Abstract
Background:
The objective was to determine the risk factors, ulcer grade, and management outcome of patients with diabetic foot ulcers (DFU) managed in a tropical tertiary hospital.
Materials and Methods:
This is a prospective observational study of all consecutive diabetes mellitus (DM) patients with DFU admitted in the University of Benin Teaching Hospital, Benin City, Nigeria over a 26-month period. Data documented included age, gender, type of DM, duration of DM, risk factors of DFU, duration of DFU, Wagner's ulcer grade, and the blood glucose at presentation and management outcome.
Results:
Thirty-four (55.7%) of the 61 study subjects were females. Their mean age was 56.29±12.71 years. 85.2% had type 2 DM. 13.1% of the patients were newly diagnosed diabetic at presentation. The mean duration of DM was 7.8±6.98 years. The mean duration of DFU was 46.09±47.82 days and the casual blood glucose level at presentation was 18.41±9.31mmol/l. Risk factors of DFU included spontaneous blisters (52.46%), peripheral vascular disease (44.3%), peripheral neuropathy (42.6%), and visual impairment (21.3%). The common ulcer grades were IV (44.3%) and III (36.1%).The amputation rate was 52.2% while the mortality rate was 14.3%.The baseline ulcer grade was significantly associated with the risk of lower extremity amputation, and the odds ratio was 2.36 (95% 1.06-5.21).
Conclusions:
Spontaneous blisters, peripheral vascular disease, peripheral neuropathy,and visual impairment are common risk factors of DFUs. Many of our patients with DFUs presented with grade IV and V ulcers with the resultant high rate of lower extremity amputations (LEAs). Early presentation and treatment of DFUs will reduce LEAs.
Keywords: Diabetic foot ulcer, lower extremity amputation, Wagner's grading
INTRODUCTION
Diabetes mellitus (DM) is one of the leading causes of non-traumatic lower extremity amputations (LEA) worldwide.1 Diabetic foot ulcers (DFUs) account for prolonged hospital stay with its attendant spiralling of hospital bills. Foot lesions in DM are associated with significant morbidity and mortality2–6 yet they are one of the most preventable long–term complications of DM. Prevalence rates of DM foot lesions vary from 0.9% to 8.3% in Nigeria.6 Early diagnosis and presentation to hospital for prompt treatment of DFU is capable of reducing the significant morbidity and mortality associated with this condition.
There is shortage of trained diabetes care specialists for the ever-increasing population of diabetic patients in primary and secondary health care centres. Most patients will seek treatment from health care providers with little or no training in managing diabetic foot ulcers. Availability and affordability of facilities for rehabilitation of amputees are scarce in Nigeria. Hence there is the need to identify the risk factors for diabetic foot ulcer, factors associated with LEA, and management outcomes of patients with DFUs in order to put preventive measures in place that will reduce burden of DFU and the incidence of LEA.
MATERIALS AND METHODS
This is a prospective study. Patients admitted into the Medical Wards of the University of Benin Teaching Hospital, Benin City with diagnosis of diabetic foot ulcer between December 2007 and February 2010 were consecutively recruited into the study after obtaining informed consent. Data documented after taking a detailed history and doing a clinical examination included age, gender, type of DM, duration of DM, duration of foot ulcer before presentation to hospital, ulcer grade at presentation using Wagner's grading7 (this system was used for ulcer classification as in most Nigerian studies for ease of comparison), blood glucose at presentation and the presence of hypertension, risk and precipitating factors of DFU, history of foot care and footwear education, season when DFU occurred, and findings on plain X-rays of the feet were documented. The outcome of treatment of diabetic foot ulcers was also documented. This could be any of the following: death, discharged (healed or healing ulcers), lower extremity amputation: major LEA such as below knee amputation or minor LEA such as Ray's amputation; discharged against medical advice (DAMA) because of lack of funds for surgery/continued inpatient treatment or/and refusal of surgery. Patients with incomplete data such as age and duration of diabetes mellitus, and those whose treatment outcomes could not be ascertained because of missing medical records (case notes) were excluded from the analysis.
Patients with DFUs are managed by a multidisciplinary team comprising the endocrinologists, orthopaedic surgeons, ophthalmologists, nutritionists and nurses. Patients are admitted under the care of the endocrinologists who evaluate and investigate the patients. They control the hyperglycemia and any associated comorbid states like hypertension. They also treat foot infections and anemia. Wound debridement, incision and drainage, and amputations were done by orthopaedic surgeons. Detailed eye examinations were done by the ophthalmologists.
For the purposes of this study, peripheral neuropathy was defined as diminished or lack of perception of touch/pain stimuli, and loss of joint position sense (assessed using a 128 mHz tuning fork). Peripheral vascular disease was defined as the presence of diminished or absent lower limb arterial pulsation on palpation. Visual impairment was defined as diminished vision resulting from refractive errors, cataracts or diabetic retinopathy, assessed by direct fundoscopy. LEA was categorized as follows: major LEA: below knee amputation, above knee amputation, minor LEA: ray amputation. Diabetic foot ulcer was defined as any full thickness ulcer below the ankle in any person with diabetes mellitus.
Statistical analysis
Data were analysed using the Statistical Package for Social Sciences (SPSS) version 16 and Stata/IC 11.2 (Stata Corp., TX) 2009. Continuous variables were expressed as mean ± SD; categorical variables were described as proportions. Comparison of means was done using a t-test for continuous data and a chi-square test for categorical data. Logistic regression analysis was performed to examine the association of some risk factors for LEA (age, sex, duration of diabetes mellitus, and type of DM, ulcer grade, ulcer duration, peripheral vascular disease, peripheral neuropathy, and hypertension) with LEA. The level of statistical significance was set at P < 0.05.
RESULTS
Eighty-four patients with diabetic foot ulcers were seen during the study period. A total of 61 patients had sufficient data for analysis. Thirty four (55.7%) of these were females. Fifty two (85.2%) had type 2 DM. Eight (13.1%) patients were newly diagnosed diabetic at presentation during the study. The baseline characteristics of the study subjects are summarized in Table 1. Only one patient acknowledged receiving any form of foot care and footwear education. Of the 54 patients who could give an accurate duration of onset of ulceration, 8(14.8%) presented to hospital within 1 week of onset of foot ulceration, 15 (27.8%) within 2 weeks, 24 (44.4%) within 3 weeks and 25 (46.3%) within 4 weeks. Ten percent of the patients had presenting casual blood glucose levels less than 6.1 mmol/l while 60% of them had blood glucose level greater than 13.9 mmol/l at presentation. The risk factors and comorbidites of diabetic foot ulcers in our patients are documented in Table 2. Anemia (PCV < 30%) was present in 47(77%) patients and leucocytosis (WBC > 11,000) was noted in 22(36%) patients. The preceding events of DFU are summarized in Table 3. Foot ulcerations occurred spontaneously in 32 (50.8%) of cases. The radiograph of the feet showed evidence of one or more of osteomyelitis, osteopenia, and osteolysis in 21 (34.4%). Subcutaneous emphysema was documented in 12 (19.7%). The frequency of the grades of the ulcers at presentation is represented in Figure 1. Grade IV was the most common ulcer grade at presentation. Results of logistic regression Tables 4 and 5 showed that the baseline ulcer grade was significantly associated with the risk of LEA; the odds ratio was 2.36 (95% 1.06- 5.21). The odds ratio for PN was 1.71 though this was not significant. Gender, type of diabetes mellitus, PVD, and hypertension were not significantly associated with the risk of amputation. The frequency of the different outcome measures is depicted in Figure 2. All cases of DAMA were females.
Table 1.
Characteristics of Nigerians with diabetic foot ulcer
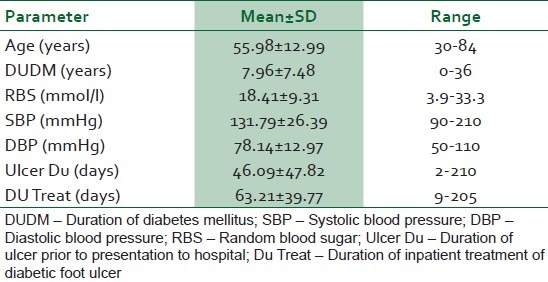
Table 2.
Frequency of risk factors and co-morbidities of diabetic foot ulcers
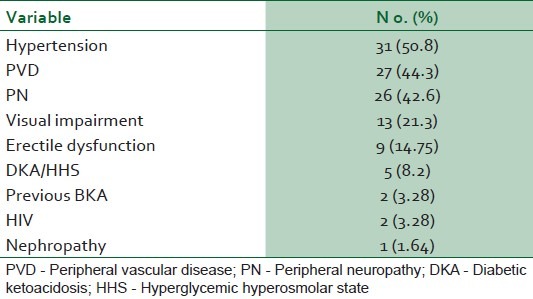
Table 3.
Frequency of preceding events of diabetic foot ulcers

Figure 1.
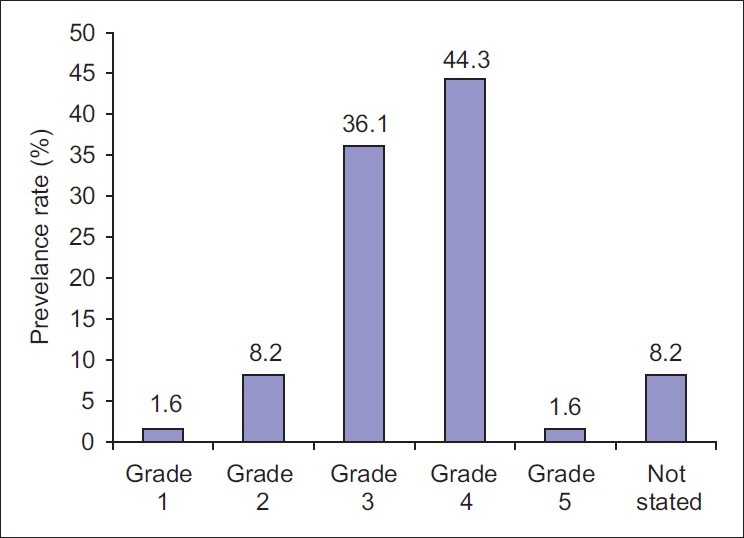
Frequency of Wagner's ulcer grade of diabetic foot ulcers among study subjects
Table 4.
The association between the Wagner's grade of ulcer and limb amputation
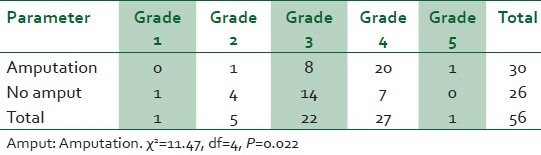
Table 5.
Logistic regression analysis – Strength of association of some risk factors with risk of LEA in 61 patients with diabetic foot ulcer
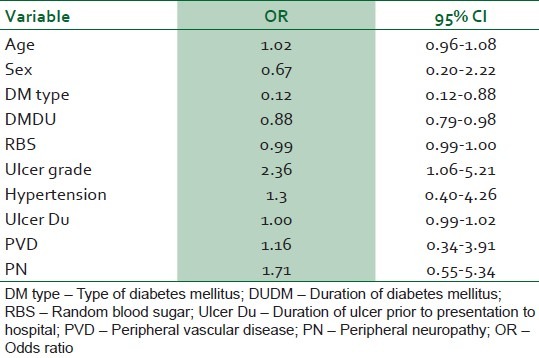
Figure 2.
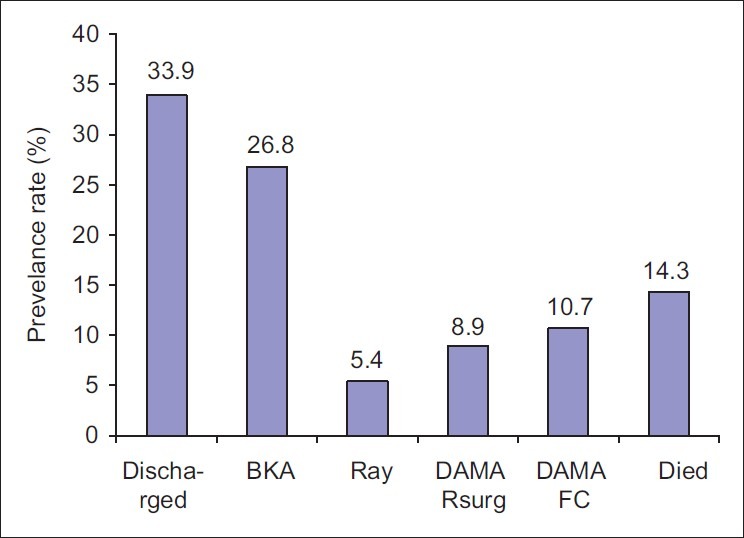
Frequency of treatment outcomes of diabetic foot ulcers among study subjects. DAMA Rsurg: discharged against medical advice because patient refused surgery (amputation); DAMA FC: Discharged against medical advice because patient lack fund for further treatment. BKA: Below knee amputation
Seasonal variation: A total of 43 patients with diabetic foot ulcer were seen during the raining season (April to October) giving an average of 6 cases per month while 39 patients with DFU were seen during the dry season (November to March) with an average of 8 cases per month. Peak admission of 13 cases was recorded in December and 11 cases in July.
DISCUSSION
Our study showed that the grade of ulcer at presentation was significantly associated with lower extremity amputation. A total of 45.9% of our patients with DFUs presented to the hospital with Wagner grade IV and V ulcers with the resultant need for some form of amputation. The amputation rate was 32/61 (52.5%); this could have been higher considering the 8.9% of patients that refused surgery. This late presentation was further confirmed by the fact that less than 10% of our patients presented to hospital within 7 days of developing a foot ulcer. Ulcers were present for up to 3 weeks in over 50% of patients before they came to the hospital for treatment. Similar presentation with limb threatening ulcers was reported by Dagogo-Jack2 in Port Harcourt, Nigeria. Ogbera et al.8 reported that grade II and III Wagner lesions were the most frequently noted grades of foot ulceration in Lagos while Unachukwu et al.9 reported grade III as the most common in Port Harcourt. Our patients thus appear to have the worst grades of foot lesions in Nigeria. Lagos and Port Harcourt are more cosmopolitan and economically more endowed than Benin City. Patients with DFU in these locales are thus more likely to report early to hospitals for treatment of their DFUs when compared to patients in Benin City.
Late presentation to hospital may result from ignorance of diabetes (13.1% of our patients with foot ulcer were previously undiagnosed of diabetes) and its complications, self care at home, patronage of chemist/pharmacist, lack of funds for hospital management, and fear of limb amputation if they came to the hospital. There is also shortage of trained Diabetes care specialists in our practice locale. Most patients will seek treatment from health care providers with little or no training in managing diabetic foot ulcers. Delays in referring patients to Diabetes care specialists also contribute to late presentation.
The study also documented a high level of poor short-term diabetes control (casual blood glucose < 6.1 mmol/l) among our patients at the point of entry with about (60%) of them having casual blood glucose above 13.8mmol/l. Five (8.2%) of the patients were managed for diabetic ketoacidosis (DKA). In addition to poor DM control delaying wound healing and providing an environment for infection to thrive, mortality can result from DKA in these poorly controlled DM patients. Glycated haemoglobin A1c (a better index of long-term glycemic control) was not available in our centre during the study period. There is research-based evidence that there is substantial increase in the risk of LEA associated with glycemia in persons with DM.10–12 Miyajima et al.13 found that blood glucose control was poor in all patients who had major amputations and their mean HbA1c was higher than those who had minor or no amputations. There was no association between casual blood glucose at presentation and LEA in this study. The odds ratio of the association between random blood sugar and LEA risk was 0.99 (95% CI 0.99-1.00). This is consistent with the finding in the UK Prospective Diabetes Study that blood glucose control alone had no significant impact on the risk of macro vascular complications in patients with type 2 diabetes.14
Spontaneous blisters accounted for 51% of DFUs. Ogbera et al.8 and Eregie and Edo15 have also noted that spontaneous blisters were the most common preceding events for DFUs. Ill-fitting foot wears in patients with PN may results in foot ulcerations in patients with insensate feet. Puncture injuries to the feet while walking bare-footed and thermal injury resulting from application of hot compress to numb feet are easily preventable preceding events for DFUs in our locale. Diabetes care givers need to educate our DM patients against the harmful practices of walking unshod and the application of hot compress to numb feet.
It was observed that a monthly average admission of DFUs was higher in the dry season. It is during this period that patients are more likely to walk bare-footed both out-doors and indoors thus increasing the risk of puncture injuries and some cases of DFUs resulting from “cracking” of the neuropathic feet (anhidrosis of the feet). This finding is in keeping with previous reports of the occurrence of DFUs and/or nontraumatic amputations of the lower limbs in the harmattan season16 and in the warm weathers of summer17 and spring.18
Visual impairment in the form of cataracts and retinopathy were common in our patients.8,19 Many patients often ascribe their visual impairment to normal aging process while others are reluctant to accept cataract extraction.
Anemia was common in our patients with DFUs as in previous reports.8,20 This could result from foot sepsis, malnutrition (poor dietary intake in ill patients) and DM nephropathy.8,21 Anemia delays wound healing and must be corrected.22 Only 30% of the anemic patients were transfused with blood. Others were given only hematinics. Some refused blood transfusion for religious or personal reasons.
In conclusion, our study showed that spontaneous blisters, PVD, PN, and visual impairment are common risk factors of DFUs in our locale. A significant proportion of our patients with DFUs presents late to tertiary care hospital, most commonly with grades IV ulcers. Of all factors studied, baseline ulcer grade has the strongest impact for the risk of LEA.
It is recommended that increasing public awareness of DFUs and encouraging patients with DFUs to present early for treatment will reduce LEAs in our environment.
LIMITATIONS OF THIS STUDY
Peripheral vascular disease was liberally defined based on the diminished or absent pedal pulsations assessed by palpation rather than on Doppler sonographic evaluation of the vessels. Peripheral sensory neuropathy was assessed with Semmes-Weinstein monofilament wire and a 128-millihertz tuning fork. A biothesiometer provides a more objective assessment of vibratory perception. The nonavailability of Doppler vascular sonography and biothesiometer at our hospital may have resulted in an underestimation of PVD and PN respectively in this study. The Wagner classification used in this study has its drawbacks. The system is based on ulcer description and depth. The presence of ischemia and infection is disregarded in this system of classification but these factors have significant impact on the outcome of treatment of DFUs.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Moses SE, Klein BE. The prevalence and incidence of lower extremity amputation in a diabetic population. Arch Intern Med. 1992;152:610–6. [PubMed] [Google Scholar]
- 2.Dagogo-Jack S. Pattern of diabetic foot ulcers in Port -Harcourt, Nigeria. Pract Diab Digest. 1991;2:75–80. [Google Scholar]
- 3.Tan MH, Gwee HM, Yeo PP, Lim P, Bose K. Diabetic amputees in Singapore. Tohoku J Exp Med. 1983;141:Suppl 575–82. doi: 10.1620/tjem.141.suppl_575. [DOI] [PubMed] [Google Scholar]
- 4.Edo AE, Eregie A. Pattern of presentation and outcomes of treatment of diabetic foot ulcers in Benin City, Nigeria. Niger J Gen Pract. 2006;7:19–22. [Google Scholar]
- 5.Akanji AO, Adetuyibi A. The pattern of presentation of foot lesions in Nigerian diabetic patients. West Afr J Med. 1990;9:1–5. [PubMed] [Google Scholar]
- 6.Ehusani FE, Giwa SO, Ohwovoriole AE. A retrospective study of diabetic foot lesions in Lagos. Nig J Int Med. 1999;2:10–2. [Google Scholar]
- 7.Wagner FW., Jr The dysvascular foot; a system for diagnosis and treatment. Foot Ankle. 1981;2:64–122. doi: 10.1177/107110078100200202. [DOI] [PubMed] [Google Scholar]
- 8.Ogbera OA, Osa E, Edo A, Chukwum E. Common clinical features of diabetic foot ulcers: perspectives from a developing nation. Int J Low Extrem Wounds. 2008;7:93–8. doi: 10.1177/1534734608318236. [DOI] [PubMed] [Google Scholar]
- 9.Unachukwu C, Babatunde S, Ihekwaba A. Diabetes, hand and/or foot ulcers: a cross-sectional hospital-based study in Port Harcourt, Nigeria. Diabetes Res Clin Pract. 2007;75:148–52. doi: 10.1016/j.diabres.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 10.Nyamu PN, Otieno CF, Amayo EO, Mcligeyo SO. Risk factors and prevalence of diabetic foot ulcers at Kenyatta National Hospital, Nairobi. East Afr Med J. 2003;80:36–43. doi: 10.4314/eamj.v80i1.8664. [DOI] [PubMed] [Google Scholar]
- 11.Aldler AL, Erqou S, Lima TA, Robinson AH. Association between glycated haemoglobin and the risk of lower extremity amputation in patients with diabetes mellitus-review and meta-analysis. Diabetologia. 2010;53:840–9. doi: 10.1007/s00125-009-1638-7. [DOI] [PubMed] [Google Scholar]
- 12.Hokkam EN. Assessment of risk factors in diabetic foot ulceration and their impact on the outcome of the disease. Prim Care Diabetes. 2009;3:219–24. doi: 10.1016/j.pcd.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 13.Miyajima S, Shirai A, Yamamoto S, Okada N, Matsushita T. Risk factors for major limb amputations in diabetic foot gangrene patients. Diabetes Res Clin Pract. 2006;71:272–9. doi: 10.1016/j.diabres.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–53. [PubMed] [Google Scholar]
- 15.Eregie A, Edo AE. Factors associated with diabetic foot ulcers in Benin City, Nigeria. Niger Med J. 2008;49:9–11. [Google Scholar]
- 16.Akanji AO. Two unusual predisposing factors for diabetic pedal ulceration in Nigerians. Trop Geogr Med. 1990;42:83–6. [PubMed] [Google Scholar]
- 17.AL-Qattan MM. The ‘Friday Mass’ burns of the feet in Saudi Arabia. Burns. 2000;26:102–5. doi: 10.1016/s0305-4179(99)00097-2. [DOI] [PubMed] [Google Scholar]
- 18.Armstrong DG, Lavery LA, van Houtum WH, Harkless LB. Seasonal variations in lower extremity amputation. J Foot Ankle Surg. 1997;36:146–50. doi: 10.1016/s1067-2516(97)80062-2. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention (CDC). Self-reported visual impairment among persons with diagnosed diabetes --- United States, 1997--2010. MMWR Morb Mortal Wkly Rep. 2011;60:1549–53. [PubMed] [Google Scholar]
- 20.Ekpebegh CO, Iwuala SO, Fasanmade OA, Ogbera AO, Igunbor O, Ohwovoriole AE. Diabetes foot ulceration in a Nigeria hospital: in-hospital mortality in relation to the presenting demographic, clinical and laboratory features. Int Wound J. 2009;6:381–5. doi: 10.1111/j.1742-481X.2009.00627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Almoznino-Sarafian D, Shteinshnaider M, Tzur I, Bar-Chaim A, Iskhakov E, Berman S, et al. Anemia in diabetic patients at an internal medicine ward: clinical correlates and prognostic significance. Eur J Intern Med. 2010;21:91–6. doi: 10.1016/j.ejim.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Harwant S, Doshi HK, Moissinac K, Abdullah BT. Factors related to adverse outcome in inpatients with diabetic foot. Med J Malaysia. 2000;55:236–41. [PubMed] [Google Scholar]


