achievable but required more time for amplification, consequently higher Tp or equivalent CT values. From these results, we plotted a linear model of Tp versus copies/mL (logarithmic scale), shown in Figure 3B. Figure 3C shows an end point analysis of our results, offering a clear illustration of all K103N concentrations tested to be well above the threshold of detection after 180 minutes of amplification.
Figure 3.
SMART assay results using off-chip amplification with real-time detection. A: WT sDNA and no DNA negative controls were included and plotted as background fluorescence (gray area). WT-specific SMART probes, WT primers, and WT molecular beacon were not included in this assay. The dashed black line indicates the threshold of detection or Tp for positive samples. SD was within 5% of results (error bars not shown). B: The Tp values are plotted versus the logarithmic scale of starting K103N sDNA concentration. SD was within 5% of results (error bars not shown). C: Histogram of normalized fluorescence of all sDNA and controls after 180 minutes’ reaction time. SD was within 5% of results (error bars not shown).
Determining K103N Concentration Using SMART Probes
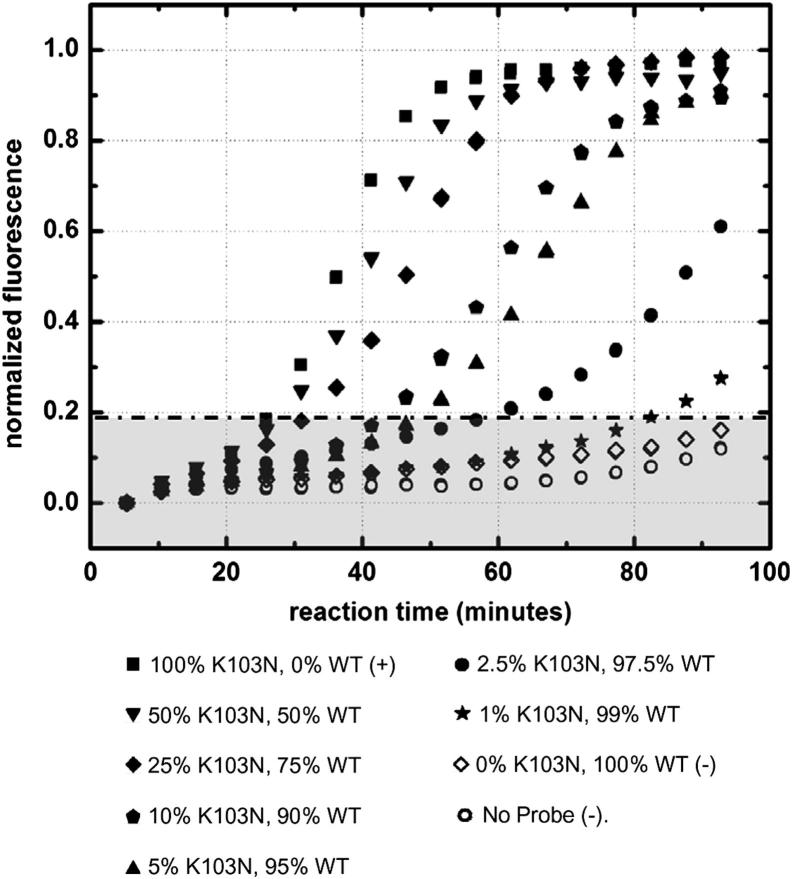
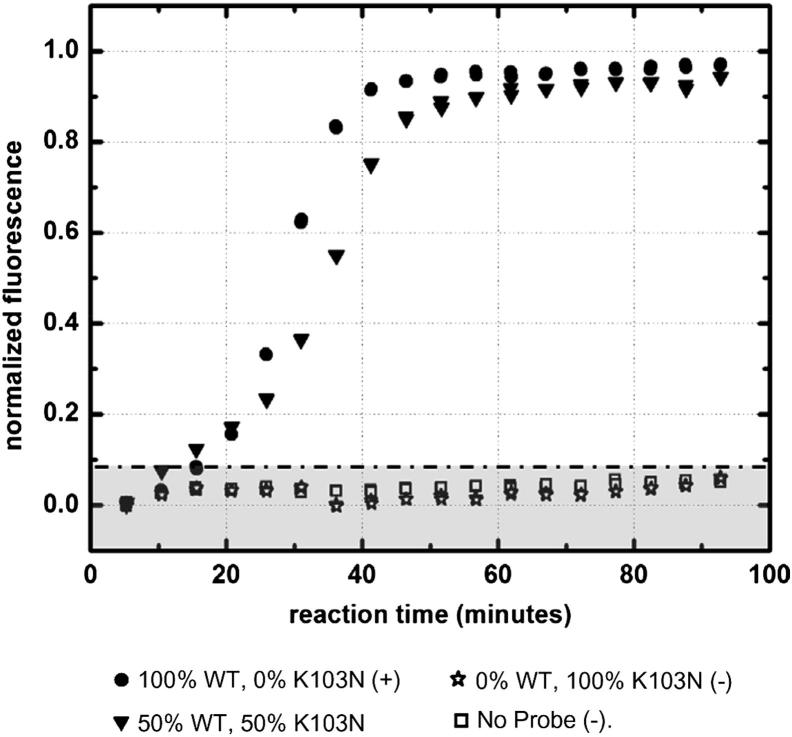
As a final implementation step, we evaluated SMART as an effective means for estimating K103N concentrations of unknown samples. The objective of this approach was to establish that we can simultaneously amplify and detect a multiple beacon system and that our SMART probes may be used in such a system as standards to determine K103N concentration of an unknown sample. We considered a group of samples with variable concentrations of WT and K103N SMART probes. We used a total of 1 pmol/L (6 × 108 copies/mL) of SMART probes (K103N + WT) to evaluate the efficacy of our assay to generate linear models based on K103N SMART probe concentrations. A variety of K103N SMART probe concentrations were used as our known standards and ranged from 100% (6 × 108 copies/mL) to 1% (6 × 106 copies/mL).
Figure 4 shows the off-chip real-time detection of the K103N probe within varying K103N-WT probe samples. There was no considerable change in baseline K103N beacon fluorescence for samples containing no DNA and for a sample containing only WT probe, confirming that the K103N beacon was stable and highly specific to its intended K103N RNA RC target. As the starting percentage of K103N probe increased, so did the rate of fluorescence. With a higher starting concentration of the K103N probe, the reaction produced more K103N RNA RC in a given amount of time. Therefore, with higher starting concentrations of K103N probes, there was faster hybridization of K103N beacons with the K103N RNA RC targets. This correlation can also be explained in its converse; as the starting concentration of K103N probes decreased, the resulting exponential fluorescence curve shifted with increasing time. These data correspond to the real-time SMART results shown in Figure 3A, in which a higher concentration of starting K103N sDNA yielded more K103N probe, which, in turn, resulted in faster amplification.
Figure 4.
Off-chip real-time fluorescence of amplified K103N probe as a function of time. Total concentration of SMART probes (K103N + WT) were 1 pmol/L (6 × 108 copies/mL). SD was within 5% of results (error bars not shown). Background fluorescence is shown as a shaded gray area.
Figure 5 similarly shows the off-chip real-time detection of the WT probe within varying K103N-WT probe samples. There was no change in baseline WT beacon fluorescence for samples containing no DNA and for a sample containing only K103N probe, confirming that the WT beacon was stable and highly specific to its intended WT target. For samples containing 50% to 100% WT probe, the WT beacon fluorescence increased exponentially, reaching its beacon-limited maximum after approximately 45 minutes. These data indicate that the WT beacon hybridized effectively with high concentrations of WT RNA RC target; however, the concentrations were too high to achieve any distinguishable resolution between the varying concentrations of probe in this time scale. This suggests that SMART, as it is designed, is not ideal for distinguishing concentrations >3 × 108 copies/mL. This gives us a ballpark number for the upper VL limit of our assay, which compares well with commercially available VL kits (approximately 107 copies/mL).
Figure 5.
Off-chip real-time fluorescence of amplified WT probe as a function of time. Total concentration of SMART probes (K103N + WT) was 1 pmol/L (6 × 108 copies/mL). SD was within 5% of results (error bars not shown). Background fluorescence is shown as a shaded gray area.
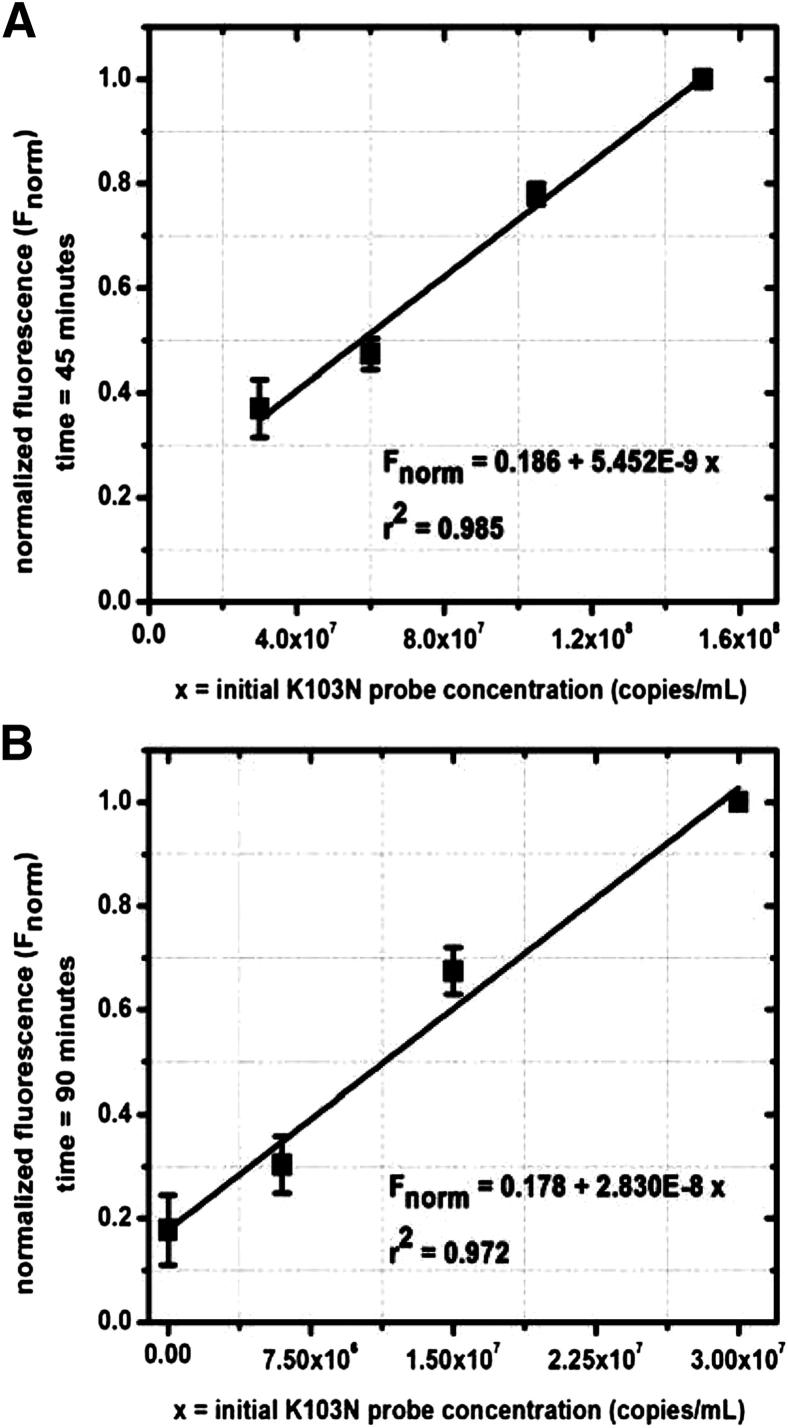
These off-chip data sets were then used to generate model fits capable of predicting starting K103N probe concentrations by standardizing, rearranging, and plotting the real-time fluorescence data. Figure 6A shows the model for high concentrations (3 × 107 to −1.5 × 108 copies/mL) of K103N SMART probes. For high starting concentrations, we were able to resolve fluorescence at an early time point during the amplification (45 minutes). These data fit a linear curve that can be used to interpolate for unknown high K103N SMART probe starting concentrations based on these measured fluorescence standard values. For low concentrations (<3 × 107 copies/μL) (Figure 6B), we are able to resolve the variations at a later time point during the amplification (90 minutes). These data fit a linear curve that can be used to interpolate for unknown low K103N SMART probe starting concentrations based on measured fluorescence values. These data also show that K103N beacons hybridized effectively with high and low concentrations of K103N RNA RC target.
Figure 6.
Off-chip modeling of fluorescence versus K103N SMART probe concentration at high concentrations (3 × 107 to 1.5 × 108 copies/mL K103N SMART probe) (A) and low concentrations (≤3 × 107 copies/mL K103N SMART probe) (B).
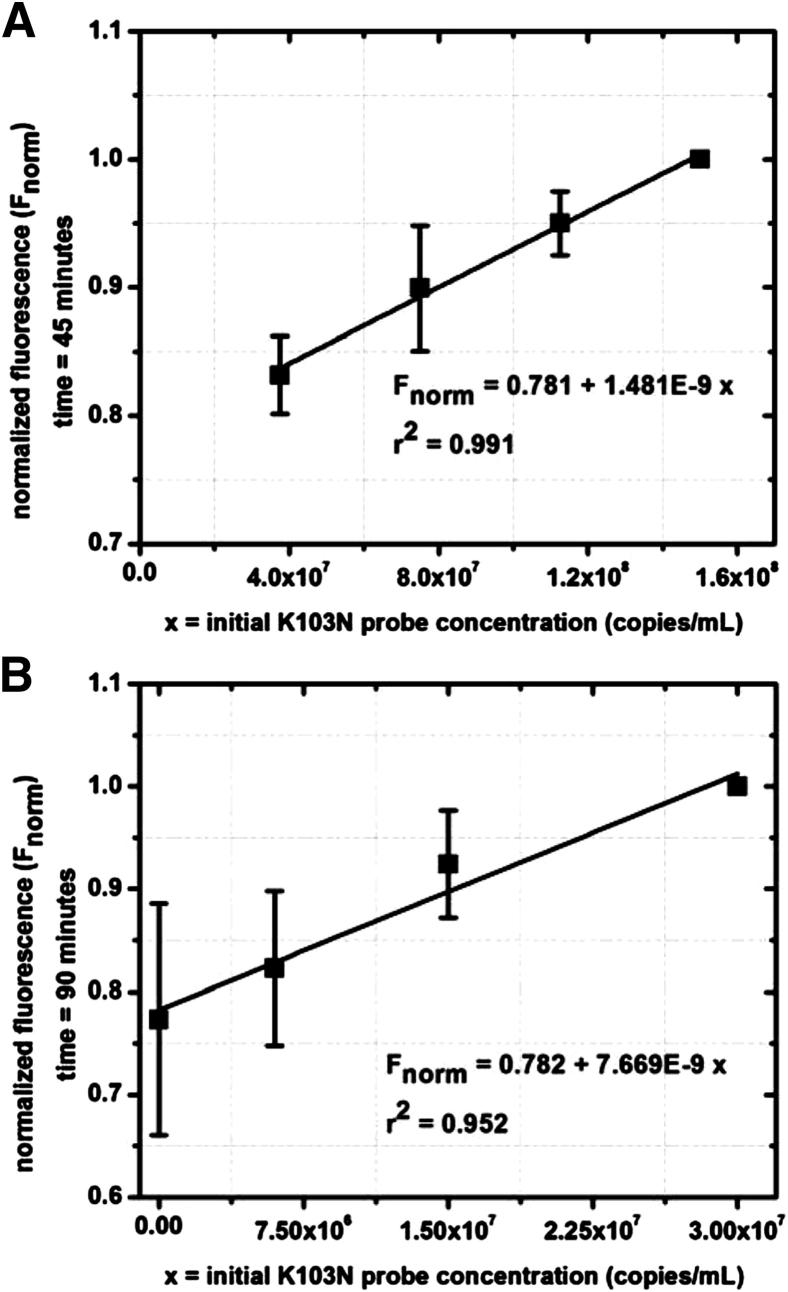
For the case of on-chip amplification, each polydimethylsiloxane chip contained four identical channels, such that three samples of known K103N concentration could be run in parallel with one unknown sample. However, to establish our theoretical model, we used all four channels with known sample quantities. Figure 7, A and B, shows similar plots for on-chip modeling.
Figure 7.
On-chip modeling of fluorescence versus K103N SMART probe concentration at high concentrations (3 × 107 to 1.5 × 108 copies/mL K103N SMART probe) (A) and low concentrations (≤3 × 107 copies/mL K103N SMART probe) (B).
Discussion
Herein, we present a novel method for RNA mutation detection, and apply it to HIV-1 drug-resistance determination. We captured, separated, amplified, and detected target mutated and WT sDNA sequences resembling an HIV-1 RT commonly mutated region, and engineered amplifiable SMART probes for optimal reaction kinetics rather than amplifying the target sDNA itself, using both off-chip and on-chip amplification and detection systems. We show that the initial 65°C step of NASBA can be removed because of our novel design, making the amplification fully isothermal, thus providing significant advantages in field-deployable and other POC settings. The hybridization of both a biotinylated oligonucleotide and an amplifiable SMART probe, instead of primers, maintained the specificity of the assay when compared with traditional methods of mutation detection. Also, the use of magnetic beads instead of a tube-based multiple wash kit allowed for simple and efficient separation of the target-bead complex from unbound SMART probe. A simple microfluidic device to facilitate separation of the amplifiable SMART probes was incorporated, avoiding the need for a flow apparatus or major heating component. This resulted in a significantly reduced assay cost and is a promising practical platform for POC use.
Existing methods for HIV resistance mutation detection are not intended for POC use. In addition, existing detection methods potentially suitable for POC use do not incorporate drug-resistance mutation determination. Sensitive commercial VL nonmicrofluidic assays are not specifically designed to detect drug-resistance sequences and appear to lack the practicality for POC settings. Microfluidic methods, which are only developed using immunobased chemistry, are promising VL methods for POC use, but fail to address drug-resistance monitoring.10 Many drug-resistance mutation detection assays either are commercially available (e.g., TRUGENE and ViroSeq) or used in the research setting (e.g., pyrosequencing, single-genome sequencing, allele-specific PCR, and ligation amplification), but they are not yet fully viable for POC and do not take advantage of microfluidics. SMART is a promising means of merging these methods for POC settings, using a simple microfluidic platform for detecting resistance-mutant sDNA with high specificity and distinguishing between mutant/WT sequences simultaneously. With further development, SMART could be used as a VL monitoring technique in addition to determining the concentration of resistance mutations such as K103N within a given sample.
In our experiments, we observed that the addition of molecular beacons had a significant effect on amplification, particularly at lower concentrations. This is likely attributed to the combination of two inhibitory events: the competition between primer 2 and beacon 1 during the amplification cycle and the premature abortion of T7 polymerase. McCalla et al38 reported that the premature abortion did not affect their detection methods because the beacon targeted the middle of the transcribed influenza RNA. However, herein, the beacon targeted the end of the transcribed RNA, which would result in decreased beacon-RNA hybrids for the prematurely aborted RNA products. Several smaller peaks in the electropherogram support this suggestion. Targeting the end of the RNA, rather than the middle, negatively affects the speed of the assay, but is a necessary assay design trade-off for simultaneous detection of many K103N SMART probes while only using two primers and one molecular beacon.
Results of the B+ versus B− experiments supported our hypothesis that a beacon-free system would amplify larger quantities of product. By our design, the beacons were in competition with Seq2 and Seq3 primers (Table 1) to hybridize with the RNA RC products during NASBA (Supplemental Figure S1C). When comparing the K103N samples containing 6 × 108 copies/mL, we saw approximately a 12% increase in fluorescence when the beacon was removed. For K103N samples containing 6 × 106 copies/mL, we saw approximately a 50% increase when the beacon was removed. This suggests that the beacon is inhibitory to the amplification cycle and supports the argument that inhibitory events are of a greater magnitude at lower K103N probe concentrations.
Nonetheless, the beacons are essential for detection in our assay and our POC design criteria; thus, the purpose of this comparison was to better understand the kinetics of the system, not to reach a decision point to eliminate or keep the molecular beacons. Rather than remove beacons from our one-step master mix, we seek to further optimize the NASBA buffer conditions for faster amplification and reduce carryover of unbound SMART probes for greater specificity. Although the sensitivity of 6000 copies/mL, shown herein, must be improved before clinical use, this work successfully establishes the feasibility of using a new technique (SMART) for detection of drug-resistance mutations, such as K103N.
The results of our real-time off-chip amplification experiments indicate that detection for lower target concentrations (6 × 106 through 6000 copies/mL) is achievable, but requires longer amplification times and, consequently, higher Tp or equivalent CT values. This strongly suggests that detection is possible for low, clinically relevant concentrations of K103N (≤1000 copies/mL) using SMART. A slightly longer amplification time is required (approximately 4 hours) to achieve comparable results in lower concentrations, although Tp is achievable within 180 minutes. Off-chip amplification achieved a much greater degree of sensitivity than could be achieved using on-chip optics, demonstrating the greatest sensitivity of our assay as it stands. From our linear model (Figure 3B) of Tp versus copies/mL (logarithmic scale), the current SMART assay is theoretically sensitive enough to detect as low as 1000 copies/mL of K103N sDNA.
By using the real-time fluorescence data from known standards (ie, samples with known K103N SMART probe concentrations), we were able to accurately predict the K103N probe concentration (copies/mL) of an unknown multiprobe sample. Off-chip amplification was used to establish this method of modeling, with a greater degree of sensitivity than could be achieved with on-chip amplification. However, the off-chip amplification was a less practical method because we could only amplify one sample at a time and required a cuvette. On-chip real-time detection of sDNA samples required a small (only 5 μL) amount of reaction volume, as opposed to conventional PCR volumes, which usually start at a minimum of 20 μL. The small reaction volume concentrated the target and probe molecules, which may have decreased the time to positive results. Linear models for on-chip amplification were produced, but with a larger SE, resulting in reduced specificity of the on-chip model.
Through this study, we were able to observe the limitations of SMART. First, the use of molecular beacons inhibited the amplification process, but the Tp values were similar to commercial and noncommercial PCR-based techniques. This is, therefore, only a minor issue and a worthwhile trade-off for the POC attributes that accompany the use of molecular beacons. Second, SMART assay design parameters may be adapted and further optimized for smaller Tp values that would reduce the reaction time beyond existing techniques. Third, SMART at its current stage lacks an integrated isolation step. This is an important aspect of its POC applicability and is in development. Fourth, the optics used for detection were laboratory grade, and a transition to field-deployable optics to achieve full POC and RLS status is still needed. Fifth, we only demonstrated SMART to be effective for targeting one K103N and one WT sequence. Future work will include assaying multiple sequences simultaneously to further prove the validity and applicability of our design. Last, the sensitivity of SMART is within the commercially relevant range (2000 to 10,000,000 copies/mL), but additional work is needed to improve the sensitivity to match the lower VL limit of commercial kits (approximately 50 copies/mL), and for sensitive detection of drug-resistance mutations. We anticipate achieving greater sensitivity by incorporating our own on-chip isolation technique, in which larger volumes of starting sample containing RNA will be processed and concentrated into a smaller volume. This would effectively increase the VL, a benefit that our synthetic samples did not have.
In summary, we introduce a novel HIV drug-resistance detection assay and show that it can theoretically detect, quantify, and estimate unknown HIV RT K103N concentrations. These methods and platform have great potential for their eventual application to clinical, global HIV care. In our study, we demonstrated that sDNA, based on actual K103N and WT HIV-1 viral RNA sequences, hybridized efficiently with SMART probes at clinically relevant concentrations. In addition, the sample-to-result time of our assay meets or improves on most commercially available assays.10 The flexibility of the SMART probe sequence allows this method to be easily adopted for other HIV drug-resistance mutations. Moving forward, we would like to achieve similar results with clinical HIV samples, incorporate the RNA isolation step, and use RLS capable optics, such as light-emitting diodes and suitable cameras. Our ultimate goal of providing costly, timely, and effective POC HIV quantification and drug-resistance detection methods in RLS, with no mandatory laboratory support, will allow clinicians to more adequately and effectively treat HIV-infected patients, whether before or after exposure to antiretroviral therapy. In our future work, we will apply these methods to clinical HIV-1 samples, other RNA targets, such as the influenza virus, and biothreats. Doing so will require extensive optimization of our assay so that it is tuned to overcome the technical challenges in sensitivity and specificity that clinical samples may present.
Acknowledgments
We thank The RNA Institute, College of Arts and Sciences, The State University of New York at Albany, for use of their DINAMelt web server; Stephanie McCalla, Aartik Sarma, and Carmichael Ong for their previous work inventing SMART and developing the technology; and Dr. Mia Coetzer for scientific contribution and technical assistance.
Footnotes
Supported by NIH grants 1R21 A1073808-01 A1, P30AI042853, and R01AI066922.
Supplemental Data
Overview of SMART: capture, separation, amplification, and detection. A: SMART capture step using sDNA. B: Microfluidic separation microchip design. A magnet is used to transfer the beads through a channel from W1 to W2. A transparent heater is attached for post-separation amplification. C: Modified isothermal (41°C) NASBA probe amplification. K103N/WT SMART probes are used as templates, undergoing cyclic isothermal amplification, to exponentially produce their RNA RC products for detection. The 3′ arm of the probe binds to the first primer, which contains the T7 promoter sequence. AMV-RT elongates primer 1 to generate double-stranded DNA capable of transcription by T7 RNA polymerase. The resulting RNA binds to primer 2, and AMV-RT generates a DNA-RNA hybrid. RNase H degrades the RNA, leaving a copy of the single-stranded DNA–amplifiable probe. This reaction is, thus, cyclic and exponential. D: Real-time fluorescence detection using molecular beacons. Beacons bind to their respective target on the RNA RC products. Excitation/emission profile for the beacon that binds to K103N RNA RC product is 491/515 nm, whereas that for the beacon that binds to WT RNA RC product is 643/667 nm.
Supplemental Data
Supplemental material for this article can be found at http://dx.doi.org/10.1016/j.jmoldx.2013.02.005.
References
- 1.Levy J.A., Autran B., Coutinho R.A., Phair J.P. 25 Years of AIDS: recording progress and future challenges. AIDS. 2012;26:1187–1189. doi: 10.1097/QAD.0b013e328354f602. [DOI] [PubMed] [Google Scholar]
- 2.Clavel F., Hance A.J. HIV drug resistance. N Engl J Med. 2004;350:1023–1035. doi: 10.1056/NEJMra025195. [DOI] [PubMed] [Google Scholar]
- 3.Johnson V.A., Calvez V., Günthard H.F., Paredes R., Pillay D., Shafer R., Wensing A.M., Richman D.D. 2011 Update of the drug resistance mutations in HIV-1. Top Antivir Med. 2011;19:156–164. [PMC free article] [PubMed] [Google Scholar]
- 4.Gilks C.F., Crowley S., Ekpini R., Gove S., Perriens J., Souteyrand Y., Sutherland D., Vitoria M., Guerma T., De Cock K. The WHO public-health approach to antiretroviral treatment against HIV in resource-limited settings. Lancet. 2006;368:505–510. doi: 10.1016/S0140-6736(06)69158-7. [DOI] [PubMed] [Google Scholar]
- 5.Gianella S., Delport W., Pacold M.E., Young J.A., Choi J.Y., Little S.J., Richman D.D., Pond S.L.K., Smith D.M. Detection of minority resistance during early HIV-1 infection: natural variation and spurious detection rather than transmission and evolution of multiple viral variants. J Virol. 2011;85:8359–8367. doi: 10.1128/JVI.02582-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gianella S., Richman D.D. Minority variants of drug-resistant HIV. J Infect Dis. 2010;202:657–666. doi: 10.1086/655397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fiscus S.A., Cheng B., Crowe S.M., Demeter L., Jennings C., Miller V., Respess R., Stevens W., Forum for Collaborative HIV Research Alternative Viral Load Assay Working Group HIV-1 viral load assays for resource-limited settings. PLoS Med. 2006;3:e417. doi: 10.1371/journal.pmed.0030417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chin C.D., Laksanasopin T., Cheung Y.K., Steinmiller D., Linder V., Parsa H., Wang J., Moore H., Rouse R., Umviligihozo G., Karita E., Mwambarangwe L., Braunstein S.L., van de Wijgert J., Sahabo R., Justman J.E., El-Sadr W., Sia S.K. Microfluidics-based diagnostics of infectious diseases in the developing world. Nat Med. 2011;17:1015–1019. doi: 10.1038/nm.2408. [DOI] [PubMed] [Google Scholar]
- 9.O’Connell R.J., Merritt T.M., Malia J.A., VanCott T.C., Dolan M.J., Zahwa H., Bradley W.P., Branson B.M., Michael N.L., De Witt C.C. Performance of the OraQuick rapid antibody test for diagnosis of human immunodeficiency virus type 1 infection in patients with various levels of exposure to highly active antiretroviral therapy. J Clin Microbiol. 2003;41:2153–2155. doi: 10.1128/JCM.41.5.2153-2155.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang S., Xu F., Demirci U. Advances in developing HIV-1 viral load assays for resource-limited settings. Biotechnol Adv. 2010;28:770–781. doi: 10.1016/j.biotechadv.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cabrera C., Marfil S., Poveda E., Puig T., Bonjoch A., Ziermann R., Soriano V., Clotet B., Ruiz L. Comparative evaluation of the TRUGENE HIV-1 envelope (gp41) genotyping assay on clinical samples. Antiviral Ther. 2005;10:S48. [Google Scholar]
- 12.Church J.D., Jones D., Flys T., Hoover D., Marlowe N., Chen S., Shi C.J., Eshleman J.R., Guay L.A., Jackson J.B., Kumwenda N., Taha T.E., Eshleman S.H. Sensitivity of the ViroSeq HIV-1 genotyping system for detection of the K103N resistance mutation in HIV-1 subtypes A, C, and D. J Mol Diagn. 2006;8:430–432. doi: 10.2353/jmoldx.2006.050148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grant R.M., Kuritzkes D.R., Johnson V.A., Mellors J.W., Sullivan J.L., Swanstrom R., D’Aquila R.T., Van Gorder M., Holodniy M., Lloyd R.M., Reid C., Morgan G.F., Winslow D.L. Accuracy of the TRUGENE HIV-1 genotyping kit. J Clin Microbiol. 2003;41:1586–1593. doi: 10.1128/JCM.41.4.1586-1593.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bile E.C., Adje-Toure C., Borget M.Y., Kalou M., Diomande F., Chorba T., Nkengasong J.N. Performance of drug-resistance genotypic assays among HIV-1 infected patients with predominantly CRF02_AG strains of HIV-1 in Abidjan, Cote d’Ivoire. J Clin Virol. 2005;32:60–66. doi: 10.1016/j.jcv.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 15.Halvas E.K., Aldrovandi G.M., Balfe P., Beck I.A., Boltz V.F., Coffin J.M., Frenkel L.M., Hazelwood J.D., Johnson V.A., Kearney M., Kovacs A., Kuritzkes D.R., Metzner K.J., Nissley D.V., Nowicki M., Palmer S., Ziermann R., Zhao R.Y., Jennings C.L., Bremer J., Brambilla D., Mellors J.W. Blinded, multicenter comparison of methods to detect a drug-resistant mutant of human immunodeficiency virus type 1 at low frequency. J Clin Microbiol. 2006;44:2612–2614. doi: 10.1128/JCM.00449-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J.Z., Paredes R., Ribaudo H.J., Svarovskaia E.S., Metzner K.J., Kozal M.J., Hullsiek K.H., Balduin M., Jakobsen M.R., Geretti A.M., Thiebaut R., Ostergaard L., Masquelier B., Johnson J.A., Miller M.D., Kuritzkes D.R. Low-frequency HIV-1 drug resistance mutations and risk of NNRTI-based antiretroviral treatment failure: a systematic review and pooled analysis. JAMA. 2011;305:1327–1335. doi: 10.1001/jama.2011.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li J.Z., Paredes R., Ribaudo H., Svarooskaia E.S., Kozal M.J., Hullsiek K.H., Miller M.D., Bangsberg D., Kuritzkes D.R. Relationship between NNRTI adherence and low-frequency resistance mutations with the risk of virological failure. Antiviral Ther. 2011;16:A16. [Google Scholar]
- 18.Wang C.L., Mitsuya Y., Gharizadeh B., Ronaghi M., Shafer R.W. Characterization of mutation spectra with ultra-deep pyrosequencing: application to HIV-1 drug resistance. Genome Res. 2007;17:1195–1201. doi: 10.1101/gr.6468307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ellis G.M., Mahalanabis M., Beck I.A., Pepper G., Wright A., Hamilton S., Holte S., Naugler W.E., Pawluk D.M., Li C.C., Frenkel L.M. Comparison of oligonucleotide ligation assay and consensus sequencing for detection of drug-resistant mutants of human immunodeficiency virus type 1 in peripheral blood mononuclear cells and plasma. J Clin Microbiol. 2004;42:3670–3674. doi: 10.1128/JCM.42.8.3670-3674.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gale H.B., Kan V.L., Shinol R.C. Performance of the TruGene human immunodeficiency virus type 1 genotyping kit and OpenGene DNA sequencing system on clinical samples diluted to approximately 100 copies per milliliter. Clin Vaccine Immunol. 2006;13:235–238. doi: 10.1128/CVI.13.2.235-238.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kapoor A., Jones M., Shafer R.W., Rhee S.Y., Kazanjian P., Delwart E.L. Sequencing-based detection of low-frequency human immunodeficiency virus type 1 drug-resistant mutants by an RNA/DNA heteroduplex generator-tracking assay. J Virol. 2004;78:7112–7123. doi: 10.1128/JVI.78.13.7112-7123.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palmer S., Kearney M., Maldarelli F., Halvas E.K., Bixby C.J., Bazmi H., Rock D., Falloon J., Davey R.T., Dewar R.L., Metcalf J.A., Hammer S., Mellors J.W., Coffin J.M. Multiple, linked human immunodeficiency virus type 1 drug resistance mutations in treatment-experienced patients are missed by standard genotype analysis. J Clin Microbiol. 2005;43:406–413. doi: 10.1128/JCM.43.1.406-413.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi C., Eshleman S.H., Jones D., Fukushima N., Hua L., Parker A.R., Yeo C.J., Hruban R.H., Goggins M.G., Eshleman J.R. LigAmp: sensitive detection of single nucleotide differences. Nat Methods. 2004;1:141–147. doi: 10.1038/nmeth713. [DOI] [PubMed] [Google Scholar]
- 24.Wang B., Dwyer D.E., Chew C.B., Kol C., He Z.P., Joshi H., Steain M.C., Cunningham A.L., Saksena N.K. Sensitive detection of the K103N non-nucleoside reverse transcriptase inhibitor resistance mutation in treatment-naive HIV-1 infected individuals by rolling circle amplification. J Virol Methods. 2009;161:128–135. doi: 10.1016/j.jviromet.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi N., Tamura K., Aotsuka T. PCR error and molecular population genetics. Biochem Genet. 1999;37:317–321. doi: 10.1023/a:1018759210666. [DOI] [PubMed] [Google Scholar]
- 26.Dyer J.R., Gilliam B.L., Eron J.J., Grosso L., Cohen M.S., Fiscus S.A. Quantitation of human immunodeficiency virus type 1 RNA in cell free seminal plasma: comparison of NASBA(TM) with Amplicor(TM) reverse transcription-PCR amplification and correlation with quantitative culture. J Virol Methods. 1996;60:161–170. doi: 10.1016/0166-0934(96)02063-0. [DOI] [PubMed] [Google Scholar]
- 27.Vandamme A.M., Van Dooren S., Kok W., Goubau P., Fransen K., Kievits T., Schmit J.C., De Clercq E., Desmyter J. Detection of HIV-1 RNA in plasma and serum samples using the NASBA amplification system compared to RNA-PCR. J Virol Methods. 1995;52:121–132. doi: 10.1016/0166-0934(94)00151-6. [DOI] [PubMed] [Google Scholar]
- 28.McCalla S.E., Tripathi A. Microfluidic reactors for diagnostics applications. Ann Rev Biomed Eng. 2011;13:321–343. doi: 10.1146/annurev-bioeng-070909-105312. [DOI] [PubMed] [Google Scholar]
- 29.Luebke K.J., Balog R.P., Garner H.R. Prioritized selection of oligodeoxyribonucleotide probes for efficient hybridization to RNA transcripts. Nucleic Acids Res. 2003;31:750–758. doi: 10.1093/nar/gkg133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guatelli J.C., Whitfield K.M., Kwoh D.Y., Barringer K.J., Richman D.D., Gingeras T.R. Isothermal, in vitro amplification of nucleic acids by a multienzyme reaction modeled after retroviral replication. Proc Natl Acad Sci U S A. 1990;87:1874–1878. doi: 10.1073/pnas.87.5.1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deiman B., van Aarle P., Sillekens P. Characteristics and applications of nucleic acid sequence-based amplification (NASBA) Mol Biotechnol. 2002;20:163–179. doi: 10.1385/MB:20:2:163. [DOI] [PubMed] [Google Scholar]
- 32.Compton J. Nucleic-acid sequence-based amplification. Nature. 1991;350:91–92. doi: 10.1038/350091a0. [DOI] [PubMed] [Google Scholar]
- 33.Gupta P., Mellors J., Kingsley L., Riddler S., Singh M.K., Schreiber S., Cronin M., Rinaldo C.R. High viral load in semen of human immunodeficiency virus type 1-infected men at all stages of disease and its reduction by therapy with protease and nonnucleoside reverse transcriptase inhibitors. J Virol. 1997;71:6271–6275. doi: 10.1128/jvi.71.8.6271-6275.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Gemen B., van Beuningen R., Nabbe A., van Strijp D., Jurriaans S., Lens P., Kievits T. A one-tube quantitative HIV-1 RNA NASBA nucleic-acid amplification assay using electrochemiluminescent (ECL) labelled probes. J Virol Methods. 1994;49:157–167. doi: 10.1016/0166-0934(94)90040-x. [DOI] [PubMed] [Google Scholar]
- 35.Vandamme A.M., Schmit J.C., Van Dooren S., Van Laethem K., Gobbers E., Kok W., Goubau P., Witvrouw M., Peetermans W., DeClercq E., Desmyter J. Quantification of HIV-1 RNA in plasma: comparable results with the NASBA HIV-1 RNA QT and the AMPLICOR HIV monitor test. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;13:127–139. doi: 10.1097/00042560-199610010-00003. [DOI] [PubMed] [Google Scholar]
- 36.Segondy M., Ly T.D., Lapeyre M., Montes B. Evaluation of the nuclisens HIV-1 QT assay for quantitation of human immunodeficiency virus type 1 RNA levels in plasma. J Clin Microbiol. 1998;36:3372–3374. doi: 10.1128/jcm.36.11.3372-3374.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yao J., Liu Z., Ko L.S., Pan G., Jiang Y. Quantitative detection of HIV-1 RNA using NucliSens EasyQ HIV-1 assay. J Virol Methods. 2005;129:40–46. doi: 10.1016/j.jviromet.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 38.McCalla S.E., Ong C., Sarma A., Opal S.M., Artenstein A.W., Tripathi A. A simple method for amplifying RNA targets (SMART) J Mol Diagn. 2012;14:328–335. doi: 10.1016/j.jmoldx.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kantor R. Impact of HIV-1 pol diversity on drug resistance and its clinical implications. Curr Opin Infect Dis. 2006;19:594–606. doi: 10.1097/QCO.0b013e3280109122. [DOI] [PubMed] [Google Scholar]
- 40.Kantor R., Katzenstein D.A., Efron B., Carvalho A.P., Wynhoven B., Cane P., Clarke J., Sirivichayakul S., Soares M.A., Snoeck J., Pillay C., Rudich H., Rodrigues R., Holguin A., Ariyoshi K., Bouzas M.B., Cahn P., Sugiura W., Soriano V., Brigido L.F., Grossman Z., Morris L., Vandamme A.M., Tanuri A., Phanuphak P., Weber J.N., Pillay D., Harrigan P.R., Camacho R., Schapiro J.M., Shafer R.W. Impact of HIV-1 subtype and antiretroviral therapy on protease and reverse transcriptase genotype: results of a global collaboration. PLoS Med. 2005;2:e112. doi: 10.1371/journal.pmed.0020112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Overview of SMART: capture, separation, amplification, and detection. A: SMART capture step using sDNA. B: Microfluidic separation microchip design. A magnet is used to transfer the beads through a channel from W1 to W2. A transparent heater is attached for post-separation amplification. C: Modified isothermal (41°C) NASBA probe amplification. K103N/WT SMART probes are used as templates, undergoing cyclic isothermal amplification, to exponentially produce their RNA RC products for detection. The 3′ arm of the probe binds to the first primer, which contains the T7 promoter sequence. AMV-RT elongates primer 1 to generate double-stranded DNA capable of transcription by T7 RNA polymerase. The resulting RNA binds to primer 2, and AMV-RT generates a DNA-RNA hybrid. RNase H degrades the RNA, leaving a copy of the single-stranded DNA–amplifiable probe. This reaction is, thus, cyclic and exponential. D: Real-time fluorescence detection using molecular beacons. Beacons bind to their respective target on the RNA RC products. Excitation/emission profile for the beacon that binds to K103N RNA RC product is 491/515 nm, whereas that for the beacon that binds to WT RNA RC product is 643/667 nm.