Abstract
Background:
Pre-delivery haemoglobin and serum ferritin concentrations of anaemic and non-anaemic mothers were determined, and cord blood haemoglobin and serum ferritin concentrations of their newborns were compared. This is to establish the mean values for pre-delivery haemoglobin and serum ferritin concentrations of anaemic and non-anaemic mothers and the cord blood haemoglobin and serum ferritin concentrations of their newborns at term.
Materials and Methods:
A case–control study was done involving 142 pregnant women and their newborns. They were divided into two groups - the anaemic group (n = 65) and the non-anaemic (n = 77) group. Five millilitres of blood was collected from each woman and 2 ml was collected from the cord of their newborns into ethylenediaminetetraacetic acid (EDTA) bottle and plain bottle for full blood count analysis and ferritin assay, respectively.
Results:
The mean pre-delivery haemoglobin concentrations of the women in anaemic group and non-anaemic group were 9.5 ± 1.01 g/dl and 12.15 ± 1.07 g/dl, respectively, and their mean serum ferritin concentrations were 64.45 ± 138.76 μg/l and 32.83 ± 35.36 μg/l, respectively. The mean cord blood haemoglobin concentrations for anaemic and for non-anaemic groups were 12.54 ± 2.54 g/dl and 13.44 ± 2.23 g/dl (P = 0.02), respectively, and the mean cord blood serum ferritin concentrations (non-anaemic, 69.38 ± 78.88 μg/l; anaemic, 7.26 ± 115.60 μg/l) (P = 0.00) were higher in the newborns of non-anaemic than of anaemic mothers. Significant association was found between maternal anaemia and cord blood ferritin concentrations (P = 0.025).
Conclusion:
Maternal anaemia has significant effects on cord blood haemoglobin and serum ferritin concentrations.
Keywords: Anaemia, haemoglobin, newborn babies, pregnant women, serum ferritin
INTRODUCTION
Anaemia in pregnancy is a major challenge to obstetric care in developing countries where the prevalence rate varies between 33 and 75%1,2 when compared with figures from developed countries with a prevalence rate of 14%.3 Since the prevalence of anaemia in non-pregnant women in developing countries is also high (43%),4 it is possible that many of these women were already anaemic at the time of conception.
Causes of anaemia during pregnancy in developing countries are multi-factorial. This include nutritional deficiencies (iron, folate and vitamin B12), and parasitic diseases such as malaria and hookworm infestation.5 However, micronutrient deficiency, especially iron deficiency, is believed to be the main underlying cause for anaemia in pregnancy.6 Pregnant women are particularly vulnerable to iron deficiency as a result of the increased demand for iron. The expansion of plasma volume, increase in erythropoiesis and increased demand of the foetoplacental unit for iron occur throughout gestation and can vary markedly between individuals.7
Due to haemodilution and mobilisation of iron, serum ferritin concentration in women with adequate iron stores at conception initially rises, then falls progressively by 32 weeks to about 50% pre-pregnancy levels, to rise again mildly in the third trimester.8
The placental transfer of iron from maternal plasma to the foetal circulation during pregnancy is controlled by hepcidin. When hepcidin concentrations are low, iron enters blood plasma at a high rate; when hepcidin concentrations are high, ferroportin is internalised, and iron is trapped in enterocytes, macrophages and hepatocytes.3 Though falsely high values may be found in acute and chronic inflammatory conditions, measurement of serum ferritin concentrations has been shown to be a good index of iron store.9
This is preffered to examination of bone marrow aspirates for haemosiderin, a “gold standard” for iron store.10
Iron transfer to the foetus occurs maximally after 30 weeks of gestation corresponding to the time of peak efficiency of maternal iron absorption following a considerable fall in serum ferritin level which occurs between 12 and 25 weeks of gestation.11 This probably occurs as a result of iron utilisation for expansion of the maternal and foetal red cell masses.11
Harrison and Ibeziako12 reported that red cell haemolysis caused indirectly due to Plasmodium falciparum was the main aetiologic factor of anaemia in pregnancy in Nigeria, followed by folate deficiency and haemoglobinopathy in that order. They concluded that iron deficiency was rare.
A report by Lamina13 also agreed with that of Harrison and Ibeziako that malaria is still major problem among pregnant women in malaria-endemic areas. However, in a study conducted by Van der Jagt et al.5 in 2007 in northern Nigeria, iron deficiency ranked first as a major cause of anaemia in pregnancy, malaria accounting for only 9.4%.
Whatever the cause, maternal anaemia has adverse consequences on the outcome of pregnancy as anaemia in pregnancies is associated with preterm deliveries, low birth weights, morbidity and perinatal mortality due to the impairment of oxygen delivery to placenta.14
The aim of this study was to establish the mean values for pre-delivery haemoglobin and serum ferritin concentrations of anaemic and non-anaemic mothers and to compare these values with the cord blood haemoglobin and serum ferritin concentrations of their newborns.
MATERIALS AND METHODS
A case–control study was carried out at the Lagos State University Teaching Hospital (LASUTH) Maternity Centre, Ikeja, Lagos, Nigeria, between June 2009 and February 2010. The study was approved by Ethics and Research Committee of LASUTH.
One hundred and forty-two consenting non-smoking and HIV-negative pregnant women at term were divided into two groups based on cut-off Hb concentration of 11 g/dl. All pregnant women enrolled belonged to the age range of 17-41 years. Some who were below 18 years of age were legally counseled, and consent was obtained from their mothers or husbands. The two groups consisted of 65 anaemic pregnant women (Hb < 11 g/dl) and 77 non-anaemic pregnant women (Hb ≥ 11 g/dl), respectively.15 In view of the fact that we did not have a similar study, this study was preceded by a pilot survey using 20 subjects in both groups in order to determine the mean and standard deviations of cord blood haemoglobin concentration using the formular proposed by Varkevisser et al.16
Though a sample size of 53 was obtained for each group, 100 participants were enrolled for each group. However, only 77 non-anaemic and 65 anaemic women participated fully in the study.
The newborns were grouped into two haemoglobin (Hb) concentrations, viz. non-anaemic (Hb ≥ 12.5 g/dl) and anaemic (Hb < 12.5 g/dl).17 The cut-off value for ferritin was 10 μg/l for the pregnant women and 60 μg/l for the newborns.18
The women recruited for the study booked at the beginning of second trimester. They received 200 mg elemental iron in three divided doses and 5 mg folic acid daily, which were commenced at the time of booking. Pregnant women with history of chronic illness, such as hepatitis, sickle cell disease, renal disorders, and those with obstetric complications, such as preterm labour, placenta previa, vaginal bleeding during pregnancy, pre-eclampsia, gestational diabetes and HIV infection, were excluded.
Demographic data including age, educational status, parity, cigarette smoking, alcohol consumption and obstetrical history were obtained with the aid of a questionnaire.
Blood sampling
Five millilitres of blood was collected from each woman early in the morning before labour and 2 ml was collected from the cord of their newborn into ethylenediaminetetraacetic acid (EDTA) bottle for full blood count analysis. The same amount of blood was collected from each participant into plain bottle for serum ferritin assays. Blood sample was collected immediately after the participants were admitted into the labour ward at 38 weeks of gestation. Haemoglobin concentration was measured using the Sysmex autoanalyser model KX-21N (Sysmex Corporation, Kobe, Japan) on the same day of collection, while blood for ferritin assay was centrifuged and sera separated and stored at –40°C before analysis. Ferittin assay was done using enzyme-linked immunosorbent assay (ELISA) technique. The ELISA kit was manufactured by TECO diagnostics (Anaheim, CA, USA).
Data analysis
Analyses were performed using SPSS, version 16. The descriptive data were expressed as mean ± SD. A probability value of P < 0.05 was considered to indicate statistical significance. Pearson Chi square was used for analytical assessment.
RESULTS
The data analysis of both anaemic and non-anaemic mothers and their newborns is presented in Table 1. In the anaemic group, the mean age [Table 1] was 28.98 ± 4.79 years (minimum age 17 years and maximum age 41 years). The age range with the highest frequency was 26-40 years. In the non-anaemic group, the mean age was 29.56 ± 4.22 years with a minimum age of 20 years and a maximum age of 39 years. The age range with the highest frequency was similar to that of the anaemic group.
Table 1.
Pregnant mothers’ parameters
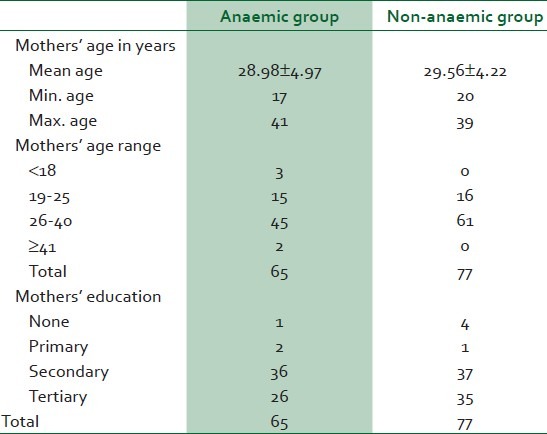
A total of 36 out of 65 (55.4%) of the anaemic pregnant women had primary and secondary education; 40% (26 of 65) had primary, secondary and tertiary education; 3% (2 of 65) had primary education only, while only 1 (1.5%) had no formal education. The pattern of education in the non-anaaemic group was similar [Table 1].
Parity of women in the non-anaemic group was also similar to that of the women in the anaemic group.
As shown in Table 2, the iron stores of both anaemic and non-anaemic women were similar as 4.6% (3 of 65) anaemic women had low iron store compared with 3.9% (3 of 77) of the non-anaemic group.
Table 2.
Pregnant mothers’ haemoglobin and ferritin parameters
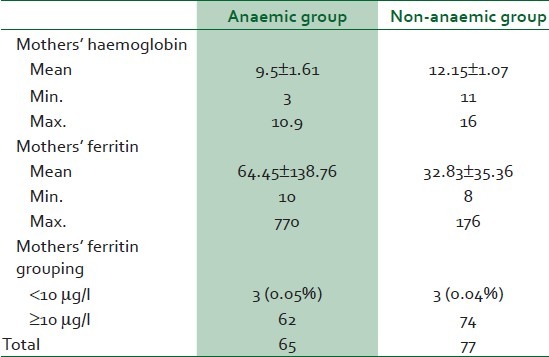
The mean Hb concentration of the anaemic women was 9.5 ± 1.01 g/dl, while their mean serum ferritin concentration was 64.45 ± 138.76 μg/l (minimum 10 and maximum 770μg/l).
The mean Hb concentration of the non-anaemic women was 12.15 ± 1.07 g/dl, while their mean serum ferritin concentration was much lower (32.83 ± 35.36 μg/l) than that of the anaemic women [Table 2]. There was no significant difference in ferritin levels in both groups as 3 of 65 (0.05%) anaemic mothers had low ferritin (<10 μg/l) while 3 of 77 (0.04%) non-anaemic mothers had low ferritin.
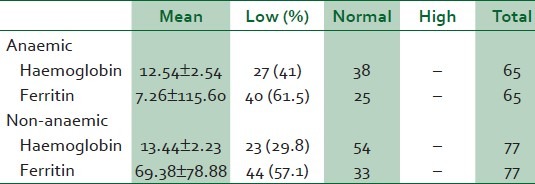
The data analysis of babies born to both anaemic and non-anaemic mothers is presented in Table 3. The mean cord blood Hb concentrations were: Non-anaemic, 13.44 ± 2.23 g/dl; anaemic, 12.54 ± 2.54 g/dl. However, the mean Hb concentrations of both groups were higher than the cut-off value of 12.5 g/dl. The mean cord blood serum ferritin (non-anaemic, 69.38 ± 78.88 μg/l; anaemic, 7.26 ± 115.60 μg/l) levels were higher in the newborns of non-anaemic than of anaemic mothers. This was significant (P = 0.025). However, majority of the newborns of both anaemic (61.5%) and non-anaemic (57.5%) mothers had low iron store.
Table 3.
Parameters of newborns of anaemic and non-anaemic mothers
DISCUSSION
With 10 μg/l as our cut-off for serum ferritin concentration to define iron deficiency, majority of the pregnant women, both anaemic and non-anaemic, had normal iron store. This is in agreement with our recent report that iron deficiency is not the major cause of anaemia in our pregnant women.19 This reports also corroborates that of Saad Dosh10 from Pakistan in which none of the samples of 40 pregnant women showed a serum ferritin concentration in the range found in iron deficiency (0-12 μg/l) and with many reports in the past which claimed that iron deficiency was rare in Nigerian pregnant women.12,20–22
That iron deficiency is rare in Nigerian pregnant women probably reflects the high iron content of Nigerian foods.21
It is, however, in contrast with a recent report from northern Nigeria on the major causes of anaemia in Nigerian pregnant women, which ranked iron deficiency as the leading cause,5 and with reports from other developing countries which also ranked iron deficiency as the leading cause.23,24
The explanation for the difference in the findings with the present study is uncertain. However, most studies comparing iron levels in pregnant mothers and their newborn babies are based on the standard tests of serum iron, total iron binding capacity and percentage transferrin saturation. Serum ferritin is a good measure of iron storage in the body, particularly of the reticuloendothelial system.
The mean serum ferritin concentration of anaemic mothers (64.45 ± 138.76) μg/l was higher than that of non-anaemic mothers (32.83 ± 35.36). This could be due to the effect of acute phase reaction which we could not establish due to limitations because one of the anaemic mothers had a very high serum ferritin level (770 μg/l) accounting for the high overall mean value.
The prevalence of foetal anaemia in the anaemic women (41.5% (27 of 65)) was higher than that in the non-anaemic group (28.9%). This observation indicates that maternal anaemia may affect foetal Hb status, and agrees with reports by Reihaneh et al25 who found significant differences between neonatal haemoglobin levels of newborns from normal and anaemic mothers and by Al-Hilli26 who also found a positive correlation between maternal haemoglobin and cord blood haemoglobin. Akaberi27 in his report on haemoglobin and serum ferritin levels in newborn babies born to anaemic Iranian women found significant differences in neonatal haemoglobin levels between normal women with serum ferritin concentration above 10 μg/l and anaemic women with serum ferritin concentration above 10 μg/l. This may further confirm that whatever the cause, anaemia in pregnancy may affect foetal haemoglobin concentration.
The prevalence of foetal anaemia obtained in this study is greater than 23.4% obtained from southern Malawi,28 but lower than 65.6% obtained from Abakaliki,29 Nigeria.
The mean cord blood haemoglobin concentration which was also lower, though not significant, in the anaemic group (12.54 ± 2.54 g/dl) than in the non-anaemic group (13.44 ± 2.23 g/dl), was slightly above the cut-off value of 12.5 g/dl used in this study. Since iron deficiency usually precedes iron-deficiency anaemia, it may mean that many of the newborns who were not anaemic at birth were born with low iron store which may not be able to sustain the babies through 6 months, accounting for the high prevalence of anaemia in 6-9-month-old children.30
As expected, the mean cord serum ferritin concentration was also observed to be significantly lower in the anaemic group (7.26 ± 115.6) than in the non-anaemic group (69.38 ± 78.88) (P = 0.025).
That majority of the newborn babies had low iron store despite the fact that majority of the women studied had normal iron store may corroborate with a report by Agarwal et al.31 who concluded that mothers with reasonably maintained ferritin and transferrin saturation levels provide sufficient iron for maintenance of cord haemoglobin, although foetal iron stores are likely depleted. We noticed that the cut-off values for serum ferritin concentrations to define iron deficiency vary with different reports. For instance, a report from Mexico used serum ferritin concentration of 30 μg/l as the cut-off and observed that 14% of their newborns had low serum serum ferritin concentration.32
In conclusion, we found out that maternal anaemia has significant effects on cord blood Hb and serum ferritin concentrations.
ACKNOWLEDGMENT
We are grateful to Mr. S. Adeyeloja who ran the serum ferritin ELISA.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Nyuke RB, Letsky EA. Etiology of anaemia in pregnancy in South Malawi. Am J Clin Nutr. 2000;72:247–56. doi: 10.1093/ajcn/72.1.247S. [DOI] [PubMed] [Google Scholar]
- 2.Ogunbode O. Anaemia in pregnancy. In: Okonofua F, Odunsi K, editors. Contemporary Obstetrics and Gynaecology For Developing Countries. Benin City Nigeria: Women's Health and Action Research Center; 2003. pp. 514–29. [Google Scholar]
- 3.Khalafallah AA, Dennis AE. Iron deficiency in pregnancy and postpartum: Pathophysiology and effect of oral versus intravenous iron therapy. J Pregnancy. 2012;2012:630519. doi: 10.1155/2012/630519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.ACC/SCN in collaboration with the International Food Policy Research Institute. Geneva: ACC/SCN; 2000. ACC/SCN. The 4th report on the world nutrition situation: Nutrition throughout the life cycle. [Google Scholar]
- 5.VanderJagt DJ, Brock HS, Melah GS, EL-Nafaty AU, Crossey MJ, Glew RH. Nutritional factors associated with anaemia in pregnant women in Northern Nigeria. J Health Popul Nutr. 2007;25:75–81. [PMC free article] [PubMed] [Google Scholar]
- 6.van den Broek NR, Letsky EA. Aetiology of anaemia in Southern Malawi. Am J Clin Nutr. 2000;72:2473–565. doi: 10.1093/ajcn/72.1.247S. [DOI] [PubMed] [Google Scholar]
- 7.van den Broek N. Anaemia and micronutrient deficiencies. Br Med Bull. 2003;67:149–60. doi: 10.1093/bmb/ldg004. [DOI] [PubMed] [Google Scholar]
- 8.Asif N, Hassan K, Mahmud S, Abbass Zaheer H, Naseem L, Zafar T, et al. Comparison of serum ferritin levels in three trimesters of pregnancy and their correlation with increasing gravidity. Int J Pathol. 2007;5:26–30. [Google Scholar]
- 9.van den Broek NR, Letsky EA, White SA, Shenkin A. Iron status in pregnant women: Which measurements are valid? Br J Heamatol. 1998;103:817–24. doi: 10.1046/j.1365-2141.1998.01035.x. [DOI] [PubMed] [Google Scholar]
- 10.Saad Dosh BA. Relationship of iron and cord blood iron stores. Kufa Med J. 2012;1:76. [Google Scholar]
- 11.Allen LH. Anemia and iron deficiency: Effects on pregnancy outcome. Am J Clin Nutr. 2000;71:1280S–4. doi: 10.1093/ajcn/71.5.1280s. [DOI] [PubMed] [Google Scholar]
- 12.Harrison KA, Ibeziako PA. Maternal anaemia and foetal birth weight. J Obstet Gynaecol Br Commonw. 1973;80:798–804. doi: 10.1111/j.1471-0528.1973.tb11221.x. [DOI] [PubMed] [Google Scholar]
- 13.Lamina MA. Prevalence of anaemia in pregnant women attending the antenatal clinic in a Nigerian University Teaching Hospital. Niger Med Pract. 2003;44:39–42. [Google Scholar]
- 14.Idowu OA, Mafiana CF, Sotiloye D. Anaemia in pregnancy: A survey of pregnant women in Abeokuta, Nigeria. Afr Health Sci. 2005;5:295–9. doi: 10.5555/afhs.2005.5.4.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.A guide for Programme Managers. Geneva: World Health Organisation; 2001. World Health Organisation. Iron Deficiency Anaemia Assessment, Prevention and Control. [Google Scholar]
- 16.Varkevisser CM, Pathmanathan I, Brownlee A. Designing and conducting health systems research projects. Ottawa, Canada: International Development Research Centre; 1991. pp. 213–6. [Google Scholar]
- 17.Kalanda BF, van Buuren S, Verhoeff FH, Brabin BJ. Anthropometry of foetal Growth in Rural Malawi in Relation to Maternal Malaria and HIV. Arch Dis Child Fetal Neonatal Ed. 2005;90:F161–5. doi: 10.1136/adc.2004.054650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ashajyothi MS, Raghavendra R, Jeffrey D, Long JA, Michael KG. The assessment of newborn iron stores at birth. A review of the literature and standards for ferritin concentrations. Neonatology. 2007;92:73–82. doi: 10.1159/000100805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adediran A, Gbadegesin A, Adeyemo TA, Akinbami AA, Akanmu AS, Osunkalu V, et al. Haemolobin and ferritin concenrations of pregnant women at term. Obstet Med. 2011;4:152–5. doi: 10.1258/om.2011.110033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fleming AF. Iron status of anaemic pregnant Nigerians. J Obstet Gynaecol Br Commonw. 1969;76:1013–7. doi: 10.1111/j.1471-0528.1969.tb09469.x. [DOI] [PubMed] [Google Scholar]
- 21.Abudu OO, Macaulay K, Oluboyede OA. Serial serum ferritin and other hematological parameters in normal Nigerian primigravidae. Int J Gynaecol Obstet. 1988;26:33–9. doi: 10.1016/0020-7292(88)90193-2. [DOI] [PubMed] [Google Scholar]
- 22.Nnatu SS, Oluboyede AO. Serum ferritin values in Nigerian pregnant women. Int J gynaecol Obstet. 1986;24:133–6. doi: 10.1016/0020-7292(86)90007-x. [DOI] [PubMed] [Google Scholar]
- 23.Okeke PU. Anaemia in pregnancy-is it a persisting public health problem in porto Novo-Cape Verde? Res J Med Sci. 2011;5:193–9. [Google Scholar]
- 24.Kalaivani K. Prevalence and consequences of anaemia in pregnancy. Indian J Med Res. 2009;130:627–33. [PubMed] [Google Scholar]
- 25.Reihaneh H, Norimah AK, Poh BK, Firoozehchian F, Raheleh H, Akaberi A. Haemoglobin and serum ferritin levels in newborn babies born to anaemic Iranian women: A cross-sectional study in an Iranian hospital. Pakistan J Nutr. 2010;9:562–6. [Google Scholar]
- 26.Al-Hilli M. The effect of maternal anaemia on cord blood haemoglobin and newborn birth weight. Karbala J Med. 2010;2:8–9. [Google Scholar]
- 27.Akaberi A. Haemoglobin and serum ferritin levels in neworn babies born to anaemic Iranian women. Pakistan J Nutrition. 2010;9:562. [Google Scholar]
- 28.Brabin BJ, Kalanda BF, Verhoeff FH, Chimsuku LH, Broadhead RL. Risk factors for fetal anaemia in a malarious area of Malawi. Ann Trop Paediatr. 2004;24:311–21. doi: 10.1179/027249304225019136. [DOI] [PubMed] [Google Scholar]
- 29.Uneke CJ, Iyare FE, Sunday-Adeoye I, Asiegu OG, Nwosu KO, Ajayi JA. Effects of maternal plasmodium falciparum malaria, anemia and HIV infection on fetal hemoglobin levels in Nigeria. Internet J Gynecol Obstet. 2009. p. 12. Available from: http://www.ispub.com/journal/the-internet-journal-ofgynecology-and-obstetrics/volume-12-number-1/effects-ofmaternal-plasmodium-falciparum-malaria-anemia-and-hivinfection-on-fetal-hemoglobin-levels-in-nigeria.html#sthash.NXd5Hlzo.dpuf .
- 30.Chapparo CM. Setting the stage for child health and development: Prevention of iron deficiency in early infancy. J Nutr. 2008;138:2529–33. doi: 10.1093/jn/138.12.2529. [DOI] [PubMed] [Google Scholar]
- 31.Agrawal RM, Tripathi AM, Agarwal KN. Cord blood haemoglobin, iron and ferritin status in maternal anaemia. Acta Paediatr Scand. 1983;72:545–8. doi: 10.1111/j.1651-2227.1983.tb09768.x. [DOI] [PubMed] [Google Scholar]
- 32.Jairne-Perez JC, Lherrera-Gaza J, Gomez-Almaguer D. Suboptimal foetal iron acqusition under a maternal environment. Arch Med Res. 2005;36:598–2002. doi: 10.1016/j.arcmed.2005.03.023. [DOI] [PubMed] [Google Scholar]


