Abstract
Epithelial host defense proteins comprise a critical component of the pulmonary innate immune response to infection. The short palate, lung, nasal epithelium clone (PLUNC) 1 (SPLUNC1) protein is a member of the bactericidal/permeability-increasing (BPI) fold-containing (BPIF) protein family, sharing structural similarities with BPI-like proteins. SPLUNC1 is a 25 kDa secretory protein that is expressed in nasal, oropharyngeal, and lung epithelia, and has been implicated in airway host defense against Pseudomonas aeruginosa and other organisms. SPLUNC1 is reported to have surfactant properties, which may contribute to anti-biofilm defenses. The objective of this study was to assess the importance of SPLUNC1 surfactant activity in airway epithelial secretions and to explore its biological relevance in the context of a bacterial infection model. Using cultured airway epithelia, we confirmed that SPLUNC1 is critically important for maintenance of low surface tension in airway fluids. Furthermore, we demonstrated that recombinant SPLUNC1 (rSPLUNC1) significantly inhibited Klebsiella pneumoniae biofilm formation on airway epithelia. We subsequently found that Splunc1−/− mice were significantly more susceptible to infection with K. pneumoniae, confirming the likely in vivo relevance of this anti-biofilm effect. Our data indicate that SPLUNC1 is a crucial component of mucosal innate immune defense against pulmonary infection by a relevant airway pathogen, and provide further support for the novel hypothesis that SPLUNC1 protein prevents bacterial biofilm formation through its ability to modulate surface tension of airway fluids.
The airway epithelium serves as a front line of pulmonary host defense. In addition to forming a mechanical barrier against the external environment, it also contributes to airway innate immunity by producing antimicrobial peptides/proteins and inflammatory cytokines in response to invading pathogens.1 These peptides and proteins exhibit direct antimicrobial activity, and in some cases also regulate the influx of inflammatory cells involved in cell-mediated host defenses.2 The airway epithelium is also adapted to remove potentially injurious particles from the airways through physical mechanisms such as cough and mucociliary clearance.
The palate, lung, nasal epithelium clone (PLUNC) or short PLUNC 1 (SPLUNC1) gene was originally found to be expressed in murine embryonic nasal epithelium and adult trachea and bronchi.3 Initially termed PLUNC, the gene product was later renamed short PLUNC 1 (SPLUNC1) and is also referred to as SPURT, LUNX, NASG, or BPIFA1. SPLUNC1 encodes a secreted protein found in abundance in human sputum and tracheobronchial secretions, as well as in saliva,4 nasal lavage fluid,5 in the apical secretions from cultured human tracheobronchial epithelial cells,6,7 and in the specific granules of human neutrophils.8 SPLUNC1 and related PLUNC family members belong to a larger protein family known as the bactericidal/permeability-increasing (BPI)-fold containing (BPIF) proteins. SPLUNC1 is structurally similar to two BPIF family members with demonstrated innate immune roles, BPI, and lipopolysaccharide-binding protein.9–11 Other members of the PLUNC family, mainly expressed in nasopharyngeal and respiratory epithelium, have been shown to mediate host defense functions in those locations.6,12
Based on these observations, it has been hypothesized that SPLUNC1 may function as an antimicrobial protein. This posited function is supported by the finding that SPLUNC1 levels are elevated in chronic airway inflammation6,13 and that mice overexpressing SPLUNC1 exhibit enhanced protection against P. aeruginosa and Mycoplasma pneumoniae.14,15 SPLUNC1 was also reported to bind the Gram-negative bacterial cell wall component lipopolysaccharide.16,17 Additionally, it has been suggested that SPLUNC1 may be required for regulation of certain physical properties of airway surface liquid, including airway surface liquid volume18 and surface tension.19,20 The latter idea is based on our previous observation that recombinant SPLUNC1 has significant surfactant activity, at concentrations that are likely to be physiologically relevant.19
Klebsiella is a common Gram-negative pathogen causing community-acquired bacterial pneumonia and 8% of all hospital-acquired infections.21,22 Lung infections with K. pneumoniae23 are often necrotic. The observed mortality rates of community-acquired K. pneumonia range from 50% to nearly 100% in alcoholic patients.24,25 Importantly, many clinical strains of K. pneumoniae are multi-drug resistant, highlighting the ineffectiveness of current therapy.26 A better understanding of innate immune response to K. pneumoniae infection could present opportunities to improve current therapeutic strategies. Although lung epithelial cells are non-phagocytic cells, they play a key role in host defense against K. pneumoniae by containing invaded K. pneumoniae through opsonization strategies to prevent severe infection.27 In addition, other epithelium-expressed antimicrobial proteins, such as lipocalin 2, are involved in the mucosal immune defense against pulmonary infection with K. pneumoniae.28
In this report, we extend on our studies of SPLUNC1 surfactant activities, using gain and loss of function approaches to demonstrate that SPLUNC1 significantly contributes to the overall surface tension of airway surface liquid (ASL), and interferes with bacterial biofilm formation in a polarized airway epithelial cell model. Furthermore, we explore the relationship between SPLUNC1 surfactant activity and airway host defense, by studying the in vivo consequences of loss of SPLUNC1 in genetically ablated Splunc1−/− mice. Here, we report that Splunc1−/− mice exhibit an impaired antimicrobial defense against K. pneumonia. These findings indicate that SPLUNC1 is important for protection against the airway pathogen K. pneumoniae, a biological activity that may be mediated in part by its unique ability to regulate surface tension in airway fluids.
Materials and Methods
Primary Culture Methods
Primary cultures of human tracheal and bronchial airway epithelia were prepared by enzymatic dispersion using established methods.29 Epithelial cells were dissociated and seeded onto collagen-coated, semi-permeable membranes with a 0.4-μm pore size (Millicell-HA, surface area, 0.6 cm2; Millipore Corp., Bedford, MA). Cells were maintained in 2% Ultroser G medium at 37°C with 5% CO2. Twenty-four hours after seeding, the mucosal medium was removed and the cells were allowed to grow at the air-liquid interface. Only well-differentiated cultures (>4-weeks old) were used in these studies. The presence of tight junctions was confirmed by trans-epithelial resistance using a volt-ohm meter (resistance >500 Ω⋅cm2; World Precision Instruments, Sarasota, FL). Primary cultures of murine tracheal epithelia were established using similar methods. Briefly, Splunc1−/− mice and Splunc1+/+ littermates were euthanized and their tracheas were immediately removed. Harvested tracheas were then cut open longitudinally and placed in a dissociation buffer to remove epithelia, as previously described. Following this step, seeding and maintenance of mouse tracheal epithelia proceeded, as described for human primary cultures.
To study SPLUNC1 expression and surface tension in primary culture secretions, human and mouse epithelia were rinsed apically using PBS containing Ca2+ and Mg2+ (Gibco, Life Technologies, Grand Island, NY). This initial rinse was discarded. Three days later, cultures were again apically rinsed (50 μL PBS with Ca2+ and Mg2+/culture) and washed material was centrifuged at 10,000 × g for 10 minutes to remove mucus, sloughed cells, and so forth. Supernatants were then transferred to fresh tubes and placed on ice. Total protein in airway epithelial washes was estimated using a Nanodrop spectrophotometer (Thermo Scientific, Wilmington, DE). Samples were diluted to a standard concentration of 50 μg/mL before surface tension measurement on the pulsating bubble surfactometer.
Generation of Airway Cell Lines Expressing Human SPLUNC1
The piggyBAC transposon system30 was used to create several clonal cell lines stably expressing human SPLUNC1. Briefly, a cDNA encoding an epitope-tagged SPLUNC1 was cloned into a piggyBAC transposon plasmid cassette. The SPLUNC1 transgene was inserted into this cassette, upstream of a puromycin resistance gene driven by the pCAGGS promoter. This plasmid was then co-transfected, along with a second plasmid encoding the piggyBAC transposase30 into the airway epithelial cell line Calu-3.31 Transfected cells were treated with 3 μg/mL puromycin for 6 days, to select for cells in which the SPLUNC1 expression cassette had been integrated into the genome. Individual surviving colonies were selected and analyzed for SPLUNC1 expression by immunoblotting of cell supernatants. SPLUNC1-positive cell lines were expanded and used for subsequent studies. In parallel, SPLUNC1-negative control cells were generated by co-transfecting Calu-3 cells with plasmids encoding the piggyBAC transposase and an empty transposon construct. To grow the cells at the air-liquid interface for surface tension experiments, cells were seeded onto Millicell supports (Millicell-HA, surface area, 0.6 cm2; Millipore Corp.) as described for human primary cultures. Cultures were maintained for approximately 14 days before apical rinsing with PBS.
Surface Tension Measurements
Surface tension in airway epithelial secretion samples was measured using a pulsating bubble surfactometer (General Transco, Inc., Largo, FL), originally described by Enhorning.32,33 To measure dynamic surface tension, each sample was loaded into a disposable sample chamber, within which a spherical air bubble was formed that maintained contact with outside air. The air bubble was pulsated at a rate of 20 pulses per minute, between a defined minimum bubble radius of 0.4 mm and a maximum bubble radius of 0.55 mm (representing a 50% surface area change). Changes in pressure across the bubble interface were recorded and used to calculate surface tension values throughout cycling according to the Law of Laplace. Data were collected using the software supplied with the instrument and transferred to a computer for analysis. The minimum and maximum surface tension values for each pulsation cycle were extracted and used to compare surface tension differences after a defined pulsation period (either 1, 2, or 10 minutes). This pulsation period was determined by the minimum amount of time needed to reach a stable equilibrium surface tension value for a given sample type. Identical pulsation periods were always used when comparing samples within an experiment.
Immunoblotting and Densitometry
To correlate surface tension with relative SPLUNC1 expression in human airway secretions, we assessed SPLUNC1 abundance in human airway epithelial wash samples by immunoblotting. Secretion samples were resolved on SDS-PAGE gels (10 μg total protein per lane) followed by immunoblotting with a monoclonal antibody recognizing human SPLUNC1 (R&D Systems, Minneapolis, MN). Each immunoblot also included a reference band containing 200 ng of recombinant human SPLUNC1 protein, produced in bacteria as described.19 Band intensities were quantitated using AlphaEaseFC densitometry software version 4.0.0 (Alpha Innotech, San Leandro, CA). To estimate relative differences in SPLUNC1 levels, the intensity of each band was normalized to the reference band on the same immunoblot. Relative SPLUNC1 abundances were then plotted against measured surface tensions for each sample. Linear regression analysis was performed using GraphPad Prism 5 (GraphPad Software, La Jolla, CA) to describe the relationship between SPLUNC1 levels and surface tension.
Determination of SPLUNC1 Antimicrobial Activity
The antimicrobial activity of SPLUNC1 was tested by incubating K. pneumoniae (ATCC #43816) with recombinant human SPLUNC1 protein. K. pneumoniae was grown in Tryptic Soy Broth (TSB, pH 7.2) at 37°C as described,34 and log-phase cultures were adjusted to an OD600 of approximately 0.18 (approximately 108 colony-forming units (CFUs)/mL). For each assay, approximately 103 cells were incubated with 1 μg or 5 μg SPLUNC1 or protein carrying buffer (50 mmol/L Hepes, pH 7.4 and 150 mmol/L NaCl) for 2 hours, a typical time for bactericidal assays against Gram-negative bacteria.35 Aliquots were collected every 30 minutes, serially diluted, and plated on tryptic soy broth agar. CFUs were counted after overnight growth to determine the effect of SPLUNC1 on bacterial growth. All experiments were performed at least three times and data are presented as means ± SD.
Biofilm Assay
A slightly modified version of the microtiter plate assay developed by O’Toole and Kolter36 was used. Briefly, overnight planktonic cultures of K. pneumoniae were inoculated into 100 μL of Dulbecco’s modified Eagle’s medium in a 96-well culture-treated polystyrene microtiter plate (Fisher Scientific, Pittsburgh, PA) with or without recombinant SPLUNC1 or antibiotic controls. Wells filled with growth medium alone were included as negative controls. After 3 hour incubation at 37°C, surface-adherent biofilm formation was measured by staining bound cells for 15 minutes with a 0.5% (w/v) aqueous solution of crystal violet. After rinsing with distilled water, the bound dye was released from the stained cells using 95% ethanol, and optical density was determined at 590 nm.
Static Bacteria-Epithelial Cell Co-Culture Biofilm Assay
A human bronchial epithelial cell line (CFBE41o−) homozygous for the ΔF508-cystic fibrosis transmembrane conductance regulator mutation and stably overexpressing wild-type cystic fibrosis transmembrane conductance regulator (hereafter called CFBE cells; these cells have characteristics similar to those of normal human bronchial epithelial cells) was maintained in minimal essential medium supplemented with 10% fetal bovine serum, 2 mmol/L l-glutamine, 2 μg/mL puromycin, 5 μg/mL plasmocin, 50 U/mL penicillin, and 50 μg/mL streptomycin in a 5% CO2-− 95% air incubator at 37°C.37,38 CFBE cells were seeded at 1 × 106 cells on 24-mm permeable filter inserts (Snapwell; Corning Costar, Kennebunk, ME) and grown in air–liquid interface culture at 37°C for 8 to 10 days to establish confluent monolayers, as previously described.39
To assess the viability of bacteria after recombinant protein treatment, biofilms were grown on polarized and confluent CFBE cells under static conditions as previously described.40 Briefly, K. pneumoniae were inoculated on the apical surface of epithelial cells grown on filters (multiplicity of infection = 25). After 1 hour of incubation at 37°C, unattached bacteria were removed. This was done by gently removing the supernatant and replacing it with Minimal Earle’s medium supplemented with 0.4% arginine. Filters containing epithelial cells and the attached bacteria were returned to 37°C and 5% CO2 for the duration of each experiment (5 hours). Arginine was added to the medium to prolong the viability of airway cells incubated with bacteria under static conditions, as previously described.40 At the end of treatment, biofilms remaining at the apical side of airway cells were washed once with Minimal Earle’s medium, and then 0.1% Triton X-100 was added to the medium for 15 minutes to lyse the epithelial cells and dissociate the biofilms. The lysate was vortexed for 3 minutes and serial dilutions were spot titered onto LB plates to determine the CFUs/well.
Generation of Splunc1−/− Mice
Screening of an ENU mutagenized sperm archive generated in a C3HeB/FeJ background41 revealed a nonsense mutation within the mSplunc1 exon 2 resulting in a stop codon at amino acid residue position 50 in the mouse (m)Splunc1 protein (L50X). Splunc1 L50X mice were generated by in vitro fertilization by The Jackson Laboratory (Bar Harbor, ME) on an inbred genetic background. Splunc1+/− F1 mice were intercrossed, and F2 breeding pairs were established from Splunc1+/+ mice and Splunc1−/− mice. To determine mouse genotypes, polymerase chain reaction was performed with the primer sets for mSplunc1 (Set1: forward, 5′-CAGGGCATACAGTGCAGAGA-3′, and reverse, 5′-CACACTTGAACATCCCCTGA-3′; Set2: forward, 5′-AGCCAGGGGCAACAGCT-3′, and reverse, 5′-GGCCAGCTGTAGCCC-3′), using genomic DNA from tail clips. The primer sets were designed to correspond to the upstream and downstream end of the mutation site. The PCR amplicons were extracted and verified by DNA sequencing by the Genomics and Proteomics Core Laboratories at the University of Pittsburgh.
SDS-PAGE and Immunoblot Analysis
Twenty five μL bronchoalveolar lavage fluid (BALF), 40 μg trachea homogenized in PBS, or 0.1 μg recombinant mouse Splunc1 (rmSplunc1) were diluted with sample loading buffer, heated (5 minutes; 95°C), and separated on a NuPAGE 12% Bis-Tris Gel (Invitrogen, Grand Island, NY). For immunoblot analysis, sheep anti-mPLUNC Ab (R&D Systems, Minneapolis, MN) was used as primary antibody, followed by donkey anti–sheep-HRP antibody (Abcam, Cambridge, MA), and signal was visualized using SuperSignal West Pico Chemiluminescent Substrate (Pierce Biotechnology, Rockford, IL).
Animal Husbandry
Mice were maintained in specific pathogen-free conditions with 12-hour light/dark cycles. All procedures were conducted using mice at 8 to 12 weeks of age, maintained in ventilated microisolator cages housed in animal facilities accredited by the American Association for Accreditation of Laboratory Animal Care, which were located at the University of Pittsburgh and the University of Iowa. Protocols and studies involving animals were conducted in accordance with guidelines of the National Institutes of Health and approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh and the University of Iowa.
Experimental K. pneumoniae Infection
All animal infections were performed using K. pneumoniae (ATCC 43816). K. pneumoniae was grown and prepared as previously described.34 For intratracheal induction of experimental pneumonia, mice were anesthetized with isoflurane inhalation and 1 × 103 CFUs of bacteria were delivered by retropharyngeal instillation. Female Splunc1+/+ mice were challenged with K. pneumoniae and sacrificed immediately and at 6, 24, and 48 hours after infection. The results from each cohort represent the findings from four to six mice per cohort.
Real-Time PCR Analysis
Total RNA was isolated from mouse tissues by a single-step acid guanidinium thiocyanate extraction method.42 Quantitative real-time PCR (ABI7700; Applied Biosystems, Foster City, CA) was performed using mouse-specific mSplunc1 primers (forward: 5′-TGGGATTCTCAGCGGTTTGGATGT-3′; reverse: 5′-TCAGCCAAGATAGCCTTCCTTCCT-3′; probe: 5′-/56-FAM/CACCCTGGTGCACAACATTGCTGAAT/TAMTph/-3′). Validation tests were performed to confirm equivalent PCR efficiencies for the target genes. Test and calibrator lung RNAs (Ambion, Austin, TX) were reverse transcribed using a Superscript III kit (Invitrogen), and PCR was amplified as follows: 95°C for 12 minutes, 40 cycles; 95°C for 15 seconds; 60°C for 1 minute. Three replicates were used to calculate the average cycle threshold for the transcript of interest and for a transcript for normalization [β-glucuronidase (GUS-B); Assays on Demand; Applied Biosystems]. Relative mRNA abundance was calculated by the ΔΔ cycle threshold (Ct) method.
Bronchoalveolar Lavage and Cell Differential Counts
At 24 or 48 hours after inoculation, 4 to 6 mice/group were anesthetized with 2.5% tribromoethanol (Avertin). The trachea was cannulated, the lungs were lavaged with 1 mL PBS twice, and the BALF samples were pooled (pool 1). The lungs were lavaged an additional five times with 1 mL PBS and the recovered fluid was pooled (pool 2). Cells from the two pools were recovered through centrifugation at 300 × g and resuspended in 0.5 mL PBS. A 50-μL aliquot was stained with an equal volume of 0.4% trypan blue (Invitrogen, San Diego, CA) and cells were counted with a hemocytometer. An additional aliquot was placed onto glass microscope slides (Shanon Cytospin; Thermo Fisher, Pittsburgh, PA), stained with Diff-Quick, and cell differential counts were determined manually.
CFU Assay
To determine lung bacterial outgrowth, lungs were lavaged with 1 mL PBS twice, then right lungs were removed aseptically at 24 or 48 hours after bacterial inoculation and placed in sterile PBS. The tissues were homogenized in a tissue homogenizer. Four serial 10-fold dilutions in PBS were made and plated on LB agar plates and incubated for 18 hours at 37°C, each dilution plated in triplicate. The colonies were then counted and surviving bacteria were expressed in log10 units.
Cytokine Assay
Cytokine levels in bronchoalveolar lavage were quantified using mouse Cytokine Multiplex Panel assay (Bio-Rad, Hercules, CA). The expression of tumor necrosis factor-α, IL-1α, IL-1β, IL-6, eotaxin, KC, MCP-1, macrophage inflammatory protein-1α, and regulated on activation normal T cell expressed and secreted was analyzed using the Luminex assay system according to the manufacturer’s instructions (EMD Millipore, Billerica, MA). Absolute cytokine concentrations were calculated from the standard curve for each cytokine.
Lung Histopathology
Lung tissues were harvested at 24 and 48 hours after infection, inflation fixed in situ with 4% paraformaldehyde at 10 cm H2O for 10 minutes with the chest cavity open. The right lobe was embedded in paraffin and 5-μm sections were prepared. Sections were stained with H&E, and histological evaluation was performed to examine K. pneumoniae-induced pathological severity. The stained lung sections were evaluated in a double-blind fashion under a light microscope using a histopathological inflammatory scoring system as previously described.43 Pathological assessment of lung inflammation was graded blindly on a scale of 0 to 4 (least to most severe) based on assessment of the quantity and quality of peribronchiolar and peribronchial inflammatory infiltrates, luminal exudates, perivascular infiltrates, and parenchymal pneumonia. An average score was determined from the means ± SEM of six animals.
Data Analysis
Statistical comparisons between cohorts were made using analysis of variance followed by Dunnett’s multiple comparison test (one-way analysis of variance) or Bonferroni multiple comparison test (two-way analysis of variance). A P value <0.05 was considered to be statistically significant.
Results
SPLUNC1 Reduces Surface Tension in Airway Surface Liquid
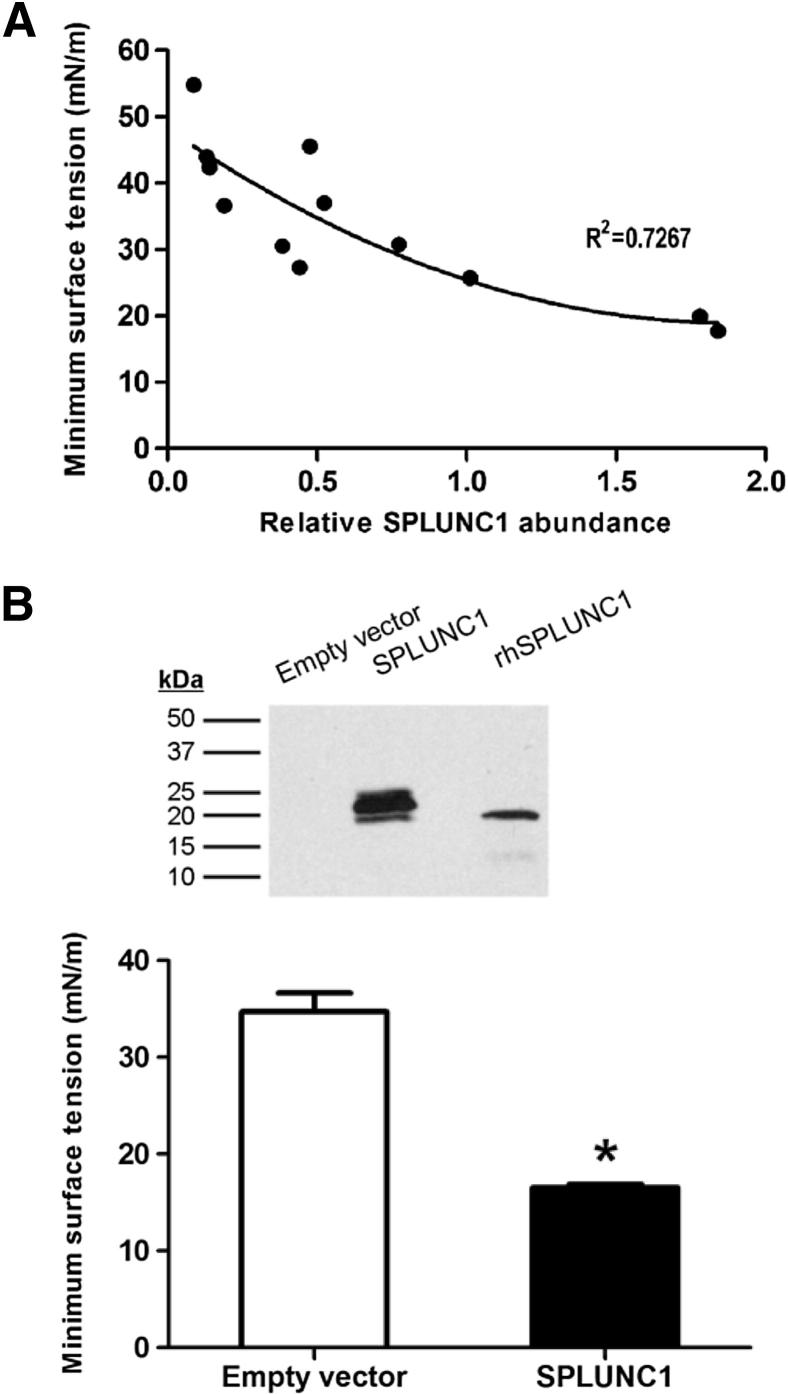
We previously reported that recombinant human SPLUNC1 protein displayed potent surfactant activity in vitro.19 To explore whether this surfactant function is relevant in a biological setting, we measured the effects of native SPLUNC1 on surface tension in secretions from primary cultures of human airway epithelia. As shown in Figure 1A, we observed that surface tension was significantly dependent (P < 0.001) on SPLUNC1 abundance in these secretions. SPLUNC1 abundance explained 72% of the variability in the measured surface tension levels. Consistent with the proposed role of SPLUNC1 protein as a surface tension-reducing agent, increases in the relative abundance of SPLUNC1 were associated with decreases in overall surface tension. To confirm that SPLUNC1 in the secretions was responsible for this effect, we then established an airway epithelial cell line stably secreting SPLUNC1 (Figure 1B). Apical secretions were collected from air-liquid interface cultures of Calu-3 cells stably expressing the human SPLUNC1 protein, along with secretions from SPLUNC1-deficient Calu-3 cells as a control, and surface tension was measured using the pulsating bubble surfactometer. As shown in Figure 1B, the secretions from the SPLUNC1-expressing cultures exhibited significantly decreased surface tensions relative to the secretions lacking SPLUNC1, suggesting that the expression of SPLUNC1 alone is sufficient to confer surface tension reducing properties to airway epithelial secretions.
Figure 1.
SPLUNC1 reduces surface tension in airway epithelial secretions. A: Linear relationship between SPLUNC1 abundance and surface activity in airway epithelial fluids. Airway secretions were collected by rinsing the apical surfaces of primary cultured human airway epithelia with PBS. Secretions were then immunoblotted to assess SPLUNC1 expression levels and relative differences in SPLUNC1 abundance were quantitated using densitometry as described in Materials and Methods. In parallel, surface tension was measured for each sample using a pulsating bubble surfactometer. Surface tension values represent the minimum surface tension achieved after 2 minutes of pulsation. Each data point represents a secretion sample from a different human donor specimen. We observed a significant linear relationship between relative SPLUNC1 levels and surface tension (*P < 0.001). For each one-unit increase in SPLUNC1 abundance (expressed as normalized pixel intensity), surface tension was decreased by an estimated 15.1 ± 7.3 mNm. B: SPLUNC1 expression confers surface tension-reducing activity to airway epithelial secretions. Calu-3 cultures stably secreting SPLUNC1 protein, as well as negative control cells (empty vector), were grown at an air-liquid interface and rinsed apically with PBS to collect secretions. The presence of SPLUNC1 in the secretions was confirmed by immunoblot (top panel). In this blot, 250 ng recombinant human SPLUNC1 protein (rhSPLUNC1) serves as a positive control for the SPLUNC1 antibody. Overall surface tension in the wash samples was measured using the pulsating bubble surfactometer (bottom panel). Minimum surface tension after 10 minutes pulsation was significantly reduced in the SPLUNC1-containing washes, supporting the idea that surfactant activity by SPLUNC1 contributes significantly to the surface activity in airway epithelial washes (n = 3). *P = 0.01.
Assessment of Splunc1 Expression in Splunc1−/− Mice
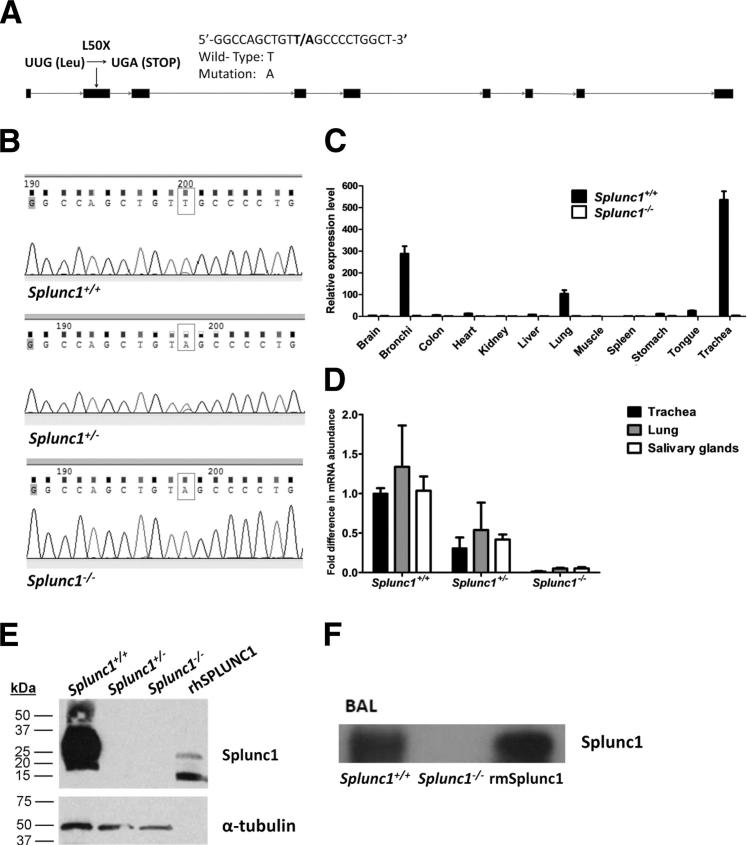
To better understand the importance of surface tension regulation by SPLUNC1 in airway fluids, we explored the consequences of loss of SPLUNC1 using a Splunc1-deficient mouse model. Mice with a genetic ablation of Splunc1 were generated (Figure 2A), and a homozygous point mutation in Splunc1-deficient mice was confirmed using DNA sequencing (Figure 2B). Examination of mSplunc1 gene expression in various mouse tissues by TaqMan-based quantitative real-time PCR indicated that mSplunc1 is expressed primarily in airway tissues including the trachea, bronchi, and lungs, and confirmed successful ablation of mSplunc1 in the Splunc1−/− mice (Figure 2C). Additional quantitative real-time PCR analysis performed on relevant respiratory and oral tissues (trachea, lung, and salivary glands) revealed that mSplunc1 mRNA levels were decreased as predicted in Splunc1+/− and Splunc1−/− mice (Figure 2D), and immunoblotting was used to verify that mSplunc1 protein was undetectable in tracheal homogenates from Splunc1−/− mice (Figure 2E). Because SPLUNC1 is a secreted protein,6 we anticipated that mSplunc1 would be present in extracellular compartments. We examined the expression of mSplunc1 protein in mouse lung, by immunoblotting BALF from Splunc1 +/+ and Splunc1 −/− mice. The mSplunc1 protein was not detected in the BALF from Splunc1−/− mice (Figure 2F).
Figure 2.
Generation of Splunc1−/− mice and assessment of Splunc1 expression. A: Schematic of the mSplunc1 gene, depicting the location of a T→A point mutation in exon 2 in the knockout mice. Boxes represent exons. B: Sequencing based genotyping of Splunc1−/− mice. The PCR products amplified using genomic DNA from Splunc1+/+, Splunc1+/-, and Splunc1−/− mouse tail clips were verified by DNA sequencing. Top panel: Sequence of DNA products from a Splunc1+/+ mouse. Middle panel: Sequence of DNA products from a Splunc1+/- mouse. Bottom panel: Sequence of DNA products from a Splunc1−/− mouse. The boxes indicate the T→A mutation in the DNA molecules. C: Relative expression of mSplunc1 in various mouse tissues was analyzed by real time PCR and determined by the ΔΔCt method using mouse glucuronidase-β RNA as a control. D: The mSplunc1 mRNA expression was assessed by quantitative real-time PCR in mouse oral and respiratory tissues including trachea, total lung, and salivary glands. The mSplunc1 expression in Splunc1+/- and Splunc1−/− mice is expressed as fold decrease relative to Splunc1+/+ expression levels (n = 3 Splunc1+/+ animals, 3 Splunc1+/- animals, and 4 Splunc1−/− animals). E: Tracheal homogenates from Splunc1+/+ and Splunc1−/− mice were resolved on an SDS-PAGE gel (40 μg total protein/lane) and immunoblotted for mSplunc1 (upper blots). The last lane contains 250 ng of recombinant human SPLUNC1 protein (rhSPLUNC1) as a positive control for the SPLUNC1 antibody. Lower blots: As a loading control, the immunoblot was stripped and re-probed with an antibody recognizing mouse α-tubulin. F: BALF from Splunc1+/+ and Splunc1−/− mice was analyzed by immunoblot using anti-mSplunc1. The mSplunc1 protein was not detected in Splunc1−/− BALF. BAL, bronchoalveolar lavage; BALF, bronchoalveolar lavage fluid.
Splunc1-Deficient Airway Secretions Display Decreased Surface Activity
We observed normal Mendelian ratios in the progeny of the Splunc1−/− mouse colony (data not shown), indicating that lack of mSplunc1 does not impact viability in utero. We also observed that Splunc1−/− mice have normal lifespans, and that body weights are not significantly different between Splunc1−/− mice and their Splunc1+/+ littermates, as shown in Supplemental Figure S1. Similarly, Splunc1−/− mice showed no differences from C3HeB/FeJ control mice in terms of gross anatomy or overt behavior. In our efforts to define the phenotype for the Splunc1−/− mouse, we predicted that the loss of a surfactant protein from the secretions of the Splunc1−/− mice would result in a measurable loss of surface activity in airway surface liquid. To test this, we collected apical secretions from well-differentiated air-liquid interface cultures of tracheal airway epithelia derived from Splunc1−/− mice and Splunc1+/+ littermates, and we assessed surface tension using the pulsating bubble surfactometer. As expected, secretions from the Splunc1−/− airway epithelia exhibited a significantly increased surface tension relative to those from Splunc1+/+ controls, indicating loss of a surface active agent (Figure 3). From this result, we concluded that SPLUNC1 is necessary for maintenance of normal surface tension in airway epithelial secretions.
Figure 3.
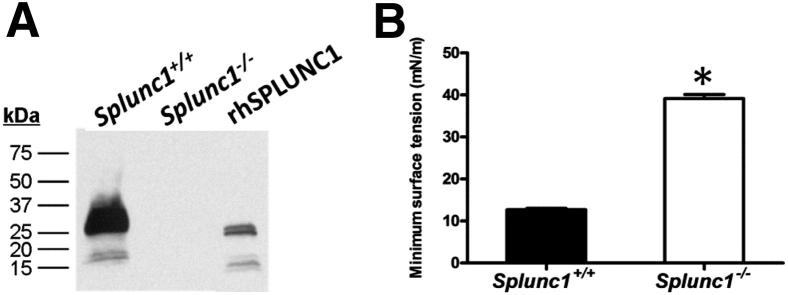
Loss of surface activity in mSplunc1-deficient airway secretions. Tracheal epithelia from Splunc1+/+ and Splunc1−/− mice were grown at an air-liquid interface and ASL was collected by rinsing the apical surfaces with PBS. A: The mSplunc1 protein was readily detected by immunoblotting of the apical washes from Splunc1+/+, but not Splunc1−/−, cultures. B: Surface activity in the samples was assessed by observing the minimum surface tension after 1 minute of pulsation in the pulsating bubble surfactometer. The mSplunc1-deficient washes displayed significantly increased surface tension relative to washes from Splunc1+/+ animals (n = 3). *P < 0.0000001.
SPLUNC1 Inhibits Biofilm Formation by Gram-Negative Bacteria
What might be the biological role of this surfactant protein in the upper respiratory tract and conducting airways? Although surfactants are known to be important for promoting compliance and preventing airway collapse in the lower airways, we found no evidence of impaired lung mechanics in the Splunc1−/− mice, as shown in Supplemental Figure S2. Therefore, we considered a second function that has been attributed to biological surfactants: inhibition of bacterial biofilm formation. There is a growing list of examples of biosurfactants (generally of microbial origin) with the capacity to inhibit biofilm formation by competing bacteria.44–47 In our own earlier studies, we demonstrated that the surface tension-modulating activity of SPLUNC1 significantly inhibits biofilm formation by P. aeruginosa on a liquid surface (Luria-Bertani broth),19 a finding which suggests that SPLUNC1 may represent an example of a mammalian biosurfactant adapted for dispersion of microbes in the airways.
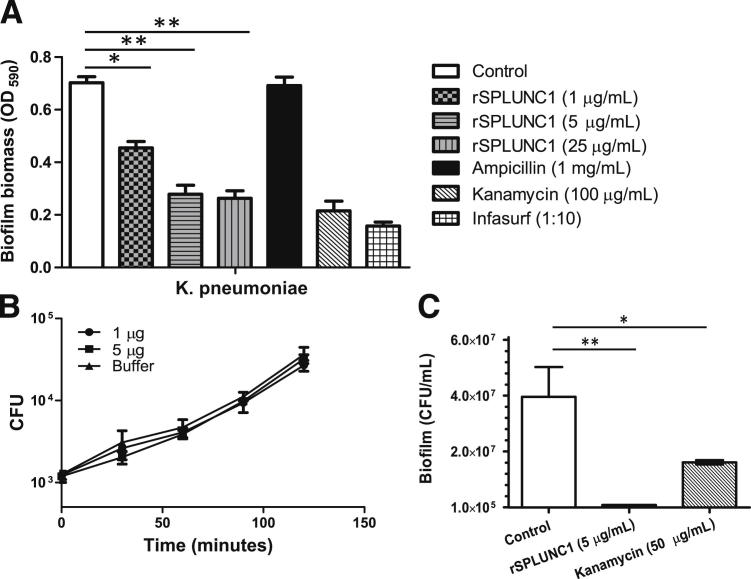
To determine whether SPLUNC1-dependent changes in surface tension affect Gram-negative bacterial biofilm formation, we performed standard microtiter plate-based biofilm assays designed to measure biofilm biomass for K. pneumoniae.23 We found that when K. pneumoniae was incubated with recombinant SPLUNC1 (rSPLUNC1) at increasing concentrations, biofilm biomass decreased and was inversely related to the rSPLUNC1 concentration (Figure 4A). As positive controls, we tested the effects of kanamycin, known to be bactericidal against K. pneumoniae, as well as Infasurf (calfactant); an extract of natural surfactant from calf lungs that includes phospholipids, neutral lipids, and the hydrophobic surfactant-associated proteins (surfactant proteins B and C). These rSPLUNC1-induced decreases in bacterial biomass could not be explained by direct killing of the bacteria by rSPLUNC1, as we failed to observe antimicrobial effects against the bacteria in traditional bacterial viability assays (Figure 4B). To ensure that the bacterial biomass in this assay represented biofilms, we treated the bacteria with ampicillin, as it has been shown that the resistance of K. pneumoniae to antibiotic treatments significantly increases once a biofilm is established. It was previously reported that planktonic K. pneumoniae can be killed by a 4-hour ampicillin treatment at a concentration of 5000 μg/mL (10 times the minimum inhibitory concentration of approximately 500 μg/mL), with an approximately 4-log reduction in the total number of CFU48; in K. pneumoniae biofilms challenged with 5000 μg/mL of ampicillin, the number of CFU remained virtually unchanged after 4 hours of treatment.48 We observed similar results in our study, with K. pneumoniae biofilms showing complete resistance to ampicillin treatment (Figure 4A).
Figure 4.
The rSPLUNC1 significantly inhibits biofilm formation of Gram-negative bacteria. Determination of anti-biofilm effects of SPLUNC1 on K. pneumoniae. Quantifications were performed by measuring the biofilm biomass on abiotic surfaces via crystal violet staining (OD590) or by counting CFU representing biofilm forming bacteria recovered from the surface of cultured epithelial cells. Kanamycin and Infasurf were used as positive controls to disrupt biofilm formation. A: K. pneumoniae biofilm biomass measurement after treatment with increasing concentrations of rSPLUNC1. B: K. pneumoniae growth in the presence or absence of rSPLUNC1. Bacteria were incubated with 1 μg and 5 μg of rSPLUNC1, or in the presence of buffer alone, and bacterial viability was assessed by CFU counting. Treatment with rSPLUNC1 did not produce a statistically significant reduction in the growth of K. pneumoniae. C: CFUs of biofilm forming K. pneumoniae on epithelial cells after treatment with SPLUNC1. Results are means ± SEM from three independent experiments. *P < 0.05, **P < 0.01 for the treatment to control comparison.
To determine whether the effect of SPLUNC1 on abiotic K. pneumoniae biofilms grown on plastic surfaces translated to a biotic biofilm setting, we co-cultured bacteria on the apical surface of polarized human airway epithelial cells. K. pneumoniae forms surface-associated bacterial biofilms on polarized human airway epithelial cells when co-cultured for 6 hours at 37°C, as measured by colony forming units. The addition of 5 μg/mL SPLUNC1 for the final 5 hours of the biofilm assay significantly reduced K. pneumoniae biofilm formation (Figure 4C).
Splunc1 Is Upregulated in the Lung after K. pneumoniae Infection
Our observation that SPLUNC1 inhibits bacterial biofilm formation in several in vitro models led us to predict that the absence of functional mSplunc1 protein should result in increased susceptibility to bacterial pathogens in Splunc1−/− mice. However, we have noted that Splunc1−/− mice are not predisposed to spontaneous development of lung disease or infection, and histopathological analysis of Splunc1−/− airways failed to detect evidence of structural abnormalities or increased inflammatory markers relative to controls (data not shown). Therefore, we hypothesized that the consequences of mSplunc1 ablation might be more readily apparent in the context of an acute airway infection. We tested this hypothesis by observing the immune response of Splunc1−/− mice to a bacterial challenge using K. pneumoniae. We first assessed mSplunc1 expression in the lungs of Splunc1+/+ mice after K. pneumoniae infection and found that mSplunc1 mRNA expression was induced in response to K. pneumoniae infection. The mSplunc1 mRNA levels gradually increased and peaked at 24-hours postinfection, when a 3.4-fold increase was observed (Figure 5).
Figure 5.
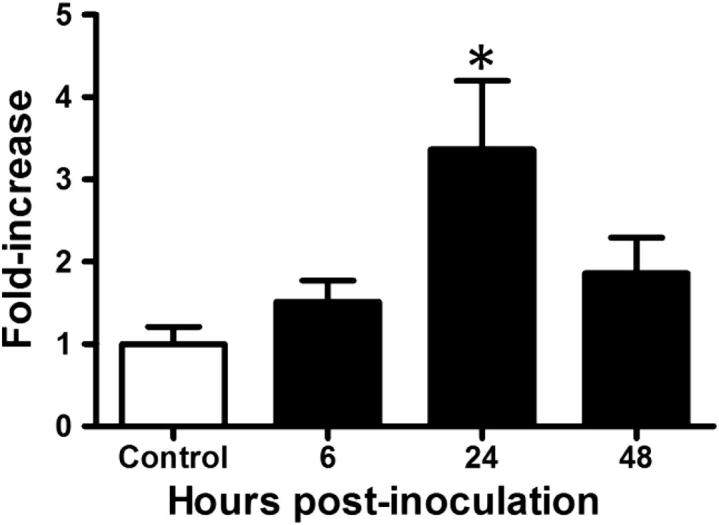
The mSplunc1 expression is upregulated in the mouse lung after an induced respiratory infection. Wild-type C3HeB/FeJ mice were infected with 1000 CFUs/mouse lung K. pneumoniae and sacrificed at 6, 24, and 48 hours after infection. Murine Splunc1 mRNA expression was quantified in lung homogenates by quantitative PCR and determined by the ΔΔCt method using mouse glucuronidase-β RNA as a control. All data are expressed as fold induction compared to 0 hour time point. Results are means ± SEM from three separate experiments (n = 6 mice for each group). *P < 0.05.
Splunc1 Deficiency Results in an Enhanced Outgrowth of K. pneumoniae
To determine whether the ablation of mSplunc1 could alter bacterial infection in vivo, Splunc1−/− mice and Splunc1+/+ littermates were infected and bacterial burden was assessed. Immediately after K. pneumoniae challenge, bacterial deposition was equivalent between Splunc1−/− mice and their Splunc1+/+ control littermates (data not shown). However, we observed a significant difference in lung bacterial burden at 24 hours and 48 hours after infection (Figure 6). Splunc1−/− mice demonstrated increased susceptibility to K. pneumoniae infection, with significantly higher bacterial CFUs in both BALF and lungs.
Figure 6.
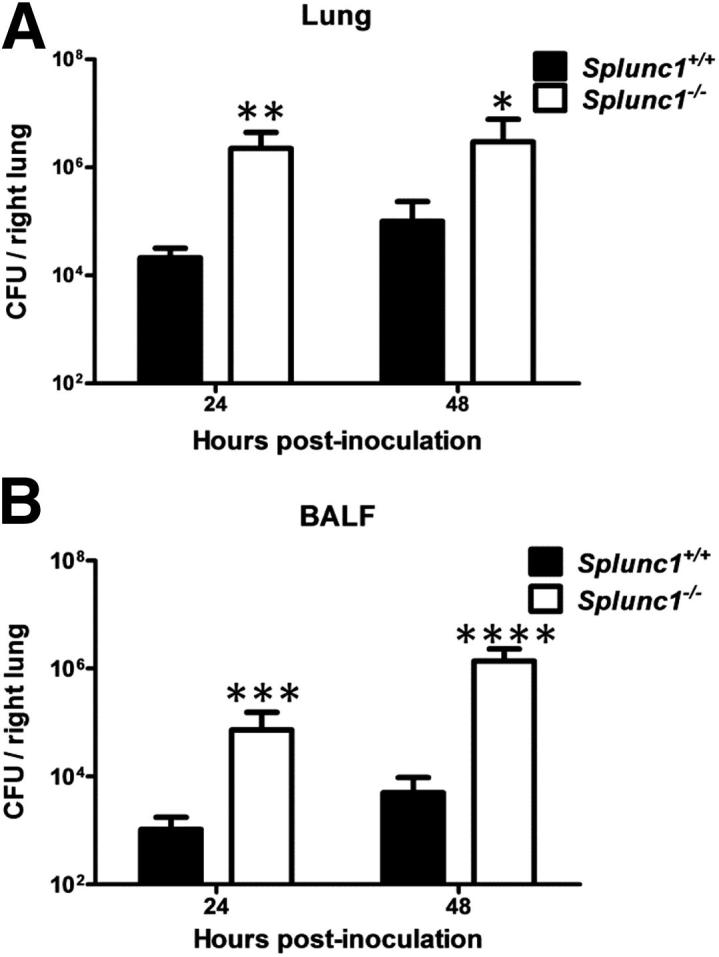
Splunc1−/− mice are susceptible to induced respiratory infection. Splunc1−/− mice and littermate controls were inoculated with 103 CFUs K. pneumoniae per mouse. CFUs in lung homogenates and BALF were determined at the indicated recovery time points. A: CFU in lung homogenates. B: CFU in BALF. At both time points, Splunc1−/− mice exhibited significantly increased bacterial load in BALF and lungs. CFU are expressed as log10 units. Results are means ± SEM from three independent experiments (n = 8 mice for each group). *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 for Splunc1+/+ to Splunc1−/− comparisons at each time point.
Increased Neutrophil Infiltration in Splunc1−/− Mice after K. pneumoniae Challenge
Granulocyte recruitment to the site of infection is an important characteristic of the innate immune response and critical to defense against K. pneumoniae infection. BALF total cell counts and differentials from unexposed Splunc1−/− mice were not different from Splunc1+/+ control mice (data not shown). However, both total inflammatory cells and total neutrophils were significantly higher in Splunc1−/− (3.87 ± 0.99 × 105 and 3.46 ± 0.9 × 105 for total cells and neutrophils respectively; n = 4 to 6) mice as compared with Splunc1+/+ (1.17 ± 0.24 × 105 and 7.28 ± 2.34 × 104 for total cells and neutrophils, respectively; n = 4 to approximately 6) at 24 hours after bacterial challenge (Figure 7).
Figure 7.
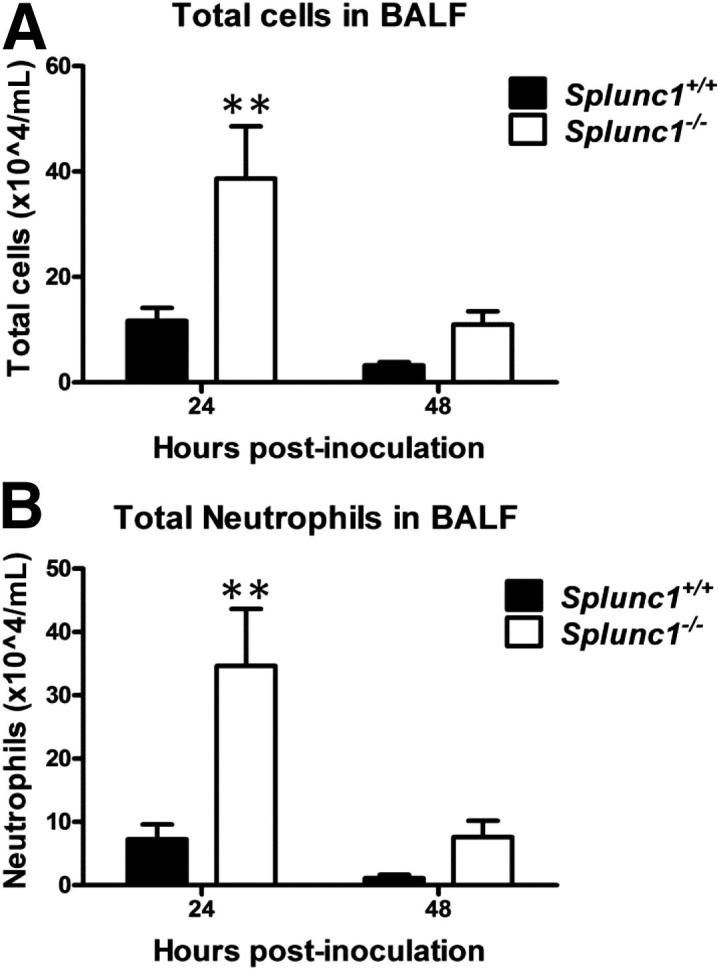
Splunc1−/− mice show increased inflammatory cell recruitment after infection. Splunc1+/+ and Splunc1−/− mice were infected with K. pneumoniae. At 24 or 48 hours after inoculation, the lungs were lavaged, and a manual differential was determined on the BALF cytospins. A: Total inflammatory cells in BALF after inoculation. B: Total neutrophils in BALF after inoculation. Both total inflammatory cells and neutrophils were significantly higher in Splunc1−/− mice at 24 hours after challenge. Results are means ± SEM from three independent experiments (n = 8 mice for each group). **P < 0.01 for Splunc1+/+ to Splunc1−/− comparisons at each time point.
Increase of Pro-Inflammatory Cytokine Production in Splunc1−/− Mice after K. pneumoniae Challenge
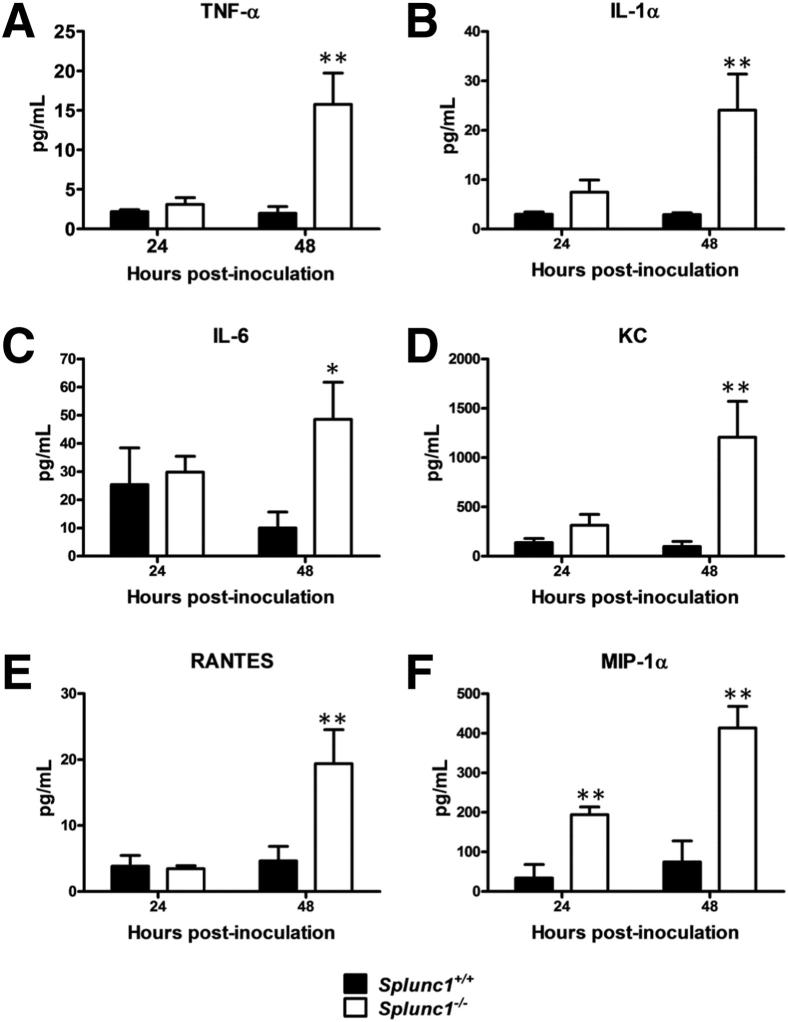
BALF samples were collected and cytokine concentrations were determined by Bio-Plex assay to determine whether production of inflammatory cytokines varied at 24 or 48 hours postinoculation. The spectrum of BALF cytokines changed did not vary by genotype, but the magnitude of the response was consistently higher in BALF from Splunc1−/− mice compared with that from Splunc1+/+ mice (Figure 8). Pro-inflammatory and neutrophil chemotactic cytokines were significantly elevated in Splunc1−/− mice compared to their Splunc1+/+ littermates. At 24 hours after bacterial inoculation, chemokine macrophage inflammatory protein-1α (also known as chemokine [C-C motif] ligand 3, CCL3) levels increased significantly in BALF from Splunc1−/− mice. At 48-hours postinoculation, cytokines, including tumor necrosis factor-α, IL-1α, IL-6, and the chemotactic cytokines chemokine KC, regulated on activation normal T cell expressed and secreted, and macrophage inflammatory protein-1α were significantly higher in BALF from Splunc1−/− mice compared with that from Splunc1+/+ control mice.
Figure 8.
Increased pro-inflammatory cytokine production in Splunc1−/− mice after challenge. Splunc1+/+ and Splunc1−/− mice were infected as described. Cytokine concentrations in BALF were measured using a Luminex assay (Milliplex) and are reported in pg/mL. Tumor necrosis factor (TNF)-α (A), IL-1α (B), IL-6 (C), KC (D), RANTES (E), and MIP-1α (F) concentrations in BALF at 24 and 48 hours after K. pneumoniae inoculation. Concentrations of three additional cytokines (IL-1β, eotaxin, and monocyte chemoattractant protein-1) showed no significant changes between Splunc1−/− and Splunc1+/+ mice (not shown). Results are means ± SEM from three independent experiments (n = 8 mice for each group). *P < 0.05, **P < 0.01 for Splunc1+/+ to Splunc1−/− comparisons at each time point. RANTES, regulated on activation normal T cell expressed and secreted; MIP-1α, macrophage inflammatory protein-1α.
Increase of Severity of K. pneumoniae-Induced Pneumonia in Splunc1−/− Mice
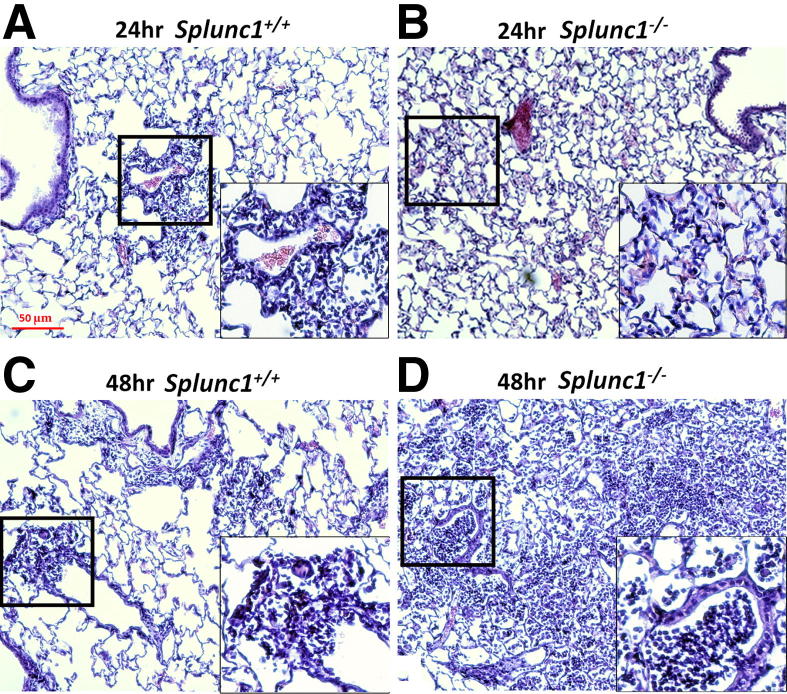
K. pneumoniae infection resulted in extensive interstitial inflammation in the lungs at both 24- and 48-hours postinfection (Figure 9). To determine whether K. pneumoniae infection-induced lung inflammation was influenced by Splunc1, lung histology was assessed after infection. Splunc1−/− mice exhibited more significant peribronchial inflammation and airway lumen leukocyte accumulation, both features characteristic of severe pneumonia. A pathological scoring system indicated more severe pneumonia and associated inflammation in Splunc1−/− mice than their wild-type littermates at 48 hours after K. pneumoniae infection (2.2 ± 0.6 versus 3.5 ± 0.7; P < 0.05).
Figure 9.
Representative histopathological findings in infected lung tissue. The lung tissues were harvested at 24 and 48 hours after infection, fixed, and H&E stained for histological evaluation. A: Representative image of lungs of Splunc1+/+ mice at 24 hours after infection. B: Representative image of lungs of Splunc1−/− mice at 24 hours after infection. C: Representative image of lungs of Splunc1+/+ mice at 48 hours after infection. D: Representative image of lungs of Splunc1−/− mice at 48 hours after infection. Splunc1−/− mice exhibited more significant peribronchial inflammation and airway lumen leukocyte accumulation and more severe pneumonia. Insets are enlarged regions of corresponding boxes in their respective figures. Scale bar = 50 μm.
Discussion
The goal of this study was to investigate the biological relevance of SPLUNC1 surfactant activity in airway epithelial secretions, by examining the effects of its loss from airway secretions both in vitro and in vivo. Our studies addressed two questions. First, does surfactant activity by native SPLUNC1 make a significant contribution to the overall surface tension in airway surface liquid? Second, we asked how maintenance of low surface tension in airway surface liquid might be connected to airway host defense. Here, we report that surfactant activity by SPLUNC1 is a significant determinant of surface tension in conducting airway secretions, and that loss of this protein is associated with increased susceptibility to infection by the airway pathogen K. pneumoniae.
Our investigation into SPLUNC1 regulation of surface tension in airway surface liquid ASL builds on earlier studies indicating that SPLUNC1 is a surface active agent, with potent and dose-dependent surface tension-reducing activity.19 These experiments were performed with purified recombinant protein. We sought to determine whether this surfactant-like activity was retained by native, fully glycosylated SPLUNC1 protein present in mammalian epithelial cell secretions. Furthermore, we wanted to assess whether SPLUNC1 could exert its surface active effects in the context of airway surface liquid, a complex mixture of secreted proteins, peptides, lipids, and electrolytes.
We found that ASL from human airway epithelia expressing SPLUNC1 displayed significantly lower surface tension than secretions from the parental SPLUNC1-deficient cell line. Consistently, ASL from Splunc1−/− mice, which lack detectable mSplunc1 protein, exhibited significantly increased surface tension relative to Splunc1+/+ secretions. These experiments confirm that native SPLUNC1 does behave as a surfactant, and that the protein is both necessary and sufficient to confer significant surface tension reduction to complex airway secretions. Together with our observation that SPLUNC1 abundance is highly correlated with surface tension in apical secretions from primary cultures of well-differentiated human airway epithelia (Figure 1A), we provide further evidence that SPLUNC1 significantly contributes to ASL surface tension.
Our studies with human airway epithelial cell secretions failed to find a complete correspondence between relative SPLUNC1 abundance and measured surface tension, indicating instead that SPLUNC1 levels could explain only approximately 72% of the variability in the observed surface activity. These results suggest that there may be additional mechanisms regulating ASL surface tension. The idea is supported by the observation that although the surface tension of secretions lacking SPLUNC1 was significantly increased relative to SPLUNC1-containing washes, surface tension in these samples was still below that of pure water (approximately 72 mN/m), implying that there are likely to be other molecules in ASL that also impact surface tension. Therefore, we cannot conclude that SPLUNC1 is the sole determinant of surface tension in these secretions; however, it does appear to be a major contributor, possibly the main one.
In this study, we further documented the inhibitory effect of SPLUNC1 on biofilm formation by K. pneumoniae, not only on the solid surface of abiotic microtiter plates, but also on the surface of polarized airway epithelial cell cultures. Interestingly, the SPLUNC1 concentrations (1 to 25 μg/mL) that displayed significant inhibitory activity against biofilm formation correspond closely with the reported physiological concentrations of SPLUNC1 in airway epithelial secretions (approximately 10 to 250 μg/mL).17,19 Furthermore, it appears that the biofilm inhibiting activity of SPLUNC1 was more robust under physiological conditions (ie, on the surface of airway epithelial cells, than on the abiotic solid surface). These results highlight the important role of SPLUNC1 in preventing Gram-negative bacteria biofilm formation – and presumably associated bacterial infections – in the conducting airways.
To better understand the relevance of this observation in vivo, we compared the susceptibility of Splunc1 knockout mice and Splunc1+/+ littermates using a K. pneumonia model. We demonstrated that mSplunc1 mRNA increases in Splunc1+/+ mice on infection with K. pneumoniae, suggesting that upregulation of SPLUNC1 is a feature of the innate immune response to Gram-negative bacteria. Splunc1 deficiency in the knockout mice infected with K. pneumoniae was associated with increased pulmonary bacterial loads and inflammatory cell recruitment. Consistent with this, we detected a profound increase in inflammatory cytokine and chemokine levels in BALF from infected Splunc1−/− mice. The significant difference in lung bacterial burden in Splunc1 knockout mice relative to Splunc1+/+ controls likely accounted for the higher production of pro-inflammatory cytokines. Finally, histological evaluation confirmed that pneumonia was more severe in Splunc1−/− mice, compared to Splunc1+/+ controls, after K. pneumoniae challenge.
Thus, this study provides in vivo evidence that SPLUNC1 plays a protective role in the context of infection with a relevant airway pathogen. Based on the profound effect that the SPLUNC1 protein has on surface tension in airway surface liquid, we speculate that loss of mSplunc1 surfactant activity likely contributed to the impaired innate immune responses we observed in the Splunc1−/− mice. We further speculate that inhibition of biofilm microcolony formation by SPLUNC1 may also increase the susceptibility of bacteria to the endogenous antimicrobials in ASL. Taken together, the increased susceptibility of the Splunc1−/− mice to K. pneumoniae-induced bacterial pneumonia, along with our in vitro data demonstrating an anti-biofilm effect by recombinant SPLUNC1 protein, suggest that the protective effects of SPLUNC1 are due, at least in part, to its ability to inhibit early biofilm establishment by invading microbial pathogens. As such, SPLUNC1 may represent the first example of a mammalian protein whose innate immune effects are mediated through an ability to alter surface tension at a mucosal surface.
Although this study focused on how modulation of surface tension might influence bacterial biofilm development in the conducting airways, it is important to note that maintenance of low surface tension has also been hypothesized to impact host defense in oral and respiratory secretions by enhancing mucociliary transport. A significant body of literature supports the idea that mucociliary transport rates in the airways are increased by the application of surfactants,49–52 an effect that may result from the ability of surfactants to act as lubricants and/or to enhance ciliary beat frequency.53 Based on these observations, we suggest that an additional role of the biosurfactant SPLUNC1 may be to aid mucociliary clearance in regions of the body that are exposed to environmental microbes. In support of this, McGillivary and Bakaletz20 reported that siRNA-mediated knockdown of Splunc1 in a chinchilla model resulted in diminished mucociliary clearance in the Eustachian tube, where bacteria are normally cleared by a mucociliary transport system very similar to that in the airways. Loss or reduction of mucociliary clearance in Splunc1−/− mouse airways awaits experimental confirmation.
Although we postulate that the increased susceptibility of the Splunc1−/− mice to K. pneumoniae infection was mediated, at least in part, through the loss of mSplunc1 surfactant activity in ASL, it is possible that the absence of mSplunc1 contributed to this outcome in other ways as well. It has been suggested that SPLUNC1 has direct antimicrobial properties. Consistently, a transgenic mouse model overexpressing human SPLUNC1 in mouse airway epithelial secretory cells displayed enhanced antimicrobial activity during P. aeruginosa infection compared to wild-type littermates,14 and additional in vitro evidence suggests that recombinant human SPLUNC1 protein reduces P. aeruginosa growth in a dose-dependent manner.17 In addition to Gram-negative bacteria, the SPLUNC1 protein has antimicrobial activity against other microorganisms. Chu et al54 showed a dose-dependent reduction of M. pneumoniae growth after incubation with recombinant mSplunc1 protein. The authors also showed increased M. pneumoniae levels and inflammatory cells in the lungs of Splunc1−/− mice compared to wild-type controls.15 Finally, Zhou et al55 found that recombinant human SPLUNC1 protein can inhibit replication of Epstein-Barr virus. We did not observe direct bacterial killing by recombinant human SPLUNC1 against K. pneumoniae in an in vitro assay (Figure 4B). However, the same purified rSPLUNC1 at similar concentrations was previously shown to have antibacterial activity against P. aeruginosa17, suggesting that different bacterial strains have differing levels of susceptibility to the bactericidal effects of SPLUNC1 protein. Overall, these results suggest that loss of direct antimicrobial activity is unlikely to contribute to the increased susceptibility of the Splunc1−/− mice in this model of K. pneumoniae-induced pneumonia.
There is also evidence that SPLUNC1 may participate in additional aspects of host defense. The BPI protein, SPLUNC1, as with its relative, has been shown to bind the bacterial cell wall component lipopolysaccharide.16,17,56 This finding suggests that SPLUNC1 may be involved in mechanisms by which host organisms sense and respond to Gram-negative bacteria, including Toll-like receptor 4- mediated inflammation. In addition, SPLUNC1 has been shown to modulate the host innate immune response by enhancing neutrophil elastase activity and increasing IL-8 secretion and leukocyte chemotaxis.15,17
In summary, we present evidence that SPLUNC1 is an endogenous surfactant that plays a significant role in maintaining low surface tension in airway epithelial secretions, an activity that appears to enhance anti-biofilm defenses at the mucosal surface. Using genetically ablated Splunc1−/− mice, we found that loss of SPLUNC1 results in an increased susceptibility to infection by the airway pathogen K. pneumoniae. Our animal studies allow us to link expression of this surface active molecule to the innate immune responses in the conducting airways, where it appears to provide protection against inhaled microbes in several novel ways. Based on our findings, we propose that SPLUNC1 is a multifunctional protein whose possible host defense functions include anti-biofilm activity, direct inhibition of bacterial growth, lipopolysaccharide binding, modulation of neutrophil activity, and facilitation of mucociliary clearance. These insights into airway epithelial defenses against bacterial infection may help inform future therapeutic strategies.
Acknowledgments
We thank Dr. Kathryn Chaloner (University of Iowa) for excellent assistance with statistical analysis, Ingenium Pharmaceuticals for generation of the Splunc1 L50X mouse model, and the University of Pittsburgh and University of Iowa DNA Core Facilities.
Footnotes
Supported by NIH grant R01 HL-091938 (Y.P.D.), P01 HL-091842 (P.B.M.), the Roy J. Carver Charitable Trust (P.B.M.), UPMC Health System Competitive Medical Research Fund (Y.P.D.), the University of Pittsburgh Cystic Fibrosis Foundation RPD (Y.P.D.), and the University of Iowa Cystic Fibrosis Foundation RDP (P.B.M.). The In Vitro Models and Cell Culture Core is partially supported by the Center for Gene Therapy for Cystic Fibrosis NIH grant P30 DK-54759, and the Cystic Fibrosis Foundation.
Y.L. and J.A.B. contributed equally to this work.
Current address of Y.L., the Department of Cell Biology and Anatomy, University of North Texas Health Science Center, and North Texas Eye Research Institute, Fort Worth, TX.
Contributor Information
Paul B. McCray, Jr., Email: paul-mccray@uiowa.edu.
Y. Peter Di, Email: peterdi@pitt.edu.
Supplemental Data
Weights of male and female mice from the Splunc1−/− mouse colony. Bars represent weights of Splunc1−/− mice and wild-type littermates at 7 weeks of age. (n = 15 Splunc1+/+ females, 25 Splunc1−/− females, 23 Splunc1+/+ males, and 21 Splunc1−/− males).
Summary of lung mechanics in Splunc1−/− mice and littermate controls. Respiratory mechanics were assessed in 7-week old Splunc1−/− mice and wild-type littermates using the flexiVent system. Shown are measurements for several different outcome parameters, including lung compliance (A), elastance (B), and resistance (C). D: depicts pressure-volume loops for individual mice. E: Graphical depiction of the average area under the curve for the pressure-volume loops shown in D (n = 4 Splunc1+/+ and 4 Splunc1−/− mice).
References
- 1.Evans S.E., Xu Y., Tuvim M.J., Dickey B.F. Inducible innate resistance of lung epithelium to infection. Annu Rev Physiol. 2010;72:413–435. doi: 10.1146/annurev-physiol-021909-135909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hiemstra P.S. Epithelial antimicrobial peptides and proteins: their role in host defence and inflammation. Paediatr Respir Rev. 2001;2:306–310. doi: 10.1053/prrv.2001.0165. [DOI] [PubMed] [Google Scholar]
- 3.Weston W.M., LeClair E.E., Trzyna W., McHugh K.M., Nugent P., Lafferty C.M., Ma L., Tuan R.S., Greene R.M. Differential display identification of plunc, a novel gene expressed in embryonic palate, nasal epithelium, and adult lung. J Biol Chem. 1999;274:13698–13703. doi: 10.1074/jbc.274.19.13698. [DOI] [PubMed] [Google Scholar]
- 4.Vitorino R., Lobo M.J., Ferrer-Correira A.J., Dubin J.R., Tomer K.B., Domingues P.M., Amado F.M. Identification of human whole saliva protein components using proteomics. Proteomics. 2004;4:1109–1115. doi: 10.1002/pmic.200300638. [DOI] [PubMed] [Google Scholar]
- 5.Ghafouri B., Kihlstrom E., Stahlbom B., Tagesson C., Lindahl M. PLUNC (palate, lung and nasal epithelial clone) proteins in human nasal lavage fluid. Biochem Soc Trans. 2003;31:810–814. doi: 10.1042/bst0310810. [DOI] [PubMed] [Google Scholar]
- 6.Di Y.P., Harper R., Zhao Y., Pahlavan N., Finkbeiner W., Wu R. Molecular cloning and characterization of spurt, a human novel gene that is retinoic acid-inducible and encodes a secretory protein specific in upper respiratory tracts. J Biol Chem. 2003;278:1165–1173. doi: 10.1074/jbc.M210523200. [DOI] [PubMed] [Google Scholar]
- 7.Campos M.A., Abreu A.R., Nlend M.C., Cobas M.A., Conner G.E., Whitney P.L. Purification and characterization of PLUNC from human tracheobronchial secretions. Am J Respir Cell Mol Biol. 2004;30:184–192. doi: 10.1165/rcmb.2003-0142OC. [DOI] [PubMed] [Google Scholar]
- 8.Bartlett J.A., Hicks B.J., Schlomann J.M., Ramachandran S., Nauseef W.M., McCray P.B., Jr. PLUNC is a secreted product of neutrophil granules. J Leukoc Biol. 2008;83:1201–1206. doi: 10.1189/jlb.0507302. [DOI] [PubMed] [Google Scholar]
- 9.Bingle C.D., Craven C.J. PLUNC: a novel family of candidate host defence proteins expressed in the upper airways and nasopharynx. Hum Mol Genet. 2002;11:937–943. doi: 10.1093/hmg/11.8.937. [DOI] [PubMed] [Google Scholar]
- 10.Elsbach P., Weiss J. Role of the bactericidal/permeability-increasing protein in host defence. Curr Opin Immunol. 1998;10:45–49. doi: 10.1016/s0952-7915(98)80030-7. [DOI] [PubMed] [Google Scholar]
- 11.Fenton M.J., Golenbock D.T. LPS-binding proteins and receptors. J Leukoc Biol. 1998;64:25–32. doi: 10.1002/jlb.64.1.25. [DOI] [PubMed] [Google Scholar]
- 12.Bingle C.D., LeClair E.E., Havard S., Bingle L., Gillingham P., Craven C.J. Phylogenetic and evolutionary analysis of the PLUNC gene family. Protein Sci. 2004;13:422–430. doi: 10.1110/ps.03332704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bingle L., Barnes F.A., Cross S.S., Rassl D., Wallace W.A., Campos M.A., Bingle C.D. Differential epithelial expression of the putative innate immune molecule SPLUNC1 in cystic fibrosis. Respir Res. 2007;8:79. doi: 10.1186/1465-9921-8-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lukinskiene L., Liu Y., Reynolds S.D., Steele C., Stripp B.R., Leikauf G.D., Kolls J.K., Di Y.P. Antimicrobial activity of PLUNC protects against Pseudomonas aeruginosa infection. J Immunol. 2011;187:382–390. doi: 10.4049/jimmunol.1001769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gally F., Di Y.P., Smith S.K., Minor M.N., Liu Y., Bratton D.L., Frasch S.C., Michels N.M., Case S.R., Chu H.W. SPLUNC1 promotes lung innate defense against Mycoplasma pneumoniae infection in mice. Am J Pathol. 2011;178:2159–2167. doi: 10.1016/j.ajpath.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghafouri B., Kihlstrom E., Tagesson C., Lindahl M. PLUNC in human nasal lavage fluid: multiple isoforms that bind to lipopolysaccharide. Biochim Biophys Acta. 2004;1699:57–63. doi: 10.1016/j.bbapap.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Sayeed S., Nistico L., St Croix C., Di Y.P. Multifunctional Role of Human SPLUNC1 in Pseudomonas aeruginosa Infection. Infect Immun. 2013;81:285–291. doi: 10.1128/IAI.00500-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia-Caballero A., Rasmussen J.E., Gaillard E., Watson M.J., Olsen J.C., Donaldson S.H., Stutts M.J., Tarran R. SPLUNC1 regulates airway surface liquid volume by protecting ENaC from proteolytic cleavage. Proc Natl Acad Sci USA. 2009;106:11412–11417. doi: 10.1073/pnas.0903609106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gakhar L., Bartlett J.A., Penterman J., Mizrachi D., Singh P.K., Mallampalli R.K., Ramaswamy S., McCray P.B., Jr. PLUNC is a novel airway surfactant protein with anti-biofilm activity. PloS one. 2010;5:e9098. doi: 10.1371/journal.pone.0009098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGillivary G., Bakaletz L.O. The multifunctional host defense peptide SPLUNC1 is critical for homeostasis of the mammalian upper airway. PLoS One. 2010;5:e13224. doi: 10.1371/journal.pone.0013224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Podschun R., Ullmann U. Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev. 1998;11:589–603. doi: 10.1128/cmr.11.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sahly H., Podschun R. Clinical, bacteriological, and serological aspects of Klebsiella infections and their spondylarthropathic sequelae. Clin Diagn Lab Immunol. 1997;4:393–399. doi: 10.1128/cdli.4.4.393-399.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin C.Y., Wheelock A.M., Morin D., Baldwin R.M., Lee M.G., Taff A., Plopper C., Buckpitt A., Rohde A. Toxicity and metabolism of methylnaphthalenes: comparison with naphthalene and 1-nitronaphthalene. Toxicology. 2009;260:16–27. doi: 10.1016/j.tox.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feldman C., Ross S., Mahomed A.G., Omar J., Smith C. The aetiology of severe community-acquired pneumonia and its impact on initial, empiric, antimicrobial chemotherapy. Respir Med. 1995;89:187–192. doi: 10.1016/0954-6111(95)90246-5. [DOI] [PubMed] [Google Scholar]
- 25.Jong G.M., Hsiue T.R., Chen C.R., Chang H.Y., Chen C.W. Rapidly fatal outcome of bacteremic Klebsiella pneumoniae pneumonia in alcoholics. Chest. 1995;107:214–217. doi: 10.1378/chest.107.1.214. [DOI] [PubMed] [Google Scholar]
- 26.Rice L.B. The clinical consequences of antimicrobial resistance. Curr Opin Microbiol. 2009;12:476–481. doi: 10.1016/j.mib.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 27.Cortes G., Alvarez D., Saus C., Alberti S. Role of lung epithelial cells in defense against Klebsiella pneumoniae pneumonia. Infect Immun. 2002;70:1075–1080. doi: 10.1128/IAI.70.3.1075-1080.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan Y.R., Liu J.S., Pociask D.A., Zheng M., Mietzner T.A., Berger T., Mak T.W., Clifton M.C., Strong R.K., Ray P., Kolls J.K. Lipocalin 2 is required for pulmonary host defense against Klebsiella infection. J Immunol. 2009;182:4947–4956. doi: 10.4049/jimmunol.0803282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karp P.H., Moninger T.O., Weber S.P., Nesselhauf T.S., Launspach J.L., Zabner J., Welsh M.J. An in vitro model of differentiated human airway epithelia. Methods for establishing primary cultures. Methods Mol Biol. 2002;188:115–137. doi: 10.1385/1-59259-185-X:115. [DOI] [PubMed] [Google Scholar]
- 30.Wu S.C., Meir Y.J., Coates C.J., Handler A.M., Pelczar P., Moisyadi S., Kaminski J.M. piggyBac is a flexible and highly active transposon as compared to sleeping beauty. Tol2, and Mos1 in mammalian cells. Proc Natl Acad Sci U S A. 2006;103:15008–15013. doi: 10.1073/pnas.0606979103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shen B.Q., Finkbeiner W.E., Wine J.J., Mrsny R.J., Widdicombe J.H. Calu-3: a human airway epithelial cell line that shows cAMP-dependent Cl- secretion. Am J Physiol. 1994;266:L493–L501. doi: 10.1152/ajplung.1994.266.5.L493. [DOI] [PubMed] [Google Scholar]
- 32.Enhorning G. Pulsating bubble technique for evaluating pulmonary surfactant. J Appl Physiol. 1977;43:198–203. doi: 10.1152/jappl.1977.43.2.198. [DOI] [PubMed] [Google Scholar]
- 33.Enhorning G. Photography of peripheral pulmonary airway expansion as affected by surfactant. J Appl Physiol. 1977;42:976–979. doi: 10.1152/jappl.1977.42.6.976. [DOI] [PubMed] [Google Scholar]
- 34.Ye P., Garvey P.B., Zhang P., Nelson S., Bagby G., Summer W.R., Schwarzenberger P., Shellito J.E., Kolls J.K. Interleukin-17 and lung host defense against Klebsiella pneumoniae infection. Am J Respir Cell Mol Biol. 2001;25:335–340. doi: 10.1165/ajrcmb.25.3.4424. [DOI] [PubMed] [Google Scholar]
- 35.Taylor P.W. Bactericidal and bacteriolytic activity of serum against gram-negative bacteria. Microbiol Rev. 1983;47:46–83. doi: 10.1128/mr.47.1.46-83.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Toole G.A., Kolter R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol Microbiol. 1998;30:295–304. doi: 10.1046/j.1365-2958.1998.01062.x. [DOI] [PubMed] [Google Scholar]
- 37.Bruscia E., Sangiuolo F., Sinibaldi P., Goncz K.K., Novelli G., Gruenert D.C. Isolation of CF cell lines corrected at DeltaF508-CFTR locus by SFHR-mediated targeting. Gene Ther. 2002;9:683–685. doi: 10.1038/sj.gt.3301741. [DOI] [PubMed] [Google Scholar]
- 38.Cozens A.L., Yezzi M.J., Kunzelmann K., Ohrui T., Chin L., Eng K., Finkbeiner W.E., Widdicombe J.H., Gruenert D.C. CFTR expression and chloride secretion in polarized immortal human bronchial epithelial cells. Am J Respir Cell Mol Biol. 1994;10:38–47. doi: 10.1165/ajrcmb.10.1.7507342. [DOI] [PubMed] [Google Scholar]
- 39.Bomberger J.M., Barnaby R.L., Stanton B.A. The deubiquitinating enzyme USP10 regulates the post-endocytic sorting of cystic fibrosis transmembrane conductance regulator in airway epithelial cells. J Biol Chem. 2009;284:18778–18789. doi: 10.1074/jbc.M109.001685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anderson G.G., Moreau-Marquis S., Stanton B.A., O’Toole G.A. In vitro analysis of tobramycin-treated Pseudomonas aeruginosa biofilms on cystic fibrosis-derived airway epithelial cells. Infect Immun. 2008;76:1423–1433. doi: 10.1128/IAI.01373-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Augustin M., Sedlmeier R., Peters T., Huffstadt U., Kochmann E., Simon D., Schoniger M., Garke-Mayerthaler S., Laufs J., Mayhaus M., Franke S., Klose M., Graupner A., Kurzmann M., Zinser C., Wolf A., Voelkel M., Kellner M., Kilian M., Seelig S., Koppius A., Teubner A., Korthaus D., Nehls M., Wattler S. Efficient and fast targeted production of murine models based on ENU mutagenesis. Mamm Genome. 2005;16:405–413. doi: 10.1007/s00335-004-3028-2. [DOI] [PubMed] [Google Scholar]
- 42.Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 43.Harrod K.S., Mounday A.D., Stripp B.R., Whitsett J.A. Clara cell secretory protein decreases lung inflammation after acute virus infection. Am J Physiol. 1998;275:L924–L930. doi: 10.1152/ajplung.1998.275.5.L924. [DOI] [PubMed] [Google Scholar]
- 44.Irie Y., O’Toole G.A., Yuk M.H. Pseudomonas aeruginosa rhamnolipids disperse Bordetella bronchiseptica biofilms. FEMS Microbiol Lett. 2005;250:237–243. doi: 10.1016/j.femsle.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 45.Pamp S.J., Tolker-Nielsen T. Multiple roles of biosurfactants in structural biofilm development by Pseudomonas aeruginosa. J Bacteriol. 2007;189:2531–2539. doi: 10.1128/JB.01515-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mireles J.R., 2nd, Toguchi A., Harshey R.M. Salmonella enterica serovar typhimurium swarming mutants with altered biofilm-forming abilities: surfactin inhibits biofilm formation. J Bacteriol. 2001;183:5848–5854. doi: 10.1128/JB.183.20.5848-5854.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walencka E., Rozalska S., Sadowska B., Rozalska B. The influence of Lactobacillus acidophilus-derived surfactants on staphylococcal adhesion and biofilm formation. Folia Microbiol. 2008;53:61–66. doi: 10.1007/s12223-008-0009-y. [DOI] [PubMed] [Google Scholar]
- 48.Anderl J.N., Franklin M.J., Stewart P.S. Role of antibiotic penetration limitation in Klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin. Antimicrob Agents Chemother. 2000;44:1818–1824. doi: 10.1128/aac.44.7.1818-1824.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Allegra L., Bossi R., Braga P. Influence of surfactant on mucociliary transport. Eur J Respir Dis Suppl. 1985;142:71–76. [PubMed] [Google Scholar]
- 50.Ballard S.T., Parker J.C., Hamm C.R. Restoration of mucociliary transport in the fluid-depleted trachea by surface-active instillates. Am J Respir Cell Mol Biol. 2006;34:500–504. doi: 10.1165/rcmb.2005-0214OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Outzen K.E., Svane-Knudsen V. Effect of surface-active substance on nasal mucociliary clearance time: a comparison of saccharin clearance time before and after the use of surface-active substance. Rhinology. 1993;31:155–157. [PubMed] [Google Scholar]
- 52.Banerjee R., Puniyani R.R., Bellare J.R. Analysis of dynamic surface properties of therapeutic surfactants and lung phospholipids. J Biomater Appl. 2000;15:140–159. doi: 10.1106/8T7E-NPCN-UN8N-JX99. [DOI] [PubMed] [Google Scholar]
- 53.Kakuta Y., Sasaki H., Takishima T. Effect of artificial surfactant on ciliary beat frequency in guinea pig trachea. Respir Physiol. 1991;83:313–321. doi: 10.1016/0034-5687(91)90050-s. [DOI] [PubMed] [Google Scholar]
- 54.Chu H.W., Thaikoottathil J., Rino J.G., Zhang G., Wu Q., Moss T., Refaeli Y., Bowler R., Wenzel S.E., Chen Z., Zdunek J., Breed R., Young R., Allaire E., Martin R.J. Function and regulation of SPLUNC1 protein in Mycoplasma infection and allergic inflammation. J Immunol. 2007;179:3995–4002. doi: 10.4049/jimmunol.179.6.3995. [DOI] [PubMed] [Google Scholar]
- 55.Zhou H.D., Li X.L., Li G.Y., Zhou M., Liu H.Y., Yang Y.X., Deng T., Ma J., Sheng S.R. Effect of SPLUNC1 protein on the Pseudomonas aeruginosa and Epstein-Barr virus. Mol Cell Biochem. 2008;309:191–197. doi: 10.1007/s11010-007-9659-3. [DOI] [PubMed] [Google Scholar]
- 56.Zhou H.D., Wu M.H., Shi L., Zhou M., Yang Y.X., Zhao J., Deng T., Li X.L., Sheng S.R., Li G.Y. [Effect of growth inhibition of the secretory protein SPLUNC1 on Pseudomonas aeruginosa] Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2006;31:464–469. Chinese. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Weights of male and female mice from the Splunc1−/− mouse colony. Bars represent weights of Splunc1−/− mice and wild-type littermates at 7 weeks of age. (n = 15 Splunc1+/+ females, 25 Splunc1−/− females, 23 Splunc1+/+ males, and 21 Splunc1−/− males).
Summary of lung mechanics in Splunc1−/− mice and littermate controls. Respiratory mechanics were assessed in 7-week old Splunc1−/− mice and wild-type littermates using the flexiVent system. Shown are measurements for several different outcome parameters, including lung compliance (A), elastance (B), and resistance (C). D: depicts pressure-volume loops for individual mice. E: Graphical depiction of the average area under the curve for the pressure-volume loops shown in D (n = 4 Splunc1+/+ and 4 Splunc1−/− mice).