Abstract
The Guthrie 903 card archived dried blood spots (DBSs) are a unique but terminal resource amenable for individual and population-wide genomic profiling. The limited amounts of DBS-derived genomic DNA (gDNA) can be whole genome amplified, producing sufficient gDNA for genomic applications, albeit with variable success; optimizing the isolation of high-quality DNA from these finite, low-yield specimens is essential. Agarose gel electrophoresis and spectrophotometry are established postextraction quality control (QC) methods but lack the power to disclose detailed structural, qualitative, or quantitative aspects that underlie gDNA failure in downstream applications. Visual automated fluorescence electrophoresis (VAFE) is a novel QC technology that affords precise quality, quantity, and molecular weight of double-stranded DNA from a single microliter of sample. We extracted DNA from 3-mm DBSs archived in the Swedish Neonatal Repository for >30 years and performed the first quantitative and qualitative analyses of DBS-derived DNA on VAFE, before and after whole genome amplified, in parallel with traditional QC methods. The VAFE QC data were correlated with subsequent sample performance in PCR, sequencing, and high-density comparative genome hybridization array. We observed improved standardization of nucleic acid quantity, quality and integrity, and high performance in the downstream genomic technologies. Addition of VAFE measures in QC increases confidence in the validity of genetic data and allows cost-effective downstream analysis of gDNA for investigational and diagnostic applications.
Sudden unexpected death in epilepsy (SUDEP) is the most common cause of mortality in people with seizure disorders, and the mechanisms of SUDEP are incompletely understood. Candidate molecular risk factors include defects in ion channel genes linked to long QT syndrome,1 epilepsy,2 and genes of the serotonergic pathways involved in arousal and respiration.3 Precision molecular autopsy4,5 and diagnostics,6,7 as well as personalized or familial risk prediction8–11 based on candidate gene profiling, are critically dependent on the availability of representative high-quality tissue samples.12 However, procurement of postmortem biological specimens often poses logistical, ethical, or cultural obstacles.13–15
The Guthrie 903 card archived dried blood spots (DBSs) are an established enduring medium frequently collected by neonatal registries and forensic laboratories.16,17 They are a unique resource amenable for individual and population-wide genomic profiling,18–21 considering the easy and cost-effective specimen collection, simple and stable long-term storage, and straightforward processing in nucleic acid extraction.15,22 In cases of sudden death among otherwise healthy individuals, the DBSs collected at birth may become the sole high-quality genomic sample to allow molecular autopsy, personalized diagnostics, and familial risk prediction.13,23 Because the DBSs represent a nonrenewable reserve, they require judicious allocation to projects of considerable public health impact with well-informed methods that maximize the source utility.17,18,22
Currently, the actual DBS-derived DNA performance often fluctuates disproportionately to the qualitative and quantitative values predicted by the established methods of gel electrophoresis and spectrophotometry, respectively.15,22,24 Moreover, the visual estimate of DNA integrity derived from agarose gel is imprecise and consumes much of the precious original specimen. A new visual automated fluorescence electrophoresis (VAFE) technology used by the Agilent 2200 TapeStation (Agilent Technologies, Inc., Santa Clara, CA) enables precise and visible postextraction quality control (QC) of nucleic acids, including high-molecular-weight genomic DNA (gDNA). It allows qualitative and quantitative standardization of nucleic acids before their terminal commitment to downstream diagnostic or profiling technologies while requiring a negligible amount of DNA for accurate molecular characterization of the template. This translates into cost-effective use of the analytical platforms and increased confidence in the validity of the final genomic data.
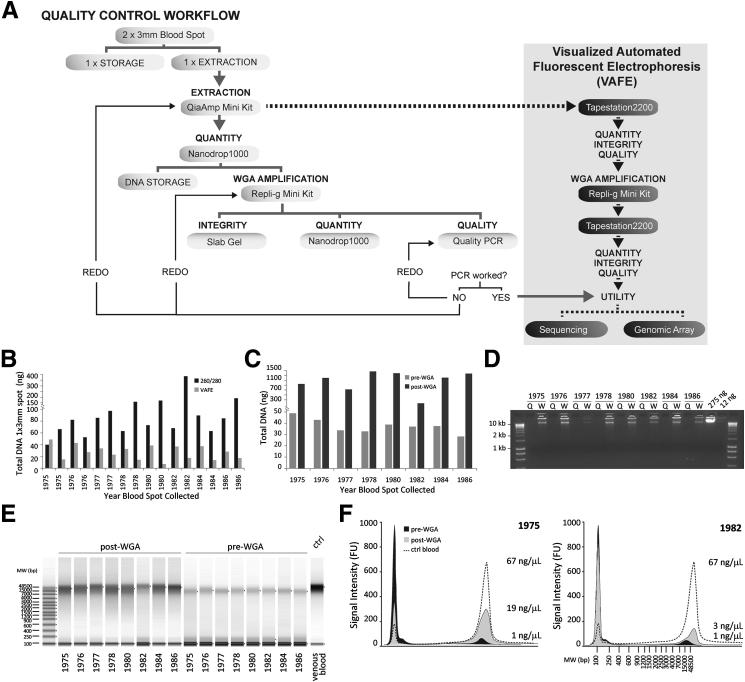
We successfully extracted serial gDNA samples from 16 individual 3-mm DBSs collected on Guthrie 903 cards and deposited at room temperature in the Swedish Repository for ≥30 years. We performed the first systematic combined quantitative and qualitative analysis of aged archived DBS-derived DNA with VAFE before and after whole genome amplification (WGA)25,26 and in parallel to our extant, traditional methods–based QC paradigm (Figure 1A).15 Finally, we correlated the novel postextraction DNA QC with sample performance in PCR, sequencing, and a high-density comparative genome hybridization array.
Figure 1.
Extraction of gDNA and WGA from decades-old 3-mm blood spots assessed for quality, quantity, and molecular-weight spectra. A: The flowchart defines our current QC paradigm (gray) to assess sample quality, quantity, and integrity of blood spot–derived gDNA samples compared with VAFE (black). The dotted black line shows the ability of VAFE to assess all QC parameters simultaneously even after the initial extraction, enabling efficient sample characterization for entry into downstream genomic applications. B: Quantification of total gDNA extracted from a single 3-mm blood spot using the QiaAmp Mini Kit measured using 260/280 ratio of a Nanodrop (black bar) and the VAFE on the TapeStation (gray bar). The 260/280 ratio overestimates dsDNA, whereas VAFE uses a fluorescent Sybr dye to calculate concentration, which is only capable of intercalating in dsDNA, resulting in a more accurate quantification. C: VAFE quantification of gDNA extracted from a single 3-mm blood spot before (gray bar) and after (dark bar) WGA. The WGA yields approximately 1 μg of dsDNA from a starting template of approximately 10 ng. D: Slab gel analysis of gDNA integrity reveals that pre-WGA DNA is not detectable on the slab gel, whereas all post-WGA samples are of high molecular weight comparable to reference DNA samples (275 and 12.5 ng) extracted from venous blood. The extracted gDNA spot (Q) is run in parallel with the post-WGA amplification (W) of the same template. E: Visualization of pre- and post-WGA blood spot samples using VAFE shows high-molecular-weight DNA is extracted from the decades-old blood spot. This high-molecular-weight DNA (>48,500 bp) is enriched in the post-WGA sample. F: Fluorescent electropherograms of the DNA extracted from a blood spot collected in 1975 and 1982. The pre-WGA DNA (black) contains high-molecular-weight (7000 to 48,500 bp) DNA. In both blood spots the post-WGA (gray) sample shows an enrichment of heavy fragments, but the relative amount of small to large fragments differs greatly between the samples, suggesting that samples must be evaluated for quality on an individual basis.
Materials and Methods
DBS Samples
The Swedish Repository of DBS samples collected on Guthrie 903 cards for neonatal screening of inborn diseases provided 2 × 3-mm blood spot punches in deidentified sterile microfuge tubes. There were total of 16 samples representing two decades: the 1970s (1975, 1976, 1977, and 1978) and the 1980s (1980, 1982, 1984, and 1986).
gDNA Extraction from 3-mm Blood Spot
A single 3-mm DBS from each sample was transferred to a fresh 1.5-mL microfuge tube. Using the QiaAmp Mini Spin Kit (Qiagen, Hilden, Germany), gDNA was extracted according to the manufacturer's protocol for DBSs. In brief, the DBS was incubated for 10 minutes at 85°C in buffer ATL and then proteinase K solution was added. The sample was vortexed and incubated for 1 hour at 56°C to facilitate enzymatic digestion of the sample. Buffer AL was then added, the sample was incubated at 70°C for 10 minutes, absolute ethanol was added, and the sample was vortexed thoroughly to mix. The supernatant solution was applied to the QIAamp mini spin column and was bound to the column via a 1-minute centrifugation at 6000 × g. Filtrate was discarded, and the sample was washed on the column by the application of buffer AW1, followed by a 1-minute 6000 × g spin where the filtrate was discarded. Buffer AW2 was then applied, the sample was spun at 20,000 × g for 3 minutes, the filtrate was discarded, and the spin column was centrifuged again for 1 minute at 20,000 × g to remove all trace ethanol. The DNA-containing column was then moved to a clean 1.5-mL microcentrifuge tube, and 50 μL of DNAase/RNAase free water (Qiagen) prewarmed to 55°C was applied to the column. The columns were incubated with water for 2 minutes and collected by centrifugation. The 50-μL elutant was immediately reapplied to the column and recollected by centrifugation to maximally recover and concentrate the gDNA in a small end volume.
Whole Genome Amplification
In the Repli-g Ultrafast WGA mini kit (Qiagen) a total input of 10 ng of DNA from each DBS was used per reaction according to the manufacturer's protocol. Denaturation buffer D1 was added to template gDNA, vortexed to mix, and centrifuged to collect. The samples were incubated at room temperature (approximately 22°C) for 3 minutes. The reaction was then neutralized by the addition of buffer N1. A master mix containing reaction buffer and DNA polymerase was applied to the gDNA and incubated for 1.5 hours at 30°C, followed by a heat inactivation step of 3 minutes at 65°C. Samples were then moved into quality analysis.
In the Genomeplex WGA Kit (Sigma-Aldrich, St. Louis, MO) template DNA was fragmented by applying the 10× fragmentation buffer followed by an incubation at 94°C for 4 minutes. The library preparation buffer and library stabilization solution were added to the fragmented gDNA template, vortexed to mix, and centrifuged to collect, followed by 2-minute incubation at 95°C. The sample was cooled on ice and the library preparation enzyme was added. The reactions were placed in a PCR thermocycler and subjected to a cycle of 20 minutes at the following temperatures in succession: 16°C, 24°C, and 37°C, followed by 5-minute 72°C final elongation step. Amplification was performed using the WGA DNA polymerase and amplification master mix provided. The reactions were cycled 14 times in a PCR thermocycler after denaturation at 95°C for 3 minutes, denaturation at 94°C for 15 seconds, and a 65°C 5-minute anneal and extension step. Samples were moved into quality analysis.
Quantification and Integrity Analysis of Genomic Samples
Sample quantification was performed in parallel using the traditional QC algorithm versus a fluorescence-based VAFE method.
The Traditional QC Algorithm
Two microliters of pre- and post-WGA DNA from each sample was quantified using a 260/280 absorbance ratio on a Nanodrop1000 (ThermoFisher, Waltham, MA). After quantification, 2 μL of DNA was stored in a 0.2-mL sterile PCR tube for visualization on a slab gel. To assess gDNA integrity before and after WGA, 1% agarose/ethidium bromide gel using Tris-acetate-EDTA buffer was prepared. Samples reserved from quantification were prepared by the addition of 10 μL of sterile water and 3 μL of loading dye. Samples were loaded pairwise onto the gel with two known concentrations (275 and 12 ng) of reference gDNA extracted from venous blood. A total of 5 μL of Hyperladder I (Bioline, Taunton, MA) was used to identify samples of high (>10 kb) molecular weight.
The VAFE QC Algorithm
A fresh 1-μL aliquot of the sample was added to 10 μL of sample buffer, quantified, visualized, and qualitatively assessed on the Agilent 2200 TapeStation. The DNA molecular weight spectra were reviewed, and all data were saved and stored for future reference and comparative analysis.
PCR Analysis
Pre and post-WGA gDNA from DBSs of all ages was assessed by quality PCR amplification. Standard 50-μL PCRs using Apex Taq Polymerase (Genesee Scientific, San Diego, CA) were performed with oligonucleotide primers targeting known polymorphisms that contained exons from ion channelopathy genes. Amplicons ranging from 300 to 1000 bp in size were generated using 1 μL of input DNA (concentration range, 0.6 to 12.5 ng/μL) using a PCR program with 10-minute initial denaturation at 94°C, followed by 40 cycles of 94°C for 30 seconds, 56°C for 1 minute, 72°C for 1 minute, and a final elongation step of 10 minutes at 72°C. A positive control reaction using 12.5 ng/μL of reference DNA extracted from venous blood was included for comparison. Reactions were size resolved on a 2% agarose/ethidium bromide Tris-acetate-EDTA gel using a 10-kb ladder for size comparison.
Sanger Sequencing
The individual PCRs that yielded a single 1000-bp amplicon from the RYR2 gene were purified using the QiaQuick PCR Purification Spin Kit (Qiagen). Templates were quantified according to the specifications of the vendor on the Nanodrop1000, and approximately 10 to 15 ng of template was submitted for commercial Sanger sequencing (GeneWiz, South Plainfield, NJ). The resulting traces were visually validated, and chromatograms were aligned pairwise with pre- and post-WGA DBS data and compared across all samples.
Comparative Genome Hybridization Copy Number Variant Analysis
We assessed the utility of post-WGA DBS samples for comparative genomic hybridization (CGH) arrays for detection of genomic copy number variation (CNV) using our custom-designed, high-resolution epilepsy candidate gene array (Agilent 019033). The array has even coverage along the whole genome backbone and additional high-density exomic probe coverage (approximately 3 to 5 probes per exon) across roughly 250 ion channel genes, along with additional monogenic epilepsy genes with variable density coverage of intronic probes. All CGH experiments were performed using the manufacturer's recommended protocol and the Agilent Genome Enzymatic Label Kit. Briefly, 1.125 μg of each post-WGA sample and 1.125 μg of control female reference gDNA were digested with RsaI and AluI restriction enzymes for 2 hours at 37°C, followed by heat inactivation at 65°C for 20 minutes. The complete digestion was confirmed visually by running 2 μL of each sample on a 1% agarose/ethidium bromide Tris-acetate-EDTA gel. The remaining digested DNA was subsequently purified and labeled with either Cy-3-dUTP (reference DNA) or Cy-5-dUTP (post-WGA DNA). DNA was hybridized for 40 hours at 65°C under constant rotation in a light impermeable hybridization oven (Agilent Technologies). Arrays were then washed and stabilized before image acquisition on the SureScan D Scanner (Agilent Technologies). The resulting image was imported into Agilent Feature Extraction Software version 10.7.3.1 (Agilent Technologies) and analyzed for fluorescent signal log ratio generating a feature extraction file for each individual genome profile. The feature extraction files were analyzed in Genome Workbench (Agilent Technologies) using the built-in ADM-2 detection settings and the default aberration filter version 2. Data for this experiment and corresponding platform files can be viewed at http://www.ncbi.nlm.nih.gov/geo (accession number GSE44249).
Results
VAFE Provides Superior Accuracy in the Measurement of DBS-Derived DNA Concentration
The total DNA extracted from each DBS was quantified in parallel using a spectrophotometer (ThermoFisher Nanodrop1000) and a fluorescence-based system (Agilent 2200 TapeStation) (Figure 1A). Both methods detected measurable amounts of nucleic acids in all samples, and there was no definite correlation between the sample age and the DNA concentration, which is in agreement with data reported previously.17 However, a statistically significant difference was found in the total DNA per blood spot as measured by individual platforms (40 to 384 ng per the Nanodrop1000 versus 12 to 49 ng per the Agilent 2200 TapeStation; t = 3.68, P = 0.002) (Figure 1B). We confirmed measurement accuracy by using high-molecular-weight gDNA standards of known concentrations on both systems. We found that both performed well when quantifying high-quality double-stranded DNA (dsDNA). The sample detection limit was >1 ng/μL by the Nanodrop1000 and >0.5 ng/μL by VAFE (Supplemental Figure S1A). Using high-molecular-weight quantitative PCR standards, we found that the minimal concentration necessary for fluorescence-based reliable visual profiling of gDNA molecular-weight spectra was ≥2 ng/μL (Supplemental Figure S1B). These results support our earlier observation15 that the quantification based on absorbance ratio tends to overestimate DNA concentration in suboptimal or degraded samples. The ratiometric measure provides an estimate of sample purity, but it is blind to the nucleic acid integrity, length, or proportional representation of single- versus double-stranded helices, both of which can act as a template for WGA amplification. In contrast, the VAFE sample buffer contains a SYBR green-based fluorophore, which intercalates with dsDNA molecules,27 giving an accurate measure of dsDNA below the concentration of <100 ng/μL.
VAFE Measures of Quantity and Quality Outperform Traditional Platforms and Facilitate Systematic WGA Amplification of gDNA from Archived Neonatal DBS Samples
A single 3-mm DBS averaged 26.3 ± 13.1 ng of total gDNA, an amount insufficient for many commercial or diagnostic high-throughput whole genome or whole exome sequencing or structural variant profiling assays, which typically require >1 μg of high-molecular-weight DNA to interrogate the entire genome. To overcome this limitation, we performed WGA on the archived DBSs using 10 ng of extracted blood spot DNA as a template. The resulting amplified DNA samples were VAFE quantified, and all demonstrated an increase in total DNA amount (Figure 1C). Most samples (7 of 8) showed a 20 to 50 times increase, resulting in approximately 1 μg of total DNA content. A single sample dated 1982 relatively underperformed, and the final total amount was 154 ng of gDNA. Although sufficient sample quantity is important, sample utility critically depends on the proportional representation and availability of DNA fragments of usable length. To assess the integrity and molecular weight of the samples, pairs of the pre- and post-WGA samples were run on a traditional agarose slab gel in parallel with a 10-kb ladder and reference DNA extracted from venous blood (Figure 1D). As expected, the DBS-extracted original gDNA was not visible on the slab gel because of the low concentration (approximately 1 to 3 ng/μL) of DNA in these samples. However, the post-WGA samples were clearly observed and ran at the same apparent molecular weight as the reference venous blood–derived DNA. Notably, none of WGA blood spots showed any discernible signs of degradation smear at the lower size range.
The identical sample set was run on VAFE, which uses microelectrophoresis of the DNA sample through a precast gel tape using a high-molecular-weight ladder for size comparison. We then compared the results between the two systems. Unlike the slab gel, VAFE clearly shows a high-molecular-weight band >15 kb in size in all of the pre-WGA DNA extractions from decades-old blood spots. Analysis of the post-WGA sample confirmed that not only did the amplification produce substantially more dsDNA, but it also occurred preferentially in the high-molecular-weight range, and the post-WGA samples uniformly resembled the venous blood–derived gDNA (Figure 1E). Individual samples were then analyzed with regard to the size profile of the DNA fragments (Figure 1F). Unlike the agarose slab gel, the VAFE clearly discriminated small but individually variable and age-independent degradation in all samples, as evidenced by the presence of lower-molecular-weight DNA fragments. The relative abundance of the high- versus lower-molecular-weight fragments was also variable between samples, suggesting that the pre- and post-amplification sample quality is better evaluated on an individual basis. In summary, the integrated quantity and quality measures from VAFE analysis define the integrity of archived DBS-derived gDNA, providing a QC parameter that is predictive for yielding high-molecular-weight gDNA after WGA.
VAFE-Established Sample Quality Confirms Utility in PCR, Sanger Sequencing, and CGH
Polymerase Chain Reaction
Our current QC paradigm for gDNA integrity involves an experimental assessment of DNA utility because samples may be variably visible on a traditional slab gel and the established 260/280 absorbance ratio–based quantification method may overestimate the content and integrity of the specimen.15 In contrast, VAFE can assess quality, quantity, and integrity of a gDNA sample simultaneously using a minimal volume of a discrete sample. This is extremely important when samples are limited and is critical in retrospective archival postmortem molecular diagnostic autopsy or research on genomic causality of sudden unexpected death. We successfully PCR amplified all pre- and post-WGA samples except for two samples that failed to amplify two different amplicons. A 1975 DBS sample failed to amplify the 300-bp amplicon of the 32nd exon of the RYR2 gene encoding the cardiac ryanodine receptor (Supplemental Figure S2). However, we saw the 1000-bp amplicon for exon 37 in the same gene in this sample, suggesting that the WGA DNA from 1975 was of sufficient quality and quantity to act as a template across the gene region. Similarly, the WGA sample from the 1986 blood spot failed to amplify the 1000-bp exon in HTR2A while producing a faint 1000-bp amplicon for RYR2 exon 37 and a robust 300-bp amplicon for exon 32 in RYR2 (Supplemental Figure S2). Our quality PCR standard operating procedure requires that any failed amplicons be repeated with the failed DNA sample to differentiate failure due to intrinsic (sample quality) versus extrinsic (technical flaw) factors (Figure 1A). All initially failed amplicons amplified on successive quality PCR assays, thus eliminating concerns regarding sample quality. It was reassuring to know that the inherently low concentration of the freshly extracted gDNA is sufficient to produce robust PCR amplification. This ensures that genetic variation identified in the WGA samples can be validated in the original blood spot gDNA to remove any potential false-positive variants that result from the WGA.
Sanger Sequencing
The 1000-bp fragment from RYR2 exon 37 was the template for commercial Sanger sequencing reactions (Supplemental Figure S3). All pre- and post-WGA blood spot samples were successfully sequenced with clear bp calls from the chromatogram. We consistently observed higher background signal in pre-WGA samples in the <250-bp range; however, this background noise was insufficient to prevent the identification of heterozygous half height chromatogram peak reductions. In summary, our work supports the literature10–12,28 in that gDNA from decades-old infantile blood spots can be used successfully in traditional medical genetic resequencing studies to detect single-nucleotide polymorphism (SNP) variants that possibly contribute to the risk of epilepsy and SUDEP.
Comparative Genomic Hybridization
The enzymatic digestion of the post-WGA DNA was successful, showing fragment size and distribution similar to the digested reference DNA (Supplemental Figure S4A), with the notable absence of high-molecular-weight undigested DNA. After purification and labeling, all of the post-WGA samples met QC requirements for the microarray, and hybridization was performed. The results were visualized (Supplemental Figure S4B), and all 8 individual arrays underwent successful feature extraction. The QC of the array data revealed that all arrays had a moderate background signal and a signal to noise ratio requiring visual assessment of probe distribution. Seven of the eight arrays passed QC. The 1978 post-WGA sample had an unacceptably high number of nonuniform outlier probes. Using the ADM-2 default algorithm in Genomic Workbench (Agilent), CNVs were detected in all samples. Because we performed our comparative hybridization blind to sex using a female reference DNA, we were able to use the CNV algorithm to identify male samples by comparing the copy number of the X chromosome. Female samples with XX genotype showed discreet small CNVs across the chromosome, whereas male XY samples showed a relative loss of X chromosome consistent with their XY genotype (Supplemental Figure S4C). These results support the literature in that post-WGA amplified gDNA from DBS sources is amenable to high-throughput array-based technologies.19,20,24,28 Unequal whole genome amplification needs to be considered, especially in degraded samples.25 However, previous studies comparing pre- and post-WGA gDNA samples from neonatal blood spots similar to ours reported >99% SNP concordance between amplified and native samples.24
Failed VAFE QC Informs Suboptimal gDNA Extractions from DBS
The previously extracted suboptimal samples that underperformed in our traditional QC paradigm were simultaneously analyzed with VAFE to compare and define the informative value of each platform and the reasons for sample failure. The 260/280 absorbance–based measurement suggested 6.8 to 11.8 ng/μL of gDNA across the four samples. However, the low concentration of the initial template was below the limit of visualization on the traditional agarose gel, indicating a lower gDNA concentration than indicated by the absorbance-based quantification. We performed WGA using two separate systems using different amplification strategies. The Repli-g Ultrafast WGA kit uses a multidisplacement amplification method, resulting in long DNA fragments (>10 kb), whereas the Genomeplex WGA kit uses the omniplex method producing short (500 bp) DNA fragments. The WGA kit using the Repli-g system augmented the total gDNA amount (Supplemental Figure S5A), and subsequent slab gel visualization indicated the existence of the high-molecular-weight gDNA in the presence of some degradation as evidence by smear (Supplemental Figure S5B). The Genomeplex WGA system only produced a moderate increase in DNA content (71.6 ± 29.3 ng total, n = 4) compared with the Repli-g system (1024 ± 239 ng total, n = 4) when using the same input template. Following our traditional QC protocol, the quality PCR was performed targeting amplicons ranging in size from 250 to 1000 bp in parallel on pre- and post-WGA samples. However, it failed across all samples except for the 250-bp fragment of the RYR2 gene generated on the pre-WGA blood spot extracted DNA (Supplemental Figure S5C). Examination of the suboptimal samples pre- and post-WGA by VAFE revealed lesser than traditionally measured quantity of the starting template. Importantly, the electropherograms produced by the fluorescent signal clearly showed the substantial degradation of the initial gDNA template (Supplemental Figure S5, D and E). Both WGA systems tested increased the total gDNA amount after amplification. As predicted by the kit method, the Genomeplex WGA samples were composed of <500-bp DNA fragments. In contrast, most DNA molecules produced using the Repli-g system remained of low molecular weight, ranging in size from 400 to 4000 bp, indicating a lesser quality, fragmented input template from suboptimal DBS gDNA extractions (Supplemental Figure S5E). These smaller post-WGA fragments subsequently failed in downstream applications as demonstrated by the failed quality PCR. This outcome is also in agreement with our earlier observations.16 Analysis of the suboptimal samples showed that VAFE clarifies important parameters related to sample quality, quantity, and integrity to be considered before proceeding with further costly and time-consuming downstream genomic investigation.
Discussion
The DBSs collected within the framework of neonatal screening program are an attractive alternative source of gDNA because of their low cost and ease of collection and long-term storage.17,18,22,29 The DBSs preserved and protected in the neonatal biobanks represent a multigenerational archive of populations and offer an unparalleled opportunity to assess both personal and population-based profiles of genetic variation.20,21,30 Moreover, they may be the only resource for a personal genomic profile in cases of sudden unexpected death.4,5,12,13,15,23,31 However, DBSs are a finite reserve. They need to be judiciously allocated to projects and used with utmost efficiency to maximize and extend the possibilities for use of individual samples.8,10,29
The VAFE is an efficient QC technology that is ideally suited for projects that work with samples of minimal quantity or uncertain quality. Our data indicate that VAFE delivers reliable quantification of precious, much diluted gDNA samples extracted from decades-old DBS samples. An additional unique attribute of this novel system is the capacity to provide complementary information simultaneously through the visualization of sample quality, integrity, and molecular-weight spectrum from a single microliter of gDNA. This platform demonstrated that gDNA extracted from decades-old infantile blood spots is composed of small fragmented DNA (<200 bp) and high-molecular-weight (>15 kb) molecules (Figure 1F), and if it is sufficient in quality and integrity, it is amenable to WGA amplification, resulting in large amounts (>1 μg) of predominantly high-molecular-weight DNA.19 The VAFE-based QC can identify suboptimal samples early in sample processing, and our follow-up experiments confirmed and correlated the failed QC with their limited utility in downstream applications, including the impervious PCR (Supplemental Figure S4C). We also observed that the quality or utility of the DNA did vary across individual blood spots,30,32,33 indicating the need for systematic integration of reliable, efficient, and highly informative QC into sample preparation and assessment to maximize the quality and reliability of final genomic data and use of resources.34,35
We have previously demonstrated a working effective QC paradigm that uses commonly available, albeit indirect and somewhat imprecise, methods for characterization of the gDNA properties extracted from alternative tissues, including DBSs.15 However, visual automated fluorescent electrophoresis represents a new-generation QC that delivers direct visualization and accuracy and that considers the imperative for minimal sample consumption. Results of this study will be applicable for projects that work with finite and limited DNA samples expected to deliver diagnostically accurate genomic data.
Acknowledgment
This work was profoundly motivated by families that lost children to SUDEP.
Footnotes
Supported by National Institute of Neurological Disorders and Stroke grants NS067013 and NS067013S (A.M.G.), NS049130, and NS076916 (J.L.N.); Citizens United for Research in Epilepsy; Fiorito Foundation and the Emma Bursick Memorial Fund (A.M.G.); the Blue Bird Circle Foundation (J.L.N.); and the Stockholm County Council, Regional Agreement on Medical Training and Clinical Research (T.T. and O.S.)
Disclosures: Agilent Technologies loaned the Agilent 2200 TapeStation and gave access to the prototype Agilent Genomic DNA ScreenTape.
Contributor Information
Tara L. Klassen, Email: klassen@bcm.edu.
Alicia M. Goldman, Email: agoldman@bcm.edu.
Supplemental Data
Comparison of VAFE and 260/280 methods used to quantifiy known concentrations of gDNA. A: Known concentrations of high-molecular-weight gDNA (Roche, Indianapolis, IN) used for quantitative PCR standard curve generation were analyzed using a NanoDrop1000 and the Agilent 2200 TapeStation. Measurement of gDNA was limited to >1 ng/μL of gDNA using the 260/280 absorbance spectrum compared with 0.5 ng/μL using VAFE. B: VAFE electropherograms of the gDNA concentration standard curve showing the relative increase in fluorescence with increasing gDNA content in the sample.
Quality PCR of pre- and post-WGA gDNA extracted from a 3-mm blood spot. Quality PCR amplification of three ion channel exons known to contain SNPs. The extracted gDNA spot (Q) is run in parallel with the post-WGA amplification (W) of the same template. All samples except the 1975 and 1986 WGA produced the expected amplicon comparable to reference DNA from venous blood. The amplicons that failed (arrow) were observed in a subsequent repeated PCR, suggesting the gDNA is of sufficiently high quality for subsequent experiments.
Sanger sequencing of PCR amplicons produced during quality PCR of pre- and post-WGA gDNA extracted from decades-old blood spots. Aligned chromatogram traces for a 50-bp region corresponding to bases 300 to 350 in exon 37 of the RYR2 gene. All pre- and post-WGA gDNA samples from blood spots produced sequences of sufficient quality for SNP detection.
Utility of post-WGA gDNA extracted from a 3-mm blood spot in CGH to detect CNVs. A: Slab gel analysis of restriction digests of post-WGA gDNA compared with digests of control DNA extracted from venous blood. An undigested high-molecular-weight DNA is shown for comparison. Most post-WGA samples, regardless of age or input DNA concentration, appear to have an enrichment of specific genome content such that clear bands are visible within the digested DNA, a phenomena clearly observed in the 1984 sample and more faintly in others. In fact, only the 1977 and 1986 post-WGA samples appear uniform, with a range of fragment sizes comparable to control DNA. B: Posthybridization immunocytochemistry arrays showing the individual blood spot post-WGA DNA fluorescence compared with the control reference DNA. All arrays had moderate levels of background fluorescence, with three arrays having derivative of log ratios (DRL) <0.3. Only the 1978 sample failed QC because of a large number of nonuniform outliers. C: Aberration detection using the default ADM-2 detection algorithm in Genomic Workbench identified CNVs in all post-WGA blood spot samples. The CGH against a female reference genome revealed a deletion of the X chromosome in four samples reflecting the XY genotype (1984). Comparatively, the XX genotype (1977) had only small gain and loss aberrations detected. Any CNVs detected in candidate genes will be screened using quantitative PCR in post-WGA samples to eliminate false-positive results before final validation in the initial extraction of QiaAmp blood spot DNA.
Poor quality pre- and post-WGA gDNA extracted from 3-mm blood spots assessed by traditional QC paradigm compared with VAFE. A: Quantification of total gDNA extracted from a single 3-mm blood spot using the QiaAmp Mini Kit (gray bar) followed by WGA (black bar) measured using a NanoDrop1000 and the Agilent 2200 TapeStation. The 260/280 ratiometric quantification overestimates the concentration of gDNA, whereas VAFE reveals the minimal amount of dsDNA extracted in these suboptimal preparations. The WGA on the poor quality template yielded approximately 500 to 3700 ng of gDNA from a starting template of <10 ng. B: Slab gel analysis of gDNA integrity reveals that pre-WGA DNA is not detectable on the slab gel, whereas post-WGA samples are composed of both high-molecular-weight DNA and fragmented or degraded DNA across the entire size range. C: Quality PCR amplification of ion channel exons known to contain SNPs. The extracted spot gDNA (Q) is run in parallel with the post-WGA amplification (W) of the same template. All samples failed to produce PCR amplicons >450 bp in size regardless of gDNA concentration. The black arrow on the right of the gel indicates the expected amplicon size. Only the small 250-bp amplicon of exon 32 of RYR2 amplified in the suboptimal samples, and that was only produced in the reactions using the initial blood spot DNA as template. These results coupled with the low amount of gDNA initially extracted and the fragmented nature of the WGA samples demonstrate the efficacy of our QC paradigm in assessing sample quality for downstream utility in genomic applications. D: VAFE of pre- and post-WGA blood spot samples shows the small amount of gDNA initially extracted and the poor quality of the post-WGA sample when using either the Repli-g or the Genomeplex WGA system. The Repli-g post-WGA samples lack a clear high-molecular-weight DNA (>48,500 bp) band and are highly degraded through the 400- to 4000-bp range. The Genomeplex post-WGA samples had only moderate amounts of total DNA, with most being <500 bp in size. A high-quality gDNA extracted from venous blood is shown for comparison. E: VAFE electropherograms of the DNA extracted from the four suboptimal sample extractions. The pre-WGA DNA (black) has substantial amounts of low molecular weight (100 to 250 bp) with limited amounts of gDNA through the larger size range even when scaled to the signal for better resolution. The Repli-g post-WGA (gray) sample shows an enrichment of fragments across the sample range with limited enrichment at the higher molecular weight. Similarly, the Genomeplex post-WGA samples (red) showed an increase in total DNA relative to the input template where the post-WGA DNA fragments are <500 bp in size. The ability of VAFE to provide quality, quantity, and integrity information on 1 μL of gDNA sample enables QC assessment at an earlier point, after initial extraction, compared with our current QC paradigm, which enabled the efficient triage of samples for downstream applications without the need for additional experimentation.
References
- 1.Goldman A., Glasscock E., Yoo J., Chen T., Klassen T., Noebels J. Arrhythmia in heart and brain: KCNQ1 mutations link epilepsy and sudden unexplained death. Sci Transl Med. 2009;1:2ra6. doi: 10.1126/scitranslmed.3000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glasscock E., Yoo J.W., Chen T.T., Klassen T.L., Noebels J.L. Kv1.1 potassium channel deficiency reveals brain-driven cardiac dysfunction as a candidate mechanism for sudden unexplained death in epilepsy. J Neurosci. 2010;30:5167–5175. doi: 10.1523/JNEUROSCI.5591-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buchanan G.F., Richerson G.B. Central serotonin neurons are required for arousal to CO2. Proc Natl Acad Sci U S A. 2010;107:16354–16359. doi: 10.1073/pnas.1004587107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skinner J.R., Crawford J., Smith W., Aitken A., Heaven D., Evans C.A., Hayes I., Neas K.R., Stables S., Koelmeyer T., Denmark L., Vuletic J., Maxwell F., White K., Yang T., Roden D.M., Leren T.P., Shelling A., Love D.R. Prospective, population-based long QT molecular autopsy study of postmortem negative sudden death in 1 to 40 year olds. Heart Rhythm. 2011;8:412–419. doi: 10.1016/j.hrthm.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 5.Johnson J.N., Tester D.J., Bass N.E., Ackerman M.J. Cardiac channel molecular autopsy for sudden unexpected death in epilepsy. J Child Neurol. 2010;25:916–921. doi: 10.1177/0883073809343722. [DOI] [PubMed] [Google Scholar]
- 6.Kapplinger J., Tester D., Salisbury B., Carr J., Harris-Kerr C., Pollevick G., Wilde A., Ackerman M. Spectrum and prevalence of mutations from the first 2,500 consecutive unrelated patients referred for the FAMILION long QT syndrome genetic test. Heart Rhythm. 2009;6:1297–1303. doi: 10.1016/j.hrthm.2009.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tester D.J., Will M.L., Haglund C.M., Ackerman M.J. Compendium of cardiac channel mutations in 541 consecutive unrelated patients referred for long QT syndrome genetic testing. Heart Rhythm. 2005;2:507–517. doi: 10.1016/j.hrthm.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 8.Klassen T., Davis C., Goldman A., Burgess D., Chen T., Wheeler D., McPherson J., Bourquin T., Lewis L., Villasana D., Morgan M., Muzny D., Gibbs R., Noebels J. Exome sequencing of ion channel genes reveals complex profiles confounding personal risk assessment in epilepsy. Cell. 2011;145:1036–1048. doi: 10.1016/j.cell.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller T.E., Estrella E., Myerburg R.J., Garcia de Viera J., Moreno N., Rusconi P., Ahearn M.E., Baumbach L., Kurlansky P., Wolff G., Bishopric N.H. Recurrent third-trimester fetal loss and maternal mosaicism for long-QT syndrome. Circulation. 2004;109:3029–3034. doi: 10.1161/01.CIR.0000130666.81539.9E. [DOI] [PubMed] [Google Scholar]
- 10.Pirmohamed M. Acceptance of biomarker-based tests for application in clinical practice: criteria and obstacles. Clin Pharmacol Ther. 2010;88:862–866. doi: 10.1038/clpt.2010.245. [DOI] [PubMed] [Google Scholar]
- 11.Verbeek N.E., van Kempen M., Gunning W.B., Renier W.O., Westland B., Lindhout D., Brilstra E.H. Adults with a history of possible Dravet syndrome: an illustration of the importance of analysis of the SCN1A gene. Epilepsia. 2011;52:e23–25. doi: 10.1111/j.1528-1167.2011.02982.x. [DOI] [PubMed] [Google Scholar]
- 12.Carturan E., Tester D.J., Brost B.C., Basso C., Thiene G., Ackerman M.J. Postmortem genetic testing for conventional autopsy-negative sudden unexplained death: an evaluation of different DNA extraction protocols and the feasibility of mutational analysis from archival paraffin-embedded heart tissue. Am J Clin Pathol. 2008;129:391–397. doi: 10.1309/VLA7TT9EQ05FFVN4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gladding P.A., Evans C.A., Crawford J., Chung S.K., Vaughan A., Webster D., Neas K., Love D.R., Rees M.I., Shelling A.N., Skinner J.R. Posthumous diagnosis of long QT syndrome from neonatal screening cards. Heart Rhythm. 2010;7:481–486. doi: 10.1016/j.hrthm.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 14.Ravid R. Standard Operating Procedures, ethical and legal regulations in BTB (Brain/Tissue/Bio) banking: what is still missing? Cell Tissue Banking. 2008;9:121–137. doi: 10.1007/s10561-007-9055-y. [DOI] [PubMed] [Google Scholar]
- 15.Klassen T.L., von Ruden E.L., Drabek J., Noebels J.L., Goldman A.M. Comparative analytical utility of DNA derived from alternative human specimens for molecular autopsy and diagnostics. J Mol Diagn. 2012;14:451–457. doi: 10.1016/j.jmoldx.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Norgaard-Pedersen B., Simonsen H. Biological specimen banks in neonatal screening. Acta Paediatr Suppl. 1999;88:106–109. doi: 10.1111/j.1651-2227.1999.tb01172.x. [DOI] [PubMed] [Google Scholar]
- 17.Hannelius U.L.C., Melén E., Malmberg A., von Dobeln U., Kere J. Phenylketonuria screening registry as a resource for population genetic studies. J Med Genet. 2005;42:e60. doi: 10.1136/jmg.2005.032987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hansen T.V., Simonsen M.K., Nielsen F.C., Hundrup Y.A. Collection of blood, saliva, and buccal cell samples in a pilot study on the Danish nurse cohort: comparison of the response rate and quality of genomic DNA. Cancer Epidemiol Biomarkers Prev. 2007;16:2072–2076. doi: 10.1158/1055-9965.EPI-07-0611. [DOI] [PubMed] [Google Scholar]
- 19.Hollegaard M.V., Thorsen P., Norgaard-Pedersen B., Hougaard D.M. Genotyping whole-genome-amplified DNA from 3- to 25-year-old neonatal dried blood spot samples with reference to fresh genomic DNA. Electrophoresis. 2009;30:2532–2535. doi: 10.1002/elps.200800655. [DOI] [PubMed] [Google Scholar]
- 20.Hollegaard M.V., Grove J., Grauholm J., Kreiner-Moller E., Bonnelykke K., Norgaard M., Benfield T.L., Norgaard-Pedersen B., Mortensen P.B., Mors O., Sorensen H.T., Harboe Z.B., Borglum A.D., Demontis D., Orntoft T.F., Bisgaard H., Hougaard D.M. Robustness of genome-wide scanning using archived dried blood spot samples as a DNA source. BMC Genet. 2011;12:58. doi: 10.1186/1471-2156-12-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hollegaard M.V., Grove J., Thorsen P., Norgaard-Pedersen B., Hougaard D.M. High-throughput genotyping on archived dried blood spot samples. Genet Test Mol Biomarkers. 2009;13:173–179. doi: 10.1089/gtmb.2008.0073. [DOI] [PubMed] [Google Scholar]
- 22.Sjoholm M.I., Dillner J., Carlson J. Assessing quality and functionality of DNA from fresh and archival dried blood spots and recommendations for quality control guidelines. Clin Chem. 2007;53:1401–1407. doi: 10.1373/clinchem.2007.087510. [DOI] [PubMed] [Google Scholar]
- 23.Winkel B.G., Larsen M.K., Berge K.E., Leren T.P., Nissen P.H., Olesen M.S., Hollegaard M.V., Jespersen T., Yuan L., Nielsen N., Haunso S., Svendsen J.H., Wang Y., Kristensen I.B., Jensen H.K., Tfelt-Hansen J., Banner J. The prevalence of mutations in KCNQ1, KCNH2, and SCN5A in an unselected national cohort of young sudden unexplained death cases. J Cardiovasc Electrophysiol. 2012;23:1092–1098. doi: 10.1111/j.1540-8167.2012.02371.x. [DOI] [PubMed] [Google Scholar]
- 24.Hardin J., Finnell R.H., Wong D., Hogan M.E., Horovitz J., Shu J., Shaw G.M. Whole genome microarray analysis, from neonatal blood cards. BMC Genet. 2009;10:38. doi: 10.1186/1471-2156-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sorensen K.M., Jespersgaard C., Vuust J., Hougaard D., Norgaard-Pedersen B., Andersen P.S. Whole genome amplification on DNA from filter paper blood spot samples: an evaluation of selected systems. Genet Test. 2007;11:65–71. doi: 10.1089/gte.2006.0503. [DOI] [PubMed] [Google Scholar]
- 26.Paynter R.A., Skibola D.R., Skibola C.F., Buffler P.A., Wiemels J.L., Smith M.T. Accuracy of multiplexed Illumina platform-based single-nucleotide polymorphism genotyping compared between genomic and whole genome amplified DNA collected from multiple sources. Cancer Epidemiol Biomarkers Prev. 2006;15:2533–2536. doi: 10.1158/1055-9965.EPI-06-0219. [DOI] [PubMed] [Google Scholar]
- 27.Rengarajan K., Cristol S.M., Mehta M., Nickerson J.M. Quantifying DNA concentrations using fluorometry: a comparison of fluorophores. Molecular Vision. 2002;8:416–421. [PubMed] [Google Scholar]
- 28.Montgomery G.W., Campbell M.J., Dickson P., Herbert S., Siemering K., Ewen-White K.R., Visscher P.M., Martin N.G. Estimation of the rate of SNP genotyping errors from DNA extracted from different tissues. Twin Res Hum Genet. 2005;8:346–352. doi: 10.1375/1832427054936673. [DOI] [PubMed] [Google Scholar]
- 29.Mei J.V., Alexander J.R., Adam B.W., Hannon W.H. Use of filter paper for the collection and analysis of human whole blood specimens. J Nutr. 2001;131:1631S–1636S. doi: 10.1093/jn/131.5.1631S. [DOI] [PubMed] [Google Scholar]
- 30.Mas S., Crescenti A., Gasso P., Vidal-Taboada J.M., Lafuente A. DNA cards: determinants of DNA yield and quality in collecting genetic samples for pharmacogenetic studies. Basic Clin Pharmacol Toxicol. 2007;101:132–137. doi: 10.1111/j.1742-7843.2007.00089.x. [DOI] [PubMed] [Google Scholar]
- 31.Skinner J.R., Chong B., Fawkner M., Webster D.R., Hegde M. Use of the newborn screening card to define cause of death in a 12-year-old diagnosed with epilepsy. J Paediatr Child Health. 2004;40:651–653. doi: 10.1111/j.1440-1754.2004.00498.x. [DOI] [PubMed] [Google Scholar]
- 32.Makowski G.S., Davis E.L., Hopfer S.M. The effect of storage on Guthrie cards: implications for deoxyribonucleic acid amplification. Ann Clin Lab Sci. 1996;26:458–469. [PubMed] [Google Scholar]
- 33.Lane J.A., Noble J.A. Maximizing deoxyribonucleic acid yield from dried blood spots. J Diabetes Sci Technol. 2010;4:250–254. doi: 10.1177/193229681000400204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mendell J.R., Shilling C., Leslie N.D., Flanigan K.M., al-Dahhak R., Gastier-Foster J., Kneile K., Dunn D.M., Duval B., Aoyagi A., Hamil C., Mahmoud M., Roush K., Bird L., Rankin C., Lilly H., Street N., Chandrasekar R., Weiss R.B. Evidence-based path to newborn screening for Duchenne muscular dystrophy. Ann Neurol. 2012;71:304–313. doi: 10.1002/ana.23528. [DOI] [PubMed] [Google Scholar]
- 35.Green N.S., Pass K.A. Neonatal screening by DNA microarray: spots and chips. Nat Rev Genet. 2005;6:147–151. doi: 10.1038/nrg1526. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of VAFE and 260/280 methods used to quantifiy known concentrations of gDNA. A: Known concentrations of high-molecular-weight gDNA (Roche, Indianapolis, IN) used for quantitative PCR standard curve generation were analyzed using a NanoDrop1000 and the Agilent 2200 TapeStation. Measurement of gDNA was limited to >1 ng/μL of gDNA using the 260/280 absorbance spectrum compared with 0.5 ng/μL using VAFE. B: VAFE electropherograms of the gDNA concentration standard curve showing the relative increase in fluorescence with increasing gDNA content in the sample.
Quality PCR of pre- and post-WGA gDNA extracted from a 3-mm blood spot. Quality PCR amplification of three ion channel exons known to contain SNPs. The extracted gDNA spot (Q) is run in parallel with the post-WGA amplification (W) of the same template. All samples except the 1975 and 1986 WGA produced the expected amplicon comparable to reference DNA from venous blood. The amplicons that failed (arrow) were observed in a subsequent repeated PCR, suggesting the gDNA is of sufficiently high quality for subsequent experiments.
Sanger sequencing of PCR amplicons produced during quality PCR of pre- and post-WGA gDNA extracted from decades-old blood spots. Aligned chromatogram traces for a 50-bp region corresponding to bases 300 to 350 in exon 37 of the RYR2 gene. All pre- and post-WGA gDNA samples from blood spots produced sequences of sufficient quality for SNP detection.
Utility of post-WGA gDNA extracted from a 3-mm blood spot in CGH to detect CNVs. A: Slab gel analysis of restriction digests of post-WGA gDNA compared with digests of control DNA extracted from venous blood. An undigested high-molecular-weight DNA is shown for comparison. Most post-WGA samples, regardless of age or input DNA concentration, appear to have an enrichment of specific genome content such that clear bands are visible within the digested DNA, a phenomena clearly observed in the 1984 sample and more faintly in others. In fact, only the 1977 and 1986 post-WGA samples appear uniform, with a range of fragment sizes comparable to control DNA. B: Posthybridization immunocytochemistry arrays showing the individual blood spot post-WGA DNA fluorescence compared with the control reference DNA. All arrays had moderate levels of background fluorescence, with three arrays having derivative of log ratios (DRL) <0.3. Only the 1978 sample failed QC because of a large number of nonuniform outliers. C: Aberration detection using the default ADM-2 detection algorithm in Genomic Workbench identified CNVs in all post-WGA blood spot samples. The CGH against a female reference genome revealed a deletion of the X chromosome in four samples reflecting the XY genotype (1984). Comparatively, the XX genotype (1977) had only small gain and loss aberrations detected. Any CNVs detected in candidate genes will be screened using quantitative PCR in post-WGA samples to eliminate false-positive results before final validation in the initial extraction of QiaAmp blood spot DNA.
Poor quality pre- and post-WGA gDNA extracted from 3-mm blood spots assessed by traditional QC paradigm compared with VAFE. A: Quantification of total gDNA extracted from a single 3-mm blood spot using the QiaAmp Mini Kit (gray bar) followed by WGA (black bar) measured using a NanoDrop1000 and the Agilent 2200 TapeStation. The 260/280 ratiometric quantification overestimates the concentration of gDNA, whereas VAFE reveals the minimal amount of dsDNA extracted in these suboptimal preparations. The WGA on the poor quality template yielded approximately 500 to 3700 ng of gDNA from a starting template of <10 ng. B: Slab gel analysis of gDNA integrity reveals that pre-WGA DNA is not detectable on the slab gel, whereas post-WGA samples are composed of both high-molecular-weight DNA and fragmented or degraded DNA across the entire size range. C: Quality PCR amplification of ion channel exons known to contain SNPs. The extracted spot gDNA (Q) is run in parallel with the post-WGA amplification (W) of the same template. All samples failed to produce PCR amplicons >450 bp in size regardless of gDNA concentration. The black arrow on the right of the gel indicates the expected amplicon size. Only the small 250-bp amplicon of exon 32 of RYR2 amplified in the suboptimal samples, and that was only produced in the reactions using the initial blood spot DNA as template. These results coupled with the low amount of gDNA initially extracted and the fragmented nature of the WGA samples demonstrate the efficacy of our QC paradigm in assessing sample quality for downstream utility in genomic applications. D: VAFE of pre- and post-WGA blood spot samples shows the small amount of gDNA initially extracted and the poor quality of the post-WGA sample when using either the Repli-g or the Genomeplex WGA system. The Repli-g post-WGA samples lack a clear high-molecular-weight DNA (>48,500 bp) band and are highly degraded through the 400- to 4000-bp range. The Genomeplex post-WGA samples had only moderate amounts of total DNA, with most being <500 bp in size. A high-quality gDNA extracted from venous blood is shown for comparison. E: VAFE electropherograms of the DNA extracted from the four suboptimal sample extractions. The pre-WGA DNA (black) has substantial amounts of low molecular weight (100 to 250 bp) with limited amounts of gDNA through the larger size range even when scaled to the signal for better resolution. The Repli-g post-WGA (gray) sample shows an enrichment of fragments across the sample range with limited enrichment at the higher molecular weight. Similarly, the Genomeplex post-WGA samples (red) showed an increase in total DNA relative to the input template where the post-WGA DNA fragments are <500 bp in size. The ability of VAFE to provide quality, quantity, and integrity information on 1 μL of gDNA sample enables QC assessment at an earlier point, after initial extraction, compared with our current QC paradigm, which enabled the efficient triage of samples for downstream applications without the need for additional experimentation.