Abstract
Osmotic demyelination syndrome resulting from postpartum hypernatremia is a recently described entity wherein young women present with hypernatremic encephalopathy and white matter hyperintensities along with quadriparesis from rhabdomyolysis. It is an acute monophasic condition with acute hypernatremia occurring during puerperium with good recovery in majority of the patients with treatment. To the best of our knowledge, recurrent postpartum hypernatremia with encephalopathy, osmotic demyelination, and rhabdomyolysis has not been described. We present a young lady who had two episodes of reversible postpartum hypernatremic encephalopathy with rhabdomyolysis. Cerebral magnetic resonance imaging (MRI) before treatment revealed osmotic demyelination on both occasions. During first admission MRI revealed hyperintensities in internal capsule and corpus callosum, and at second admission revealed more extensive white matter hyperintensity, which simulated the ‘wine glass’ appearance.
Keywords: Hypernatremia, magnetic resonance imaging, osmotic demyelination, postpartum, ‘Wine-Glass’ sign
Introduction
Neurological manifestations of hypernatremia consist of varying degrees of encephalopathy, seizures, rhabdomyolysis, and rarely subdural hematoma.[1] Cerebral magnetic resonance imaging (MRI) in hypernatremia shows features of osmotic demyelination.[2,3] Spontaneous postpartum hypernatremia resulting in osmotic myelinolysis and rhabdomyolysis was recently described where MRI revealed predominantly extrapontine lesions.[4] Distribution of these MRI abnormalities differ from the osmotic demyelination syndrome seen with hyponatremia.[5]
Recurrent postpartum hypernatremic encephalopathy with rhabdomyolysis and osmotic myelinolysis has not been described earlier to the best of our knowledge. We present a young woman who had neurological manifestations due to recurrent postpartum hypernatremia.
Case Report
A 23-year-old female presented in June 2009 twelve days after second delivery with 8 days fever followed by dysarthria with progressive quadriparesis and progressive altered sensorium. She was febrile and in altered consciousness groaning, opening eyes, and flexing limbs to pain. She had generalized hypotonia, brisk muscle stretch reflexes, and flexor plantars.
She had severe hypernatremia, elevated creatinine kinase (CK) and azotemia [Table 1]. Cerebral MRI revealed symmetrical white matter hyperintensities in FLAIR, T2 and diffusion weighted imaging (DWI) sequences, which were hypointense in T1 images [Figure 1a]. Quadriplegia (proximal 0/5, distal 2/5) was noted on day 3 when she became conscious with slow correction of hypernatremia. On discharge at 18 days, she was conscious, alert with emotional lability, moderate limb, and gait ataxia with mild quadriparesis (MRC 4/5), which recovered completely at 2 months.
Table 1.
Laboratory findings during the two admissions and at follow-up
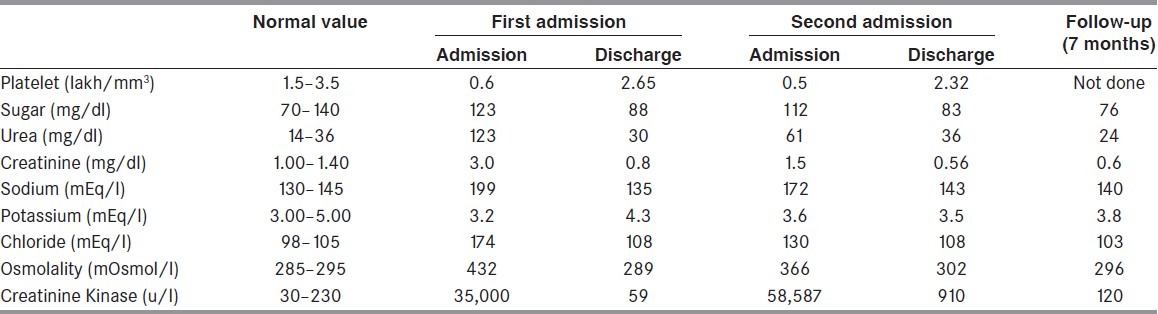
Figure 1.
Axial T2 weighted images in first admission (a) second admission (b) and at follow-up (c) Hyperintensity of the posterior limb of internal capsule (white arrow), anterolateral midbrain and anterior pons (thick black arrow) and middle cerebellar peduncle (black arrowhead) are more pronounced in second admission. Splenial hyperintensity was circumscribed in first admission while being more extensive in second admission with partial resolution at follow-up (thin black arrow)
She delivered her third baby 25 days before admission in May 2011. Moderate fever started 15 days later. Progressive quadriparesis began 5 days before admission followed by irrelevant speech, disorientation, progressively reducing word output, and incontinence 2 days later. She was febrile with dehydration and tachycardia. She lay with open eyes, obeyed simple commands, spoke irrelevantly, and was disoriented. Eyes were convergent. She had flaccid quadriplegia (upper limbs 2/5, lower limbs 1/5). Muscle stretch reflexes were normal with flexor plantars.
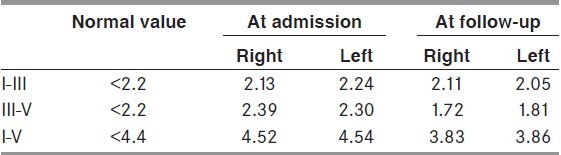
Investigations revealed recurrence of hypernatremia, azotemia and hyperCKemia [Table 1]. MRI revealed extensive symmetrical T2, FLAIR, and DWI hyperintensities of white matter with prominent changes in internal capsule extending through midbrain and pons to middle cerebellar peduncle simulating the ‘wine-glass’ Figures appearance [Figures 1b and 2a]. Auditory evoked potentials (AEP) revealed bilateral prolongation of interpeak intervals III-V and I-V while I-III intervals were normal [Table 2]. Following recovery of encephalopathy and quadriplegia with gradual hypernatremia correction, she had generalized hyperreflexia, ankle clonus with limb and gait ataxia. She had diplopia from transient bilateral lateral rectus palsy. She was discharged 12 days after admission when her biochemical abnormalities had recovered, with muscle power 4+/5 and moderate ataxia, which resolved at 1 month.
Figure 2.
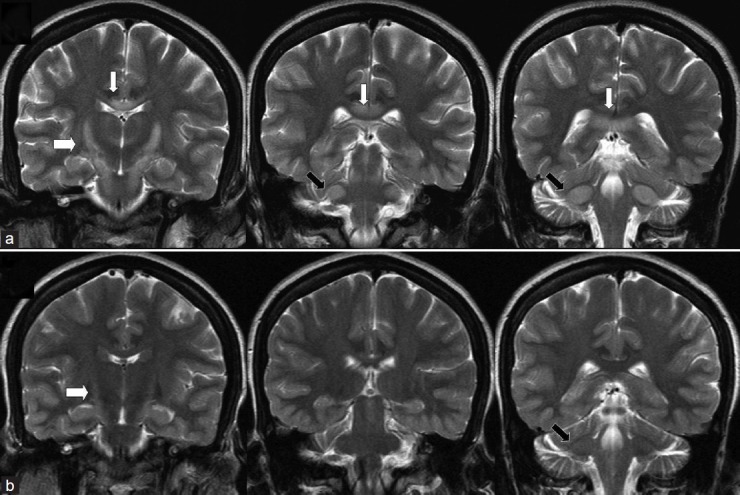
Coronal T2 weighted images at second admission (a) and at follow-up at 7 months later. There is symmetrical hyperintensity of the white matter in corticospinal tracts from internal capsule to pons producing ‘wine glass’ appearance (thick white arrow) which extends to middle cerebellar peduncle (black arrow) along with splenial hyperintensity (thin white arrows) at second admission which resolved significantly at follow-up
Table 2.
Auditory evoked potential interpeak intervals using rarefaction clicks in the second admission and at 6 months follow-up. All values are in milliseconds
At follow-up 7 months later, she was asymptomatic with normal neurological examination and biochemical values. Repeat MRI revealed partial resolution of the white matter hyperintensities [Figures 1c and 2b] whereas AEP was normal.
Discussion
Hypernatremia from diverse etiologies including pure water loss, antidiuretic hormone (ADH) deficiency, sweating, diuresis, vomiting, exogenous salt, etc., is a relatively common dyselectrolytemia encountered in hospitalized patients, especially those in the critical care units.[1,6] Hypernatremia should to be corrected slowly, especially in chronic hypernatremia as homeostatic mechanisms increase the intracellular concentration of ideogenic molecules that counter extracellular hyperosmolarity.[7] Postpartum hypernatremia with osmotic cerebral demyelination and rhabdomyolysis was recently described from India. Osmotic demyelination was documented in all 10 patients who underwent cerebral MRI and was predominantly extrapontine in distribution.[4] Rats with experimentally induced severe hypernatremia had symmetrical demyelinating lesions in thalami, basal ganglia, cerebral cortex, and hippocampi.[8]
Our patient had severe recurrent postpartum hypernatremia with symptom-free interval of 2 years between the admissions. Rhabdomyolysis evidenced by proximally dominant muscle weakness and hyperCKemia occurred during both the admissions. During the recovery phase, encephalopathy improved initially followed by gradual improvement in the ataxia and quadriparesis. Her clinical, biochemical, and radiological features of postpartum hypernatremia with encephalopathy, osmotic demyelination, and rhabdomyolysis were similar to those described earlier.[4] The point of interest in this report is that our patient had two discrete episodes of postpartum hypernatremia. Serum sodium levels at discharge after both admissions and 7 months after the second admission were normal, ruling out preexisting dyselectrolytemia.
Occurrence of hypernatremia could have been contributed by presence of fever and poor water intake. Investigations for common infective causes of fever were negative during both admissions. Similar observations were seen in the earlier study.[4] Lack of thirst sensation in the presence of dehydration or hypernatremia can occur in central adypsic hypernatremia with hypothalamic dysfunction. Partial ADH secretion defect can be unmasked during pregnancy due to the enhanced peripheral breakdown by vasopressinase.[9] A woman with gestational diabetes insipidus (GDI) manifesting in the later third trimester had encephalopathy with hypernatremia following cesarean section.[10] Recurrent GDI was reported in a patient with hemochromatosis who had low ADH levels with increased vasopressinase levels probably due to reduced hepatic breakdown of vasopressinase.[11] If aminopeptidase activity does not reduce following delivery, it could produce deficiency of ADH resulting in diabetes insipidus and can explain the hypernatremia in the postpartum state. However, our patient did not have features of diabetes insipidus and with available data cause of hypernatremia was unclear in our patient.
Osmotic demyelination syndrome (ODS) with central pontine myelinolysis and extrapontine myelinolysis is associated with rapid correction of hyponatremia. MRI abnormalities in extrapontine ODS lesions with hyponatremia are predominantly in basal ganglia.[5] Cerebral MRI abnormalities in hypernatremia in the earlier publications have been different than that seen in ODS associated with hyponatremia.[2–4] MRI abnormalities were demonstrated before commencement of treatment for hypernatremia unlike in hyponatremia associated ODS.[4,5]
MRI in postpartum hypernatremic ODS revealed involvement of corpus callosum, especially the splenium in all the patients with variable involvement of internal capsule, cerebellar peduncles, pons, and hippocampus. Follow-up MRI in one patient had revealed resolution of white matter hyperintensities.[4] The present patient had similar distribution of the hyperintensities with more pronounced abnormalities during the second admission. In addition, pontine and cerebellar peduncle involvement was pronounced in the second admission. Reversible hyperintensities in bilateral basal ganglia, internal capsules, and periventricular white matter have been reported in patients with uremic encephalopathy.[12,13] While our patient had impaired renal parameters, the renal impairment was moderate and distribution of MRI abnormalities is different than those reported in uremia and hence are more likely to be due to hypernatremic ODS.
Coronal T2 weighted images in our patient revealed symmetrical hyperintensities of the internal capsule, crus cerebri, and pons similar to the ‘wine-glass’ pattern of hyperintensities found in amyotrophic lateral sclerosis (ALS), primary lateral sclerosis, and leukodystrophies.[14–17] The ‘wine glass’ pattern depicts the involvement of corticospinal tract in these different conditions involving the white matter. Normal individuals can have subtle hyperintensity of the posterior limb of internal capsule.[18] However, in pathological states, the T2 and proton density hyperintensities are more pronounced. The ‘wine glass’ sign has not been described earlier in hypernatremic ODS. While she recovered without clinical neurological deficits, her follow-up MRI at 7 months revealed partial persistence of white matter hyperintensities.
Coronal T2 images also revealed symmetrical oval hyperintensity in the middle cerebellar peduncles, which has not been documented in hypernatremic ODS to the best of our knowledge, explains the ataxia in the patient. Hyperactive muscle stretch reflexes including jaw jerk associated with ankle clonus are from the involvement of corticospinal and corticobulbar fibers. Reversible prolongations of central interpeak intervals of AEP during the second admission suggest additional involvement of the auditory pathway in the brainstem. While the corticospinal tract and middle cerebellar peduncle hyperintensities were noted previously, the abnormalities were not as striking in the present patient.[4]
Literature survey did not reveal publications on recurrent postpartum hypernatremia or ′wine-glass′ appearance in patients with osmotic demyelination syndrome and we believe that the present report is the first of its kind in hypernatremic ODS.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil
References
- 1.Adrogué HJ, Madias NE. Hypernatremia. N Engl J Med. 2000;342:1493–9. doi: 10.1056/NEJM200005183422006. [DOI] [PubMed] [Google Scholar]
- 2.Brown WD, Caruso JM. Extrapontine myelinolysis with involvement of the hippocampus in three children with severe hypernatremia. J Child Neurol. 1999;14:428–33. doi: 10.1177/088307389901400704. [DOI] [PubMed] [Google Scholar]
- 3.Maeda M, Tsukahara H, Terada H, Nakaji S, Nakamura H, Oba H, et al. Reversible splenial lesion with restricted diffusion in a wide spectrum of diseases and conditions. J Neuroradiol. 2006;33:229–36. doi: 10.1016/s0150-9861(06)77268-6. [DOI] [PubMed] [Google Scholar]
- 4.Naik KR, Saroja AO. Seasonal postpartum hypernatremic encephalopathy with osmotic extrapontine myelinolysis and rhabdomyolysis. J Neurol Sci. 2010;291:5–11. doi: 10.1016/j.jns.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 5.Kallakatta RN, Radhakrishnan A, Fayaz RK, Unnikrishnan JP, Kesavadas C, Sarma SP. Clinical and functional outcome and factors predicting prognosis in osmotic demyelination syndrome (central pontine and/or extrapontine myelinolysis) in 25 patients. J Neurol Neurosurg Psychiatry. 2011;82:326–31. doi: 10.1136/jnnp.2009.201764. [DOI] [PubMed] [Google Scholar]
- 6.Pevelsky PM, Bagrath R, Greenberg A. Hypernatremia in hospitalized patients. Ann Intern Med. 1996;124:197–203. doi: 10.7326/0003-4819-124-2-199601150-00002. [DOI] [PubMed] [Google Scholar]
- 7.Lee JH, Arcinue C, Ross BD. Brief report: Organic osmolytes in the brain of an infant with hypernatremia. N Engl J Med. 1994;331:439–42. doi: 10.1056/NEJM199408183310704. [DOI] [PubMed] [Google Scholar]
- 8.Soupart A, Penninkx R, Namias B, Stenuit A, Perier O, Decaux G. Brain myelinolysis following hypernatremia in rats. J Neurpathol Exp Neurol. 1996;55:106–13. doi: 10.1097/00005072-199601000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Shrier RW. Systemic arterial vasodilation, vasopressin, and vasopressinase in pregnancy. J Am Soc Nephrol. 2010;21:570–2. doi: 10.1681/ASN.2009060653. [DOI] [PubMed] [Google Scholar]
- 10.Sherer DM, Cutler J, Santoso P, Angus S, Abulafia O. Severe hypernatremia after cesarean delivery secondary to transient diabetes insipidus of pregnancy. Obstet Gynecol. 2003;102:1166–8. doi: 10.1016/s0029-7844(03)00704-x. [DOI] [PubMed] [Google Scholar]
- 11.Krysiak R, Kobielusz-Gembala I, Okopien B. Recurrent pregnancy-induced diabetes insipidus in a woman with hemochormatosis. Endocr J. 2010;57:1023–8. doi: 10.1507/endocrj.k10e-125. [DOI] [PubMed] [Google Scholar]
- 12.Okada J, Yoshikawa K, Matsuo H, Kanno K, Oouchi M. Reversible MRI and CT findings in uremic encephalopathy. Neuroradiology. 1991;33:524–6. doi: 10.1007/BF00588046. [DOI] [PubMed] [Google Scholar]
- 13.Yoon CH, Seok JI, Lee DK, An GS. Bilateral basal ganglia and unilateral cortical involvement in a diabetic uremic patient. Clin Neurol Neurosurg. 2009;111:477–9. doi: 10.1016/j.clineuro.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 14.Agosta F, Chiò A, Cosottini M, De Stefano N, Falini A, Mascalchi M, et al. The present and the future of neuroimaging in amyotrophic lateral sclerosis. AJNR Am J Neuroradiol. 2010;31:1769–77. doi: 10.3174/ajnr.A2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuruvilla A, Joseph S. ′Wine Glass′ appearance: A unique MRI observation in a case of primary lateral sclerosis. Neurol India. 2002;50:306–9. [PubMed] [Google Scholar]
- 16.Kohlschûtter A, Eichler F. Childhood leukodystrophies: A clinical perspective. Exper Rev Neurother. 2011;11:1485–96. doi: 10.1586/ern.11.135. [DOI] [PubMed] [Google Scholar]
- 17.Satoh JI, Tokumoto H, Kurohara K, Yukitake M, Matsui M, Kuroda Y, et al. Adult-onset Krabbe disease with homozygous T1853C mutation in the galactocerebrosidase gene.Unusual MRI findings of corticospinal tract demyelination. Neurology. 1997;49:1392–9. doi: 10.1212/wnl.49.5.1392. [DOI] [PubMed] [Google Scholar]
- 18.Mirowitz S, Sartor K, Gado M, Torack R. Focal signal-intensity variation in the posterior internal capsule: Normal MR findings and distinction from pathological findings. Radiology. 1989;172:535–9. doi: 10.1148/radiology.172.2.2748836. [DOI] [PubMed] [Google Scholar]


