Abstract
Microglia play an essential role in innate immunity, homeostasis, and neurotropic support in the central nervous system. In Alzheimer disease (AD), these cells may affect disease progression by modulating the buildup of β-amyloid (Aβ) or releasing proinflammatory cytokines and neurotoxic substances. Discovering agents capable of increasing Aβ uptake by phagocytic cells is of potential therapeutic interest for AD. Lipoxin A4 (LXA4) is an endogenous lipid mediator with potent anti-inflammatory properties directly involved in inflammatory resolution, an active process essential for appropriate host responses, tissue protection, and the return to homeostasis. Herein, we demonstrate that aspirin-triggered LXA4 (15 μg/kg) s.c., twice a day, reduced NF-κB activation and levels of proinflammatory cytokines and chemokines, as well as increased levels of anti-inflammatory IL-10 and transforming growth factor-β. Such changes in the cerebral milieu resulted in recruitment of microglia in an alternative phenotype, as characterized by the up-regulation of YM1 and arginase-1 and the down-regulation of inducible nitric oxide synthase expression. Microglia in an alternative phenotype–positive cells demonstrated improved phagocytic function, promoting clearance of Aβ deposits and ultimately leading to reduction in synaptotoxicity and improvement in cognition. Our data indicate that activating LXA4 signaling may represent a novel therapeutic approach for AD.
Alzheimer disease (AD) is a devastating neurodegenerative disorder that impairs memory and causes cognitive and psychiatric deficits. The neuropathological hallmarks of AD are diffuse and neuritic plaques, which are predominantly composed of the β-amyloid (Aβ) peptide, and neurofibrillary tangles, which are composed of filamentous aggregates of hyperphosphorylated tau protein.1 Chronic inflammation due to recruitment of activated glial cells to amyloid plaques is another key pathological feature of AD, although its impact on disease progression and neurodegeneration remains an area of active investigation.2
Microglia play essential roles in the maintenance of homeostasis within the central nervous system, but the inflammatory program that is induced by these cells also has the potential to cause neuronal dysfunction and death if inflammatory responses are not properly resolved.3,4 Primarily, activated microglia respond to environmental stresses and immunological challenges by scavenging excess neurotoxins and exerting their phagocytic ability of engulfing damaged and dead cell debris, providing a nurturing environment for tissue healing.5 Moreover, it has been recently demonstrated that microglia exert a critical role on postnatal development, adult neuronal plasticity, and circuit function.6,7 In contrast, chronically activated microglia ignite inflammatory responses by releasing a variety of mediators that have been demonstrated to disrupt cellular function in the brain.8,9 Remarkably, such an exacerbated inflammatory response has been proposed to be a critical causal factor for the impairment in the phagocytosis of Aβ deposits by microglia in the AD brain.10,11 Therefore, modifying microglial activation, instead of inhibiting its function, seems to represent a reasonable alternative to enhance Aβ clearance and reduce amyloid deposition in the AD brain.
Recent advances in knowledge of the mechanisms of inflammatory resolution have identified lipoxins as attractive therapeutic tools to treat diseases in which inflammation is involved.12–15 Lipoxin A4 (LXA4) is generated via the lipoxygenase pathway during cell-cell interactions in inflammatory conditions, whereas aspirin-triggered LXA4 (ATL), a molecule that displays the same anti-inflammatory activities as the native lipoxins, is generated after the acetylation of cyclooxygenase-2 and is more resistant to metabolic inactivation.16 Lipoxins potentiate inflammatory resolution by means of potent agonistic actions at the G-protein–coupled receptor, termed LXA4 receptor (ALX/FPR2).17 Activation of ALX by LXA4 reduces many endogenous processes, such as neutrophil and eosinophil recruitment and activation, leukocyte migration, NF-κB translocation, and chemokine and cytokine production. Likewise, evidence shows that LXA4 signaling primes macrophages for chemotaxis and enhances phagocytosis of microorganisms and apoptotic cells.18 In the nervous system, LXA4 protects neurons against experimental stroke and Aβ42 toxicity by modulating inflammation.13,19,20 In addition, lipoxins inhibit inflammatory pain processing through their actions on astrocytic activation in the spinal cord.15 However, the ability of LXA4 signaling to modulate neuroinflammation and AD pathology in vivo has not been addressed. Given the fact that elevated neuroinflammation and altered microglial responses are common hallmarks in AD brain during the disease course, we examined the effects of ATL on AD neuropathology and behavior deficits.
Materials and Methods
Animals
Twelve-month-old male Tg2576 mice harboring the Swedish double mutation in amyloid precursor protein (APP; APPKM670/671ML) were used for all experiments.21 Animals were maintained at controlled room temperature (22°C ± 2°C) and humidity (60% to 80%) under a 12:12-hour light-dark cycle (lights on at 6 AM). All procedures used in the present study followed the Principles of Laboratory Animal Care from the NIH (Bethesda, MD), publication 85-23, and were approved by the University of California, Irvine, Institutional Animal Care and Use Committee.
Treatment with Aspirin-Triggered LXA4
Animals were treated s.c. with 15 μg/kg ATL (5S,6R,15R-trihydroxy-7,9,13-trans-11-cis-eicosatetraenoic acid; Cayman Chemical, Ann Arbor, MI), twice daily for 8 weeks.15 A separate group of animals was treated with 5% polyethylene glycol 200 and 5% Tween 20 in saline (vehicle). Injections were performed from 8 to 10 months of age and completed on the day of the euthanasia.
Behavior Studies
Mice were exposed to two identical objects placed at the opposite ends of the arena for 5 minutes. Twenty-four hours later, mice were presented for 5 minutes with one of the familiar objects and a novel object of similar dimensions. The recognition index represents the percentage of the time that mice spend exploring the novel object.
For the Morris water maze (MWM), mice were trained to swim to a platform submerged 1.5 cm beneath the surface of the water and invisible to the mice while swimming. Animals were subjected to four training trials per day for 7 days. A probe trial was assessed 24 hours after the last training session and consisted of a 60-second free swim in the pool without the platform. Performance was monitored with the EthoVision XT video-tracking system (Noldus Information Technology, Leesburg, VA).
For the contextual fear conditioning, mice were placed in the conditioning chamber (San Diego Instruments, San Diego, CA) and allowed to explore for 2 minutes before receiving three electric foot shocks (duration, 1 second; intensity, 0.2 mA; intershock interval, 2 minutes). Twenty-four hours later, freezing behavior was analyzed.
Tissue Preparation
Mice were deeply anesthetized with sodium pentobarbital and sacrificed by perfusion transcardially with 0.1 mol/L PBS (pH 7.4) solution. The right brain hemispheres were fixed for 48 hours in 4% paraformaldehyde and cryoprotected in 30% sucrose for immunohistochemical (IHC) analysis. Frozen brains were divided into sections coronally (40 μm thick) using a Leica SM2010R freezing microtome (Leica Microsystems, Bannockburn, IL), serially collected in cold 0.02% sodium azide, and stored at 4°C. The left hemispheres were snap frozen on dry ice after removal of cerebellum, brainstem, and olfactory bulb, and subjected to protein extraction sequentially using T-PER tissue protein extraction reagent (Thermo Scientific, Rockford, IL) and 70% formic acid. The supernatant was aliquoted and stored at −80°C. Protein concentration in the supernatant was determined using the Bradford assay.
Immunoblotting
Equal protein amounts were separated using 4% to 12% gradient SDS-PAGE, transferred to a nitrocellulose membrane, and incubated overnight at 4°C with primary antibody. The following primary antibodies were used in this study: postsynaptic density protein 95 (Cell Signaling Technology, Danvers, MA); human APP-CT20, A disintegrin and metalloproteinase domain-containing protein (ADAM) 10, ADAM17, and beta-secretase 1 (BACE1; Calbiochem, San Diego, CA); OC and A11 (Dr. Charles Glabe, University of California, Irvine); Aβ1-16 (6E10) (Covance Research Products, Denver, PA); arginase, inducible nitric oxide synthase (iNOS), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Santa Cruz Biotechnology, Santa Cruz, CA); ATP-binding cassette transporter 1 (ABCA1), low density lipoprotein receptor-related protein 1 (LRP1), apolipoprotein E (APOE), insulin-degrading enzyme (IDE), neprilysin, and ubiquitin (Abcam, Cambridge, MA); YM1 (Stem Cell Technologies, Vancouver, BC, Canada); phosphorylated-p65 NF-κB, liver X receptor (LXR), and peroxisome proliferator-activated receptor γ (Cell Signaling Technology, Danvers, MA); or protein phosphatase 2A (PP2A; Sigma-Aldrich, St. Louis, MO). After washing, the membranes were incubated with adjusted secondary antibodies coupled to horseradish peroxidase. The immunocomplexes were visualized using the SuperSignal West Pico Kit (Thermo Scientific). Band density measurements were made using ImageJ imaging software version 1.36b (NIH).
ELISA
Determination of the Aβ levels was performed as previously described.22 Tumor necrosis factor (TNF)-α and IL-1β were measured using commercially available enzyme-linked immunosorbent assay (ELISA) kits (Thermo Scientific), according to the manufacturer’s instructions. The levels of granulocyte-macrophage colony-stimulating factor (GM-CSF), interferon-γ, IL-6, IL-4, monocyte chemotactic protein-1, macrophage inflammatory protein-1α, regulated on activation normal T-cell expressed and secreted (RANTES), matrix metallopeptidase-9 (MMP-9), IL-10, and transforming growth factor (TGF)-β were assessed using the Aushon SearchLight Assay Services (Aushon BioSystems, Billerica, MA).
Immunohistochemistry
Sections were incubated overnight at 4°C with anti–Aβ1-16 (6E10; Covance Research Products), anti-Aβ40 (C49), anti-Aβ42 (D32; Dr. Vitaly Vasilevko and Dr. David H. Cribbs, University of California, Irvine), anti–glial fibrillary acidic protein (GFAP; Millipore, Billerica, MA), anti-CD45, anti-CD11b (AbD Serotec, Raleigh, NC), or anti–ionized calcium-binding adapter molecule 1 (Iba-1; Wako Chemicals, Richmond, VA) with 5% normal serum in Tris-buffered solution. After the appropriate biotinylated secondary antibody, sections were processed using the Vectastain Elite ABC reagent and 3,3′-diaminobenzidine (Vector Laboratories, Burlingame, CA), according to the manufacturer’s instructions. Sections from vehicle- and ATL-treated mice were processed under the same conditions. Negative control experiments included omission of primary antibody and substitution of the primary antibody by equivalent dilutions of nonimmune serum, using the same staining protocol, and were devoid of specific immunoreaction product.
The immunostaining was assessed at six brain coronal levels. Specifically, six alternate sections (40 μm thick) of the brain with an individual distance of approximately 160 μm were obtained between 1.34 and 2.54 mm posterior to the bregma. Images of stained hippocampus and entorhinal cortex were acquired using an Axiocam digital camera and AxioVision software version 4.6 connected to an Axioskop 50 microscope (Carl Zeiss MicroImaging, Thornwood, NY). Settings for image acquisition were identical for vehicle- and ATL-treated tissues.
Staining analyses were calculated as the percentage of labeled area captured (positive pixels)/the full area captured (total pixels) using ImageJ complying with strict standards.22–24 Examiners (R.M., M.K., and D.B.V.) blinded to sample identities made all histological assessments.
Immunofluorescence
Sections were incubated overnight at 4°C with the following primary antibodies: anti-synaptophysin (Sigma-Aldrich), anti–Aβ1-16 (6E10; Covance Research Products), anti-GFAP (Dako, Carpentaria, CA), anti–Iba-1 (Wako Chemicals), anti-YM1 (Stem Cell Technologies), anti–phosphorylated-p65 NF-κB (Cell Signaling Technology, Danvers, MA), anti-CD45 (AbD Serotec), anti-collagen IV (Fitzgerald Industries International, Acton, MA), anti-NeuN (Millipore), and/or anti-ALX (Novus Biologicals, Littleton, CO). Sections were then rinsed and incubated for 1 hour with secondary Alexa Fluor–conjugated antibodies (Invitrogen, Carlsbad, CA) at room temperature. Finally, sections were mounted onto gelatin-coated slides in Fluoromount-G (Southern Biotech, Birmingham, AL) and examined under a Leica DM2500 confocal laser microscope using the Leica Application Suite Advanced Fluorescence software version 2.6.0 (Leica Microsystems).
The immunofluorescence was assessed at the same brain coronal levels previously described. Confocal images were acquired by sequential scanning using a z-separation of 0.25 μm using the Leica Application Suite Advanced Fluorescence software (Leica Microsystems). Volumetric image measurements were made in the hippocampus and entorhinal cortex using Imaris software version 7.5.2 (Bitplane Inc., South Windsor, CT). For quantification of the ratio between Iba-1 or GFAP and 6E10 staining, the Iba-1 or GFAP volume was divided by the corresponding 6E10 volume for each image before calculating averages per mouse.
Thioflavin S Staining
Sections were incubated in 0.5% thioflavin S in 50% ethanol for 10 minutes, differentiated twice in 50% ethanol, and washed in PBS solution. Staining was visualized under a confocal microscope. Volumetric image measurements were made using Imaris software (Bitplane Inc.). The thioflavin S levels represent the average value obtained by the analysis of images of the hippocampus and entorhinal cortex.
Cell Culture Studies
Primary astrocytes were isolated from postnatal day 1 C57BL/6 mice. Briefly, cortex was isolated, minced, and trypsinized for 20 minutes at 37°C. Tissues were then triturated and grown in Dulbecco’s modified Eagle’s medium/F12 medium supplemented with 10% fetal bovine serum, penicillin, and streptomycin for 6 days in a humidified incubator at 37°C with 5% CO2. When cells were confluent, primary astrocytes were purified by shaking at 350 rpm for 24 hours at 37°C. Attached cells were trypsinized and cultured on slide chambers to evaluate the purity of astrocytes by staining with GFAP (astrocyte marker), Iba-1 (microglia marker), CNPase (oligodendrocyte marker), and β-tubulin (neuronal marker) antibodies or seeded on culture flasks for experiments. Cells were exposed to 1 μmol/L Aβ42 in the absence or presence of 10, 100, or 1000 nmol/L ATL for 24 hours. Some cells were incubated in the presence of 1000 nmol/L ATL plus 100 nmol/L ALX antagonist butoxycarbonyl-Phe-Leu-Phe-Leu-Phe (BOC2). Conditioned media were collected for treatments to BV2 cells, and astrocytes were homogenized with M-PER reagent containing protease and phosphatase inhibitors to collect proteins. Extracted proteins were used for Western blot analysis to measure phosphorylated-p65 NF-κB or GAPDH expression.
Murine microglia/macrophage (BV2) cells were grown in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, penicillin, and streptomycin. For Aβ phagocytosis studies, 5 × 106/mL BV2 cells were incubated with 150 nmol/L 488-labeled Aβ42 (488-Aβ42; Anaspec, Fremont, CA) and LysoTracker (Invitrogen) in conditioned media collected from primary astrocytes for 3 hours at 37°C. ALX antagonist, BOC2 (100 nmol/L), was used to avoid the effect of any residual ATL present in the conditioned media collected from primary astrocytes. Microglial Aβ phagocytosis was verified by confocal laser-scanning microscopy (Leica Microsystems). 488-Aβ42–positive cells were examined microscopically using a counting grid at ×630 magnification. In addition, YM1 expression was verified by confocal laser-scanning microscopy using anti-YM1 and anti–Iba-1. Finally, extracted proteins from BV2 cells were used for Western blot analysis to measure YM1 or GAPDH expression.
Statistical Analysis
All data are expressed as means ± SEM. The statistical evaluation of the results was performed using one- or two-way analysis of variance. After significant analyses of variance, multiple post hoc comparisons were performed using the Bonferroni’s test. Some data were analyzed using the unpaired t-test. The accepted level of significance for the tests was P < 0.05. All tests were performed using the Statistica software version 5.1 (StatSoft Inc., Tulsa, OK). All final data were presented as percentage of control (vehicle-treated samples).
Results
Aspirin-Triggered LXA4 Improves Cognition and Protects Synapses
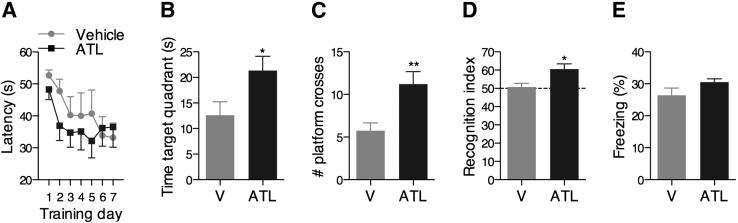
Learning and memory functions are vulnerable to several pathological processes, including AD.25 To investigate the effect of ATL on the cognitive decline associated with AD, Tg2576 mice were evaluated in cognitive tasks that rely on brain areas most affected by AD. ATL-treated mice performed significantly better than vehicle-treated mice in the hippocampal-dependent reference spatial memory version of the MWM,26 as indicated by shorter latencies to find the hidden platform in the training session (Figure 1A), increased target quadrant preference (Figure 1B), and number of platform location crosses (Figure 1C) during the probe trial. Similarly, ATL-treated mice exhibited a significant increase in the exploration of the nonfamiliar object in the cortical-dependent novel object recognition task (Figure 1D).27 However, no significant difference was found between vehicle- and ATL-treated mice in the contextual fear conditioning, which is mainly dependent on the amygdala and hippocampus (Figure 1E).28 More important, the effects of ATL on behavior were not related to overt motor impairments, because the swimming speed or total distance traveled in the MWM and the total squares crossed and rearing behavior in the open-field arena were comparable between groups (data not shown).
Figure 1.
Aspirin-triggered LXA4 reduces cognitive impairment. Learning and memory in Tg2576 mice were significantly improved after treatment with ATL. A: Each training day represents the average latency to find the hidden platform of four trials in the spatial reference version of the MWM. Time spent in the target quadrant (B) and number of platform location crosses (C) in the probe trial of the MWM. ATL-treated mice presented significantly shorter latencies to find the hidden platform during the training session and a higher preference for the target quadrant during the probe session than vehicle (V)–treated mice. D: Tg2576 mice treated with ATL exhibited a significant increase in the recognition index compared with vehicle-treated animals. The recognition index represents the percentage of the time that mice spend exploring the nonfamiliar object. E: No change in the contextual fear conditioning was found between ATL- and vehicle-treated Tg2576 mice. The values represent means ± SEM (N = 10). ∗P < 0.05, ∗∗P < 0.01.
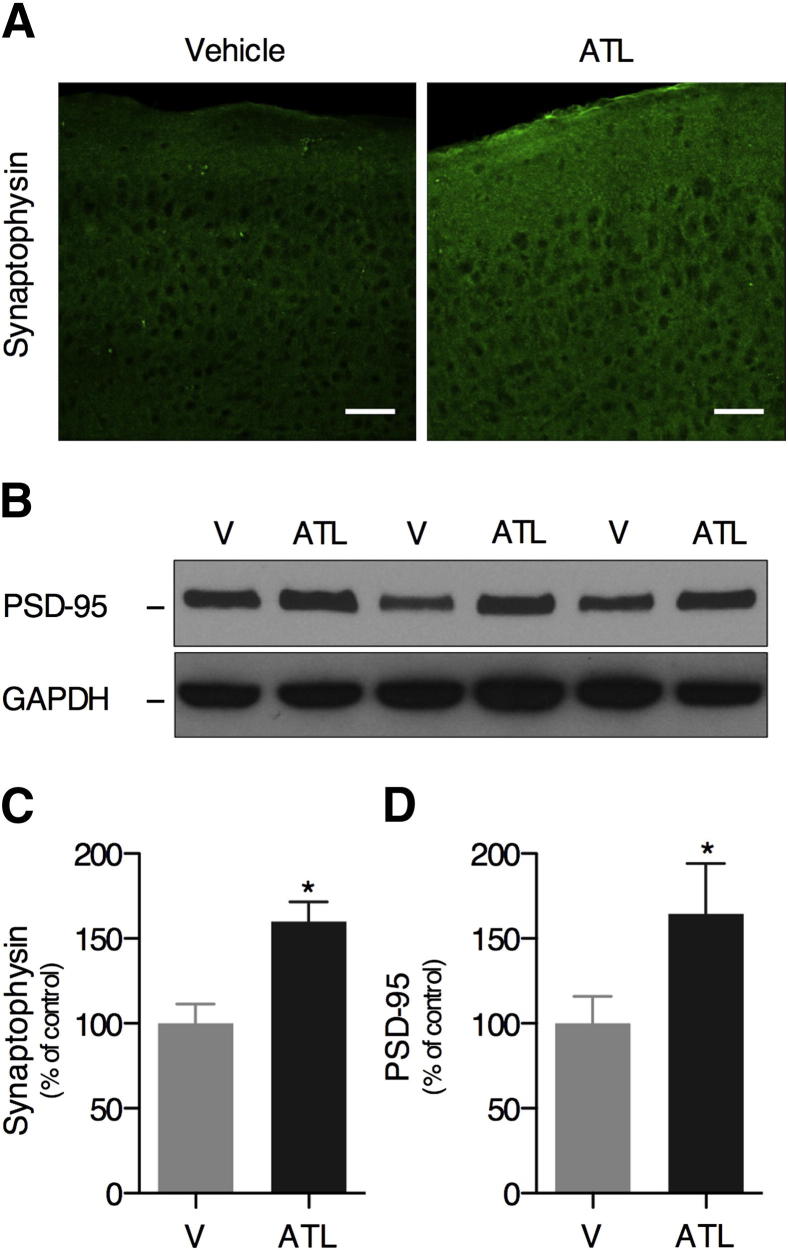
The restoration of cognitive function by the effect of ATL seems to be mediated by an overall increase in synaptic density in the hippocampus and cortex. Increased levels of presynaptic synaptophysin (Figure 2, A and C) and postsynaptic density protein 95 (Figure 2, B and D) protein were found in ATL-treated mice relative to vehicle-treated mice. These data show that the restoration of the inflammatory homeostasis by ATL leads to the increase of synaptic density and improves cognition in Tg2576 mice.
Figure 2.
Aspirin-triggered LXA4 increases the expression of synaptic proteins. Increased levels of presynaptic synaptophysin [A (IHC) and C (quantitation)] and postsynaptic density protein 95 (B and D) were found in the brains of ATL-treated mice when compared with vehicle (V)–treated mice. Representative photomicrographs were taken from mouse cortex. Scale bar = 50 μm. The values represent means ± SEM (N = 10). ∗P < 0.05.
Aspirin-Triggered LXA4 Reduces Aβ Levels through a Mechanism Independent of APP Processing
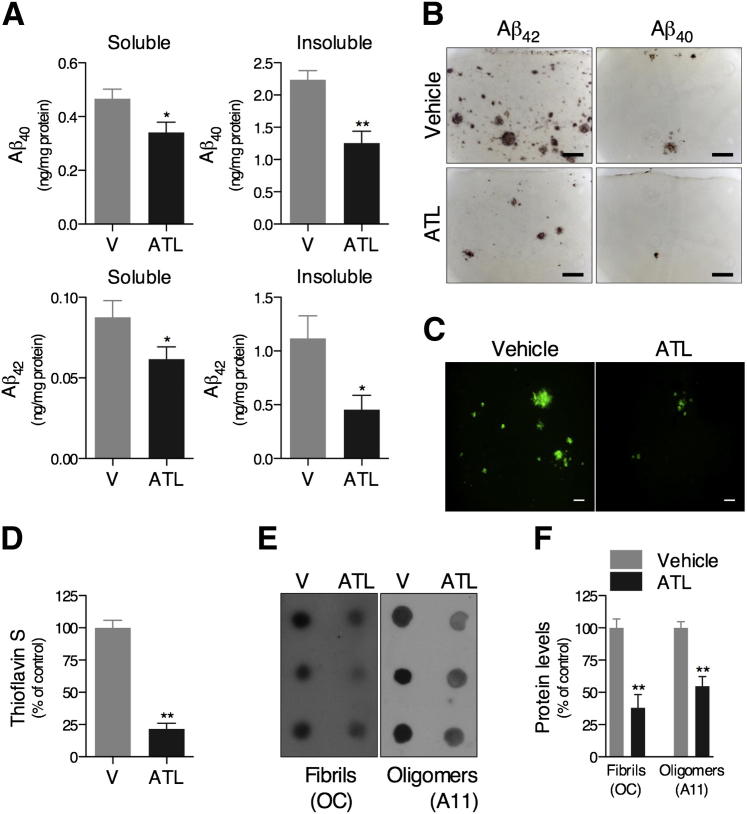
Accumulation of Aβ in the brain parenchyma as diffuse and senile plaques is a major pathological feature of AD.1 By using an ELISA, we found that levels of Aβ40 and Aβ42 were significantly reduced in both the soluble- and insoluble-detergent fractions in the brains of ATL-treated versus vehicle-treated Tg2576 mice (Figure 3A). In addition, there was diminished immunoreactivity to Aβ40 and Aβ42 antibodies (Figure 3B), and decreased thioflavin S–positive fibrillar Aβ deposits (Figure 3, C and D), in brains of ATL-treated Tg2576 mice. We also found that ATL markedly reduced the levels of both protofibrillar and fibrillar oligomers in the soluble-detergent fraction of Tg2576 mouse brains, as demonstrated by the changes in A11 and OC immunoreactivity, respectively (Figure 3, E and F).
Figure 3.
Aspirin-triggered LXA4 reduces brain Aβ levels in Tg2576 mice. Mice treated with ATL have lower levels of Aβ40 and Aβ42 peptides in both soluble- and insoluble-detergent fractions measured by ELISA (A), reduced Aβ40 and Aβ42 immunoreactivity (B), and reduced thioflavin S–positive fibrillar Aβ deposits (C and D) compared with vehicle (V)–treated mice. Representative photomicrographs were taken from the cortex. E and F: Effect of ATL on Aβ oligomer levels. Dot-blot analysis was performed using A11 and OC antibodies for protofibrillar and fibrillar Aβ oligomers, respectively, demonstrating reduced levels of both oligomeric forms in the soluble-detergent fraction of the brains from ATL-treated mice. Scale bars: 100 μm (B); 50 μm (C). The values represent means ± SEM (N = 10). ∗P < 0.05, ∗∗P < 0.01.
To elucidate the mechanism by which ATL reduced Aβ levels, we determined whether APP processing pathways were modified in Tg2576 mice. Steady-state levels of APP, α-APP cleaving enzymes ADAM10 and ADAM17, the putative β-secretase enzyme BACE1, and the C-terminal fragments of APP, C99, and C83 were unaffected by ATL, suggesting that ATL did not alter APP processing or Aβ production (Supplemental Figure S1, A and B). These results suggest that ATL might trigger changes in the Aβ levels through activation of clearance processes, which we examined by assessing the effect of ATL on the major putative Aβ clearance pathways.29–33 Interestingly, ATL did not alter the expression of ABCA1, LRP1, and APOE, nor was there a reduction in the levels of the major Aβ-degrading enzymes, IDE and neprilysin (Supplemental Figure S1, C and E). In addition, steady-state levels of ubiquitinated proteins, a critical process for proteasomal degradation,34 remained unaltered after treatment with ATL (Supplemental Figure S1, D and E).
Aspirin-Triggered LXA4 Switches the Microglia from the Classic toward the Alternative Phenotype
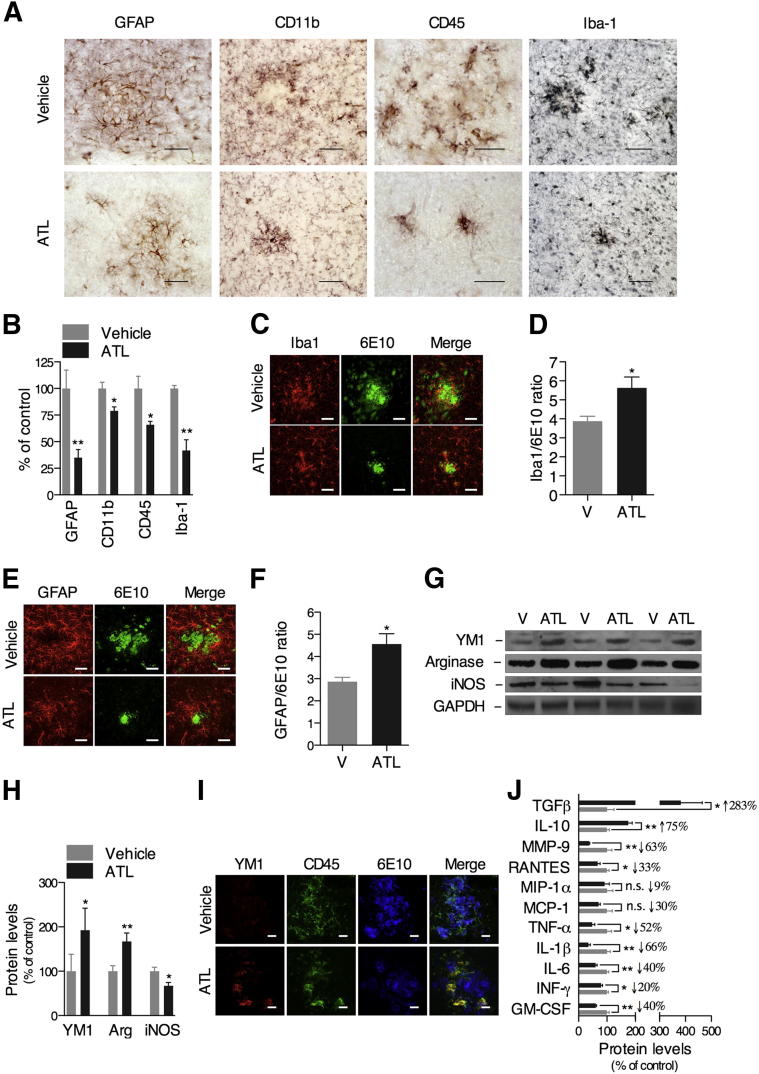
Growing evidence suggests that microglia and bone marrow–derived macrophages are capable of degrading Aβ and preventing it from accumulating in the brain.35,36 Lipoxins are critical modulators of inflammatory resolution, and have been implicated in the non-phlogistic recruitment and activation of monocytes and macrophages.18,37–40 Consequently, the activation of the lipoxin signaling pathway may facilitate the Aβ clearance through the modulation of the glial cell response. To test this hypothesis, we examined whether the effect of ATL on Aβ clearance was mediated by the modulation of the inflammatory response. On treatment with ATL, Tg2576 mice had a significant reduction in the inflammatory reaction, as evidenced by the pronounced decrease in the GFAP-positive astrocytes and CD11b-, CD45-, and Iba-1–positive microglial immunoreactivity (Figure 4, A and B). Colocalization studies demonstrated that microglia and astrocytes are directly associated with Aβ deposits (Figure 4, C and E). Surprisingly, the ratio between the immunoreactivity for Iba-1–positive microglia and 6E10-positive Aβ deposits (Figure 4D), or between GFAP-positive astrocytes and 6E10-positive Aβ deposits (Figure 4F), was significantly higher in the ATL-treated mice than vehicle-treated mice. These data indicate that ATL facilitates activation of glial cells without interfering in their recruitment to sites of Aβ deposition.
Figure 4.
Aspirin-triggered LXA4 switches microglia from the classic toward the alternative phenotype. A and B: Tg2576 mice treated with ATL exhibited a significant decrease in GFAP-positive astrocytes and CD11b-, CD45-, and Iba-1–positive microglial immunoreactivity versus vehicle-treated animals. Confocal analysis showing the colocalization of Iba-1–positive microglia (red; C) and GFAP-positive astrocytes (red; E) and with 6E10-positive Aβ deposits (green). The determination of the ratio between the immunoreactivity for Iba-1–positive microglia and 6E10-positive Aβ deposits (D) and GFAP-positive astrocytes and 6E10-positive Aβ deposits (F) demonstrated an increased cell burden in ATL-treated mice compared with vehicle (V)–treated animals. Representative photomicrographs were taken from the cortex. Representative blots (G) and quantitative results (H) of Western blot analysis showing the up-regulation of YM1 and arginase-1 and the down-regulation in iNOS expression induced by ATL treatment compared with vehicle-treated mice. Tissue amounts of GAPDH were used as loading controls. I: Confocal analysis confirming the colocalization of YM1 (red), CD45-positive microglia (green), and 6E10-positive Aβ deposits (blue) in brains of ATL-treated mice. Representative photomicrographs were taken from the cortex. J: ATL administration resulted in reduction of MMP-9, RANTES, macrophage inflammatory protein-1α, monocyte chemotactic protein-1, TNF-α, IL-1β, IL-6, interferon-γ, and GM-CSF and elevation of IL-10 and TGFβ levels. Scale bars: 50 μm (GFAP, CD11b, and Iba-1) or 25 μm (CD45) (A); 50 μm (C and E); 100 μm (I). The values represent means ± SEM (N = 10). ∗P < 0.05, ∗∗P < 0.01.
To explore mechanisms of glial cell regulation by lipoxins, we characterized the expression pattern of ALX in the mouse brain. Notably, although we detected ALX on neurons, astrocytes, and cerebrovasculature, the expression of ALX was undetectable on microglia/macrophages (Supplemental Figure S2). Thus, it is likely that ATL activates astrocytes, neurons, and/or cerebrovascular cells, which, in turn, release mediators that stimulate the recruitment of phagocytic cells to the site of Aβ deposition. To test this hypothesis, we determined the levels of molecular markers of classic and alternative activation of monocyte-macrophage cells.41–43 Of great relevance, microglia in the brains of ATL-treated Tg2576 mice presented an alternative activation phenotype, as characterized by the up-regulation in YM1 and arginase-1 and the down-regulation in iNOS expression (Figure 4, G and H). These findings were further confirmed by colocalization analysis of YM1, CD45-positive microglia, and 6E10-positive Aβ deposits, which revealed higher levels of the alternative activation marker, YM1, in the microglia of ATL-treated mice (Figure 4I). Likewise, ATL stimulated the proresolution inflammatory response by switching the mediator phenotype from generating proinflammatory to anti-inflammatory mediators, as demonstrated by the change in the levels of TGFβ, IL-10, MMP-9, RANTES, TNF-α, IL-1β, IFN-γ, IL-6, and GM-CSF (Figure 4J).
To extend these findings, we examined phagocytosis in vitro. By using conditioned media from primary astrocytes treated with Aβ42 in the absence or presence of ATL, we found that ATL was capable of potentiating the phagocytosis of 488-Aβ42 by BV2 microglial cells in a concentration-dependent manner (Supplemental Figure S3, A and C). Corroborating our in vivo studies, BV2 microglial cells also showed a more robust YM1 expression when incubated in conditioned media from astrocytes treated with ATL versus vehicle (Supplemental Figure S3, B, D, and E). More important, the effects mediated by ATL inhibited the ALX antagonist, BOC2.
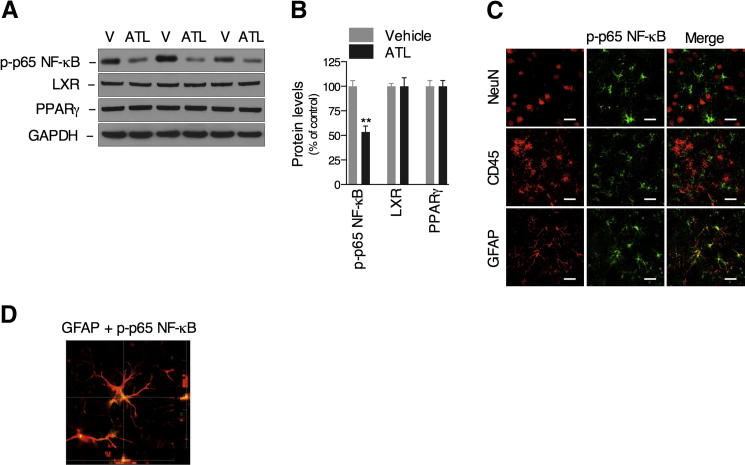
Aspirin-Triggered LXA4 Reduces the Activation of Transcription Factor NF-κB on Astrocytes
Finally, we examined the underlying molecular mechanism by which ATL promoted Aβ clearance. In this regard, the activation of transcriptional factors with particular importance for the inflammatory response was analyzed in the brains of Tg2576 mice.44–46 Our data clearly demonstrate that ATL reduces the activation of NF-κB, but not liver X receptor (LXR) and peroxisome proliferator-activated receptor γ, as designated by the decrease in the phosphorylated-p65 NF-κB levels compared with vehicle-treated mice (Figure 5, A and B). Notably, the activation of NF-κB in the Tg2576 mice was only observed in GFAP-positive astrocytes (Figure 5, C and D). Corroborating these data, ATL inhibited Aβ42-induced NF-κB activation in primary astrocytes, an effect that was diminished by ALX antagonist, BOC2 (Supplemental Figure S4). Although we cannot discard the involvement of neurons and cerebrovasculature, these data clearly suggest that stimulation of ALX in astrocytes by ATL potentiates the non-phlogistic recruitment and activation of alternative microglia, resulting in Aβ clearance.
Figure 5.
Aspirin-triggered LXA4 reduces the activation of transcriptional factor, NF-κB, on astrocytes. Representative blots (A) and quantitative results (B) of Western blot analysis demonstrating that ATL reduces the levels of phosphorylated-p65 NF-κB, but not of LXR or peroxisome proliferator-activated receptor (PPAR) γ, compared with vehicle (V)–treated mice. Tissue amounts of GAPDH were used as loading controls. C: The activation of NF-κB (green) was observed in GFAP-positive astrocytes, but not in CD45-positive microglia or NeuN-positive neurons (all red), as demonstrated by confocal analysis. Representative photomicrographs were taken from the cortex. D: Colocalization of GFAP-positive astrocytes with phosphorylated p65 NF-κB. Scale bars: 25 μm. The values represent means ± SEM (N = 10). ∗∗P < 0.01.
Discussion
Herein, we provide critical functional and molecular evidence indicating the endogenous proresolution LXA4 pathway as a potential candidate to treat AD. More important, ATL presented notable potency, because mitigation of AD-like pathological characteristics was obtained at a microgram dose of this compound versus the milligram doses of more widely used non-steroidal anti-inflammatory drugs. Mechanistically, the effects of ATL seem to be dependent on activation of ALX expressed in astrocytes, and likely on cerebrovasculature and neurons. ATL decreased activation of NF-κB and production of proinflammatory mediators, and increased levels of anti-inflammatory proteins. Remarkably, these environmental changes in the AD-like brains propitiate the activation of microglia in an alternative and phagocytic phenotype, which, in turn, results in clearance of Aβ plaques and improvement of cognition.
Accumulation of Aβ in the brain parenchyma is a major neuropathological hallmark of AD and is believed to trigger a cascade that leads to the other pathological features of AD, including synaptic loss and aberrant inflammation.47 Overproduction, altered processing, or failed clearance of Aβ may be causal factors of AD.1 Of great relevance, we demonstrated the novel findings that ATL markedly reduces Aβ deposition in AD-like brains. Notably, such an effect appears to be unrelated to changes in Aβ production, because the balance between APP proteolytic fragments, CTFβ (C99) and CTFα (C83), remained unaltered after ATL treatment. In addition, we found no changes in the expression of LRP1, APOE, IDE, and neprilysin, suggesting that LXA4 signaling is not involved in the regulation of blood-brain barrier– and protease-mediated Aβ clearance pathways. However, possible changes in degradative enzyme activity still need to be addressed.
An exacerbated inflammatory response is another important feature of AD that may trigger loss of function in cells of the central nervous system.48–50 For this reason, many studies have focused on uncovering the underlying regulatory mechanisms and strategies to down-regulate proinflammatory responses. However, recent studies showing that blockade of inflammatory responses aggravates the progression of AD raised the question about how to best manipulate the immune response to succeed in the management of neurodegenerative disorders.4,51 The discoveries that the resolution of inflammation is a highly coordinated and active process controlled by endogenous proresolving mediators and that inflammatory cells undergo classic and alternative activation highlight new potential molecular targets to modulate inflammation and treat chronic inflammatory diseases.18,41,52 Accordingly, we report the novel finding that brains of AD transgenic mice undergo dynamic modification in response to ATL, resulting in reduced NF-κB activation and switching the profile of released mediators from proinflammatory to anti-inflammatory. In addition, our studies suggest that astrocytes are key modulators in the regulation of these modifications in the cerebral milieu; however, a potential role of cerebrovascular cells and neurons cannot be disregarded. Future studies using conditional knockout strategies to selectively delete ALX gene expression in specific cells of the central nervous system are necessary to further understand the role of the LXA4 pathway in the regulation of immune responses in the diseased brain.
Microglia are the resident central nervous system immune cells that have long been recognized as rapid responders to injury and disease, playing a role in a broad range of processes, such as tissue inflammation and clearance of cellular debris.53–55 Growing evidence has strongly suggested a dichotomous role of these cells in AD. Although activation of microglia has been shown to promote Aβ clearance, excessive or dysregulated releases of proinflammatory cytokines, chemokines, and reactive oxygen species from these inflammatory cells contribute to neuronal degeneration.4,51 Such paradoxical actions are related to the plasticity of these cells in response to environmental signals, which allow them to undergo different forms of polarized activation. Cells of monocyte-macrophage lineage, including microglia, can be activated into two different states, classic and alternative.41,42,56,57 Classically activated macrophages are essentially proinflammatory, and their activation is driven by IFN-γ and Toll-like receptor ligands, which results in up-regulation of iNOS, IL-12, and major histocompatibility complex class II expression.43 Alternatively activated macrophages, however, produce higher levels of anti-inflammatory mediators, such as IL-10, and are thought to play a primary role in the resolution of inflammation and the coordination of tissue repair after the acute inflammatory response.41 In this context, because lipoxins are key modulators of inflammatory resolution and have been implicated in the non-phlogistic recruitment and activation of monocytes and macrophages, they represent a viable alternative to facilitate the Aβ clearance through the modulation of glial cell response.18,37,38 In support of this hypothesis, we found that the activation of LXA4 signaling decreases levels of TNF-α, IL-1β, interferon-γ, IL-6, GM-CSF, RANTES, and MMP-9, which have been implicated with a different degree of importance in the progression of neuroinflammation and brain degeneration in AD. In addition, IL-10 and TGFβ in the brain are increased after the treatment with ATL. Notably, it has been shown that these cytokines present potent anti-inflammatory properties and support neuronal maintenance, function, and plasticity.58 Such changes in the pattern of immune mediators released in response to ATL prompt the brain for the recruitment and activation of microglia in a non-phlogistic phenotype. Consistent with the idea that these cells may affect disease progression by modulating the buildup of Aβ through its phagocytosis, we detect a reduced accumulation of Aβ after stimulation of LXA4 signaling. More important, the reduction of Aβ leads to a robust decrease in synaptotoxicity that resulted in improvement in cognition.
Available treatment options for AD are limited, and their efficacy is minimal. Therapies under investigation involve disease-modifying strategies, although many of these are associated with prominent adverse events in humans. Therefore, discovering agents that are capable of increasing Aβ uptake by phagocytic cells is of potential therapeutic interest for AD. Given that lipoxins are a family of endogenous lipid mediators with potent anti-inflammatory and proresolution properties, they may be potential candidates for the development of novel AD therapies.
Footnotes
Supported by NIH grants, NIH/National Institute of AgingR01AG20335 (F.M.L.), Program Project Grant AG00538 (F.M.L. and D.H.C.), National Institute of Neurological Disorders and Stroke (NINDS) RO1NSO20989 (D.H.C.), and NIH/National Institute of Arthritis and Musculoskeletal and Skin DiseasesK99AR054695 (M.K.), and by Alzheimer’s Association grants IIRG-11-204855 (D.H.C.) and IIRG-91822 (D.H.C.). Antibodies and peptides were provided by the University of California, Irvine, Alzheimer’s Disease Research Center through funding from NIH/National Institute of Aging grant P50 AG16573.
Supplemental Data
Aspirin-triggered LXA4 does not affect APP processing and major mechanisms of Aβ clearance. Representative blots (A) and quantitative results (B) of Western blot analysis showing that ATL does not change levels of APP, APP C-terminal fragments C83 and C99, or APP-cleaving enzymes ADAM10, ADAM17, and BACE1 compared with vehicle (V)–treated animals. Representative Western blots (C) and quantitative analysis (E) showing that ATL is not capable of inducing changes in the expression of ABCA1, LRP1, APOE, IDE, and neprilysin. Tissue amounts of GAPDH were used as loading controls. D and E: Ubiquitin levels are unaffected by ATL treatment, when compared with vehicle-treated animals. The values represent means ± SEM (N = 10).
ALX/FPR2 is expressed on neurons, astrocytes, and cerebrovasculature, but not on microglia in the mouse central nervous system. ALX/FPR2 (green) colocalizes with the neuronal marker, NeuN, the astrocyte marker, GFAP, and the cerebrovascular marker, collagen IV, but not with the microglial marker, CD45 (all red). Representative photomicrographs were taken from Tg2576 mouse cortex (N = 10). Scale bar = 10 μm.
Aspirin-triggered LXA4 stimulates Aβ phagocytosis by alternative activated microglia. BV2 microglial cells were treated for 3 hours with 150 nmol/L 488-Aβ42 in conditioned media from primary astrocytes stimulated with 1 μmol/L Aβ42 plus vehicle, ATL (10, 100, or 1000 nmol/L) or 1000 nmol/L ATL plus 100 nmol/L ALXR antagonist, BOC2. A and B: Cells were visualized by confocal microscopy for 488-Aβ42 (green) and LysoTracker (red). A: Merged images demonstrate higher presence of Aβ on lysosomes of BV2 cells stimulated with conditioned media from astrocytes treated with 1000 nmol/L ATL versus vehicle. BV2 cells treated with conditioned media from astrocytes treated with 1000 nmol/L ATL plus 100 nmol/L BOC2 showed a reduced amount of Aβ intracellularly. B: Conditioned media from astrocytes treated with ATL also stimulated the up-regulation of YM1 (green) in the Iba-1–positive (red) BV2 cells. C: Quantification of confocal images indicates that Aβ phagocytosis was stimulated in a concentration-dependent manner by ATL, an effect significantly inhibited by ALXR antagonist, BOC2. Quantitative analysis (D) and representative Western blots (E) showing that ATL induced the YM1 up-regulation in a concentration-dependent manner in the BV2 cells. BV2 cells treated with 1000 nmol/L ATL plus 100 nmol/L BOC2-conditioned media showed reduced YM1 expression. Scale bars: 100 μm (A); 25 μm (C). The values represent means ± SEM (N = 3 to 4). ** P ≤ 0.01 versus vehicle and ††P < 0.01 versus ATL 10E3 nM.
Aspirin-triggered LXA4 suppresses p65 NF-κB activation in primary astrocytes. Primary astrocytes were stimulated with 1 μmol/L Aβ42 plus vehicle, ATL (10, 100, or 1000 nmol/L), or 1000 nmol/L ATL plus 100 nmol/L ALXR antagonist, BOC2. Representative Western blots (A) and quantitative analysis (B) showing that ATL inhibits the p65 NF-κB phosphorylation in a concentration-dependent manner. ALXR antagonist, BOC2 (100 nmol/L), significantly blocked the effect mediated by ATL. The values represent means ± SEM (N = 3). **P ≤ 0.01 versus vehicle and ††P < 0.01 versus ATL 10E3 nM.
References
- 1.Querfurth H.W., LaFerla F.M. Alzheimer’s disease. N Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 2.Akiyama H., Barger S., Barnum S., Bradt B., Bauer J., Cole G.M. Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Graeber M.B. Changing face of microglia. Science. 2010;330:783–788. doi: 10.1126/science.1190929. [DOI] [PubMed] [Google Scholar]
- 4.Lucin K.M., Wyss-Coray T. Immune activation in brain aging and neurodegeneration: too much or too little? Neuron. 2009;64:110–122. doi: 10.1016/j.neuron.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neumann H., Kotter M.R., Franklin R.J. Debris clearance by microglia: an essential link between degeneration and regeneration. Brain. 2009;132:288–295. doi: 10.1093/brain/awn109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pascual O., Ben Achour S., Rostaing P., Triller A., Bessis A. Microglia activation triggers astrocyte-mediated modulation of excitatory neurotransmission. Proc Natl Acad Sci U S A. 2012;109:E197–E205. doi: 10.1073/pnas.1111098109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tremblay M.E., Stevens B., Sierra A., Wake H., Bessis A., Nimmerjahn A. The role of microglia in the healthy brain. J Neurosci. 2011;31:16064–16069. doi: 10.1523/JNEUROSCI.4158-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Czirr E., Wyss-Coray T. The immunology of neurodegeneration. J Clin Invest. 2012;122:1156–1163. doi: 10.1172/JCI58656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prinz M., Priller J., Sisodia S.S., Ransohoff R.M. Heterogeneity of CNS myeloid cells and their roles in neurodegeneration. Nat Neurosci. 2011;14:1227–1235. doi: 10.1038/nn.2923. [DOI] [PubMed] [Google Scholar]
- 10.Koenigsknecht-Talboo J., Landreth G.E. Microglial phagocytosis induced by fibrillar beta-amyloid and IgGs are differentially regulated by proinflammatory cytokines. J Neurosci. 2005;25:8240–8249. doi: 10.1523/JNEUROSCI.1808-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mandrekar-Colucci S., Landreth G.E. Microglia and inflammation in Alzheimer’s disease. CNS Neurol Disord Drug Targets. 2010;9:156–167. doi: 10.2174/187152710791012071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Medeiros R., Rodrigues G.B., Figueiredo C.P., Rodrigues E.B., Grumman A., Jr., Menezes-de-Lima O., Jr., Passos G.F., Calixto J.B. Molecular mechanisms of topical anti-inflammatory effects of lipoxin A(4) in endotoxin-induced uveitis. Mol Pharmacol. 2008;74:154–161. doi: 10.1124/mol.108.046870. [DOI] [PubMed] [Google Scholar]
- 13.Sobrado M., Pereira M.P., Ballesteros I., Hurtado O., Fernandez-Lopez D., Pradillo J.M., Caso J.R., Vivancos J., Nombela F., Serena J., Lizasoain I., Moro M.A. Synthesis of lipoxin A4 by 5-lipoxygenase mediates PPARgamma-dependent, neuroprotective effects of rosiglitazone in experimental stroke. J Neurosci. 2009;29:3875–3884. doi: 10.1523/JNEUROSCI.5529-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haworth O., Cernadas M., Yang R., Serhan C.N., Levy B.D. Resolvin E1 regulates interleukin 23, interferon-gamma and lipoxin A4 to promote the resolution of allergic airway inflammation. Nat Immunol. 2008;9:873–879. doi: 10.1038/ni.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Svensson C.I., Zattoni M., Serhan C.N. Lipoxins and aspirin-triggered lipoxin inhibit inflammatory pain processing. J Exp Med. 2007;204:245–252. doi: 10.1084/jem.20061826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Serhan C.N. Lipoxins and aspirin-triggered 15-epi-lipoxins are the first lipid mediators of endogenous anti-inflammation and resolution. Prostaglandins Leukot Essent Fatty Acids. 2005;73:141–162. doi: 10.1016/j.plefa.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Ye R.D., Boulay F., Wang J.M., Dahlgren C., Gerard C., Parmentier M., Serhan C.N., Murphy P.M. International Union of Basic and Clinical Pharmacology, LXXIII: nomenclature for the formyl peptide receptor (FPR) family. Pharmacol Rev. 2009;61:119–161. doi: 10.1124/pr.109.001578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Serhan C.N. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu Rev Immunol. 2007;25:101–137. doi: 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- 19.Wu Y., Ye X.H., Guo P.P., Xu S.P., Wang J., Yuan S.Y., Yao S.L., Shang Y. Neuroprotective effect of lipoxin A4 methyl ester in a rat model of permanent focal cerebral ischemia. J Mol Neurosci. 2010;42:226–234. doi: 10.1007/s12031-010-9355-8. [DOI] [PubMed] [Google Scholar]
- 20.Wu J., Wang A., Min Z., Xiong Y., Yan Q., Zhang J., Xu J., Zhang S. Lipoxin A4 inhibits the production of proinflammatory cytokines induced by beta-amyloid in vitro and in vivo. Biochem Biophys Res Commun. 2011;408:382–387. doi: 10.1016/j.bbrc.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 21.Hsiao K., Chapman P., Nilsen S., Eckman C., Harigaya Y., Younkin S., Yang F., Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 22.Medeiros R., Kitazawa M., Caccamo A., Baglietto-Vargas D., Estrada-Hernandez T., Cribbs D.H., Fisher A., LaFerla F.M. Loss of muscarinic M1 receptor exacerbates Alzheimer’s disease-like pathology and cognitive decline. Am J Pathol. 2011;179:980–991. doi: 10.1016/j.ajpath.2011.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rossner M., Yamada K.M. What’s in a picture? the temptation of image manipulation. J Cell Biol. 2004;166:11–15. doi: 10.1083/jcb.200406019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Town T., Laouar Y., Pittenger C., Mori T., Szekely C.A., Tan J., Duman R.S., Flavell R.A. Blocking TGF-beta-Smad2/3 innate immune signaling mitigates Alzheimer-like pathology. Nat Med. 2008;14:681–687. doi: 10.1038/nm1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Budson A.E., Price B.H. Memory dysfunction. N Engl J Med. 2005;352:692–699. doi: 10.1056/NEJMra041071. [DOI] [PubMed] [Google Scholar]
- 26.Morris R.G., Garrud P., Rawlins J.N., O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 27.Barker G.R., Bird F., Alexander V., Warburton E.C. Recognition memory for objects, place, and temporal order: a disconnection analysis of the role of the medial prefrontal cortex and perirhinal cortex. J Neurosci. 2007;27:2948–2957. doi: 10.1523/JNEUROSCI.5289-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phillips R.G., LeDoux J.E. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- 29.Jiang Q., Lee C.Y., Mandrekar S., Wilkinson B., Cramer P., Zelcer N., Mann K., Lamb B., Willson T.M., Collins J.L., Richardson J.C., Smith J.D., Comery T.A., Riddell D., Holtzman D.M., Tontonoz P., Landreth G.E. ApoE promotes the proteolytic degradation of Abeta. Neuron. 2008;58:681–693. doi: 10.1016/j.neuron.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wahrle S.E., Jiang H., Parsadanian M., Kim J., Li A., Knoten A., Jain S., Hirsch-Reinshagen V., Wellington C.L., Bales K.R., Paul S.M., Holtzman D.M. Overexpression of ABCA1 reduces amyloid deposition in the PDAPP mouse model of Alzheimer disease. J Clin Invest. 2008;118:671–682. doi: 10.1172/JCI33622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Q., Zerbinatti C.V., Zhang J., Hoe H.S., Wang B., Cole S.L., Herz J., Muglia L., Bu G. Amyloid precursor protein regulates brain apolipoprotein E and cholesterol metabolism through lipoprotein receptor LRP1. Neuron. 2007;56:66–78. doi: 10.1016/j.neuron.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vekrellis K., Ye Z., Qiu W.Q., Walsh D., Hartley D., Chesneau V., Rosner M.R., Selkoe D.J. Neurons regulate extracellular levels of amyloid beta-protein via proteolysis by insulin-degrading enzyme. J Neurosci. 2000;20:1657–1665. doi: 10.1523/JNEUROSCI.20-05-01657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iwata N., Tsubuki S., Takaki Y., Watanabe K., Sekiguchi M., Hosoki E., Kawashima-Morishima M., Lee H.J., Hama E., Sekine-Aizawa Y., Saido T.C. Identification of the major Abeta1-42-degrading catabolic pathway in brain parenchyma: suppression leads to biochemical and pathological deposition. Nat Med. 2000;6:143–150. doi: 10.1038/72237. [DOI] [PubMed] [Google Scholar]
- 34.Marambaud P., Zhao H., Davies P. Resveratrol promotes clearance of Alzheimer’s disease amyloid-beta peptides. J Biol Chem. 2005;280:37377–37382. doi: 10.1074/jbc.M508246200. [DOI] [PubMed] [Google Scholar]
- 35.Wyss-Coray T., Lin C., Yan F., Yu G.Q., Rohde M., McConlogue L., Masliah E., Mucke L. TGF-beta1 promotes microglial amyloid-beta clearance and reduces plaque burden in transgenic mice. Nat Med. 2001;7:612–618. doi: 10.1038/87945. [DOI] [PubMed] [Google Scholar]
- 36.El Khoury J., Toft M., Hickman S.E., Means T.K., Terada K., Geula C., Luster A.D. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat Med. 2007;13:432–438. doi: 10.1038/nm1555. [DOI] [PubMed] [Google Scholar]
- 37.Maderna P., Cottell D.C., Toivonen T., Dufton N., Dalli J., Perretti M., Godson C. FPR2/ALX receptor expression and internalization are critical for lipoxin A4 and annexin-derived peptide-stimulated phagocytosis. FASEB J. 2010;24:4240–4249. doi: 10.1096/fj.10-159913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reville K., Crean J.K., Vivers S., Dransfield I., Godson C. Lipoxin A4 redistributes myosin IIA and Cdc42 in macrophages: implications for phagocytosis of apoptotic leukocytes. J Immunol. 2006;176:1878–1888. doi: 10.4049/jimmunol.176.3.1878. [DOI] [PubMed] [Google Scholar]
- 39.Maddox J.F., Serhan C.N. Lipoxin A4 and B4 are potent stimuli for human monocyte migration and adhesion: selective inactivation by dehydrogenation and reduction. J Exp Med. 1996;183:137–146. doi: 10.1084/jem.183.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Romano M., Maddox J.F., Serhan C.N. Activation of human monocytes and the acute monocytic leukemia cell line (THP-1) by lipoxins involves unique signaling pathways for lipoxin A4 versus lipoxin B4: evidence for differential Ca2+ mobilization. J Immunol. 1996;157:2149–2154. [PubMed] [Google Scholar]
- 41.Gordon S., Martinez F.O. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 42.Gordon S., Taylor P.R. Monocyte and macrophage heterogeneity. Nat Rev. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 43.Martinez F.O., Gordon S., Locati M., Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol. 2006;177:7303–7311. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- 44.Glass C.K., Saijo K. Nuclear receptor transrepression pathways that regulate inflammation in macrophages and T cells. Nat Rev Immunol. 2010;10:365–376. doi: 10.1038/nri2748. [DOI] [PubMed] [Google Scholar]
- 45.Saijo K., Crotti A., Glass C.K. Nuclear receptors, inflammation, and neurodegenerative diseases. Adv Immunol. 2010;106:21–59. doi: 10.1016/S0065-2776(10)06002-5. [DOI] [PubMed] [Google Scholar]
- 46.Bonizzi G., Karin M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25:280–288. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 47.Hardy J., Selkoe D.J. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 48.Solana R., Pawelec G., Tarazona R. Aging and innate immunity. Immunity. 2006;24:491–494. doi: 10.1016/j.immuni.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 49.Amor S., Puentes F., Baker D., van der Valk P. Inflammation in neurodegenerative diseases. Immunology. 2010;129:154–169. doi: 10.1111/j.1365-2567.2009.03225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Licastro F., Candore G., Lio D., Porcellini E., Colonna-Romano G., Franceschi C., Caruso C. Innate immunity and inflammation in ageing: a key for understanding age-related diseases. Immun Ageing. 2005;2:8. doi: 10.1186/1742-4933-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wyss-Coray T., Mucke L. Inflammation in neurodegenerative disease: a double-edged sword. Neuron. 2002;35:419–432. doi: 10.1016/s0896-6273(02)00794-8. [DOI] [PubMed] [Google Scholar]
- 52.Serhan C.N., Chiang N., Van Dyke T.E. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kreutzberg G.W. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- 54.Ransohoff R.M., Perry V.H. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol. 2009;27:119–145. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- 55.Hanisch U.K., Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- 56.Colton C.A., Wilcock D.M. Assessing activation states in microglia. CNS Neurol Disord Drug Targets. 2010;9:174–191. doi: 10.2174/187152710791012053. [DOI] [PubMed] [Google Scholar]
- 57.Jimenez S., Baglietto-Vargas D., Caballero C., Moreno-Gonzalez I., Torres M., Sanchez-Varo R., Ruano D., Vizuete M., Gutierrez A., Vitorica J. Inflammatory response in the hippocampus of PS1M146L/APP751SL mouse model of Alzheimer’s disease: age-dependent switch in the microglial phenotype from alternative to classic. J Neurosci. 2008;28:11650–11661. doi: 10.1523/JNEUROSCI.3024-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tesseur I., Zou K., Esposito L., Bard F., Berber E., Can J.V., Lin A.H., Crews L., Tremblay P., Mathews P., Mucke L., Masliah E., Wyss-Coray T. Deficiency in neuronal TGF-beta signaling promotes neurodegeneration and Alzheimer’s pathology. J Clin Invest. 2006;116:3060–3069. doi: 10.1172/JCI27341. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Aspirin-triggered LXA4 does not affect APP processing and major mechanisms of Aβ clearance. Representative blots (A) and quantitative results (B) of Western blot analysis showing that ATL does not change levels of APP, APP C-terminal fragments C83 and C99, or APP-cleaving enzymes ADAM10, ADAM17, and BACE1 compared with vehicle (V)–treated animals. Representative Western blots (C) and quantitative analysis (E) showing that ATL is not capable of inducing changes in the expression of ABCA1, LRP1, APOE, IDE, and neprilysin. Tissue amounts of GAPDH were used as loading controls. D and E: Ubiquitin levels are unaffected by ATL treatment, when compared with vehicle-treated animals. The values represent means ± SEM (N = 10).
ALX/FPR2 is expressed on neurons, astrocytes, and cerebrovasculature, but not on microglia in the mouse central nervous system. ALX/FPR2 (green) colocalizes with the neuronal marker, NeuN, the astrocyte marker, GFAP, and the cerebrovascular marker, collagen IV, but not with the microglial marker, CD45 (all red). Representative photomicrographs were taken from Tg2576 mouse cortex (N = 10). Scale bar = 10 μm.
Aspirin-triggered LXA4 stimulates Aβ phagocytosis by alternative activated microglia. BV2 microglial cells were treated for 3 hours with 150 nmol/L 488-Aβ42 in conditioned media from primary astrocytes stimulated with 1 μmol/L Aβ42 plus vehicle, ATL (10, 100, or 1000 nmol/L) or 1000 nmol/L ATL plus 100 nmol/L ALXR antagonist, BOC2. A and B: Cells were visualized by confocal microscopy for 488-Aβ42 (green) and LysoTracker (red). A: Merged images demonstrate higher presence of Aβ on lysosomes of BV2 cells stimulated with conditioned media from astrocytes treated with 1000 nmol/L ATL versus vehicle. BV2 cells treated with conditioned media from astrocytes treated with 1000 nmol/L ATL plus 100 nmol/L BOC2 showed a reduced amount of Aβ intracellularly. B: Conditioned media from astrocytes treated with ATL also stimulated the up-regulation of YM1 (green) in the Iba-1–positive (red) BV2 cells. C: Quantification of confocal images indicates that Aβ phagocytosis was stimulated in a concentration-dependent manner by ATL, an effect significantly inhibited by ALXR antagonist, BOC2. Quantitative analysis (D) and representative Western blots (E) showing that ATL induced the YM1 up-regulation in a concentration-dependent manner in the BV2 cells. BV2 cells treated with 1000 nmol/L ATL plus 100 nmol/L BOC2-conditioned media showed reduced YM1 expression. Scale bars: 100 μm (A); 25 μm (C). The values represent means ± SEM (N = 3 to 4). ** P ≤ 0.01 versus vehicle and ††P < 0.01 versus ATL 10E3 nM.
Aspirin-triggered LXA4 suppresses p65 NF-κB activation in primary astrocytes. Primary astrocytes were stimulated with 1 μmol/L Aβ42 plus vehicle, ATL (10, 100, or 1000 nmol/L), or 1000 nmol/L ATL plus 100 nmol/L ALXR antagonist, BOC2. Representative Western blots (A) and quantitative analysis (B) showing that ATL inhibits the p65 NF-κB phosphorylation in a concentration-dependent manner. ALXR antagonist, BOC2 (100 nmol/L), significantly blocked the effect mediated by ATL. The values represent means ± SEM (N = 3). **P ≤ 0.01 versus vehicle and ††P < 0.01 versus ATL 10E3 nM.