Abstract
Electrodiagnosis (EDX) is a useful test to accurately localize the site, determine the extent, identify the predominant pathophysiology, and objectively quantify the severity of brachial plexopathies. It can also be used to examine muscles not easily assessed clinically and recognize minimal defects. Post-operatively and on follow up studies, it is important for early detection of re-innervation. It can be used intra-operatively to assess conduction across a neuroma, which would help the surgeon to decide further course of action. Localization of the site of the lesion can be very challenging as there may be multiple sites of involvement and hence the electroneuromyographic evaluation must be adequate. The unaffected limb also needs to be examined for comparison. The final impression must be co-related with the type and severity of injury.
Keywords: Brachial-plexopathy, electrodiagnosis, traumatic
Introduction
Brachial plexus and nerve injuries form about 14% of total referrals to our laboratory every year. The two commonest causes for brachial plexus injuries are motor cycle and road traffic accidents. These usually result in closed traction injuries, which are severe and involve the entire brachial plexus sometimes along with root avulsions.[1] These form 57% of the total injuries in our electrodiagnosis laboratory. Other causes are cut injuries, fall from running train and heights, industrial accidents, and birth injuries. Classifying all nerve injuries by their severity based on the extent of axon loss and the elements of the brachial plexus involved, 41% showed severe, 39% moderate, and 20% mild involvement. 63% of brachial plexus injuries were severe pre- and post-ganglionic lesions involving C567 more than C8T1 fibers. EDX analysis of traumatic brachial plexopathies over the last 10 years in our department has shown that there has not been a single case of isolated cord lesion. Most lesions are pre+ post-ganglionic, involving upper more than lower plexus elements. When infra-clavicular lesions were analyzed, all 3 cords and /or the terminal nerves were involved. This may be due to the fact that we are a tertiary hospital-based practice. Except one patient, who had severe arm pain due to root avulsion injury, no patient has ever expressed a desire to amputate his non-functioning arm, very often a handicap. Most patients follow-up “patiently” post-nerve surgery, happy to get any function of their arm. Brachial plexus repair is a very pertinent need.
Plexus literally means “a network or interwoven mass, specially of nerves, blood vessels or lymphatic vessels,” and “brachial” means “pertaining or belonging to the arm” derived from the classic Latin words “plex” meaning to “plait” or “interweave” and “brachium” meaning “arm.”[2] As the name implies, it is a complex structure and its evaluation, whether clinical or electrodiagnostic, requires a thorough knowledge of its anatomy.
Electrodiagnosis (EDX) is a combination of tests that assess the function of the brachial plexus. It is time-consuming, especially when evaluating the plexus and must be customized for each patient, depending on the clinical findings. The information obtained from it helps directly in the management and hence, the emphasis should be on performing a complete and accurate study. An adequately performed EDX identifies the site of the lesion or lesions as the case maybe, provides prognosis by determining the severity and pathophysiology of the lesion. It can also identify milder lesions that are masked by the more severe ones. It can suggest nerves as potential donors for surgical procedures and is the first test to show re-innervation on follow-up. Its intra-operative use has been adequately demonstrated.[3–5] This review will focus on pre- operative electrodiagnosis (EDX) of brachial plexopathies following closed trauma in adults. Other causes of brachial plexopathies e.g., due to radiation, compression, post-operative, neoplastic infiltration, post-sternotomy, and hypertrophic inflammatory lesions can also be diagnosed using similar protocols, but they have distinct findings, which have not been mentioned here.[6]
Historical aspects
The anatomical description of the brachial plexus is best documented in the drawings of Leonardo da Vinci in the 15th century.[7] Brachial plexus injury, however, was first documented in the year 138 AD by the physician Galen, who treated a temporary weakness of the upper limb caused by traction to the neck.[7] The first clinically documented case by Smellie in 1764 of brachial plexopathy was birth palsy, but it was in 1872 that Guillaume Benjamin Armand Duchenne, a French physician, coined the term “obstetrical brachial plexus palsy;” he described 4 cases of infantile paralysis at birth, involving C567 fibers. He was the first to use electrodiagnosis to measure the severity of the weakness.[7] Erb described similar cases in adults in the year 1874.[7] In 1885, Klumpke first described a lower trunk brachial plexopathy.[7] The first iatrogenic brachial plexopathy following a reduction procedure for a dislocated shoulder was described in 1827 by Flaubert de Rouen in France. In 1910, a window cleaner fell onto the shoulder of a physician passing below who sustained a weakness of the left upper limb along with agonizing pain, now recognized as avulsion pain. This accident lead to the discovery that anterior and posterior roots of 6, 7, and 8 cervical roots form the brachial plexus as when the surgeons operated upon him they found these to be avulsed.[7]
Though clinical neurophysiology originated with experiments of Galvani in the eighteen century, Eichler was the first to report percutaneous recording of nerve action potentials in 1937.[8] The technique that is in use now was developed accidentally by Dawson in 1947.[8] Dawson also introduced orthodromic sensory nerve testing in 1956.[9] Gilliatt and Sears were the first to document that there were sensory domains, when they found absent ulnar sensory potentials in lower trunk lesions, but median sensory potentials absent in more widespread brachial plexopathy.[10] Nerve conduction velocity measurements came into clinical practice in 1960's, and the First International Congress in electromyography was held in 1961. The needle electromyography examination as we know it now came into being in 1929 when Lord Adrian and Detlov Bronk first designed the concentric needle electrode. In the last century, the techniques for both the tests have been refined and made simple with the use of computers.[8]
Review of literature for “electrodiagnosis in classifying brachial plexus injuries” revealed that most of the earlier papers were on clinical observations on non-traumatic acute brachial plexitis, (neuralgic amyotrophy) birth injuries, post-operative, post-radiation, and post-injection brachial plexopathies. In 1976, a paper titled “Some lesions of the brachial plexus” had reported “our chief diagnostic agent: myelography” for traumatic brachial plexus injuries.[11] In 1978, Kaplan reported a study using F waves for diagnosis of upper trunk lesions.[12] Daube JR described localization to individual element of the brachial plexus in 1979,[13] and a 1981 paper reports electrodiagnosis of traumatic suprascapular neuropathies.[14] Streib described conduction block in the medial cord of the brachial plexus as an unusual finding in 2 patients of breast cancer in 1982.[15] In 1984, a paper described electrodiagnostic findings in 18 patients with traumatic upper trunk injury; a common football injury called “the stinger.” They reported “conduction slowing in the proximal segments of the axillary, musculocutaneous, suprascapular, and accessory nerves. The most commonly observed electromyographic abnormalities were an increase in polyphasic waves and decreased recruitment. Spontaneous activity was sparse.”[16] In the same year, a paper using electrodiagnosis highlighted the difference between radiation-induced and recurrence of neoplastic brachial plexopathies.[17] Asa Wilbourn and later Ferrante MA have published the most comprehensive articles on electrodiagnosis in brachial plexopathies,[6,18–21,32] Subsequently, many papers have been written, based primarily on the electrodiagnosis of brachial plexopathies of various etiologies.[22–37] Of particular mention are those by Ferrante and Wilbourn who have given a comprehensive algorithm for electrodiagnostic evaluation and exact localization of the lesion at the level of each element of the brachial plexus.[6,18–21,32]
Anatomy
The anatomy described here is with reference to its requirement, especially for electrodiagnostic localization; more details are available in standard anatomic textbooks. The brachial plexus has 10200 to 16600 interlacing axons, derived from the primary anterior rami of the cervical spinal nerves 5,6,7,8, and thoracic spinal nerve 1.[1] The anterior horn cell is the primary neuron for the motor fibers, and it lies in the spinal cord. The primary neuron for the sensory fibers is the dorsal root ganglion, and it lies in the intervertebral foramen. The ventral and dorsal rootlets join to form the ventral and dorsal anatomic roots. The ventral and dorsal roots unite distal to the dorsal root ganglion to form the mixed spinal nerve. The mixed spinal nerve gives off a posterior branch, the posterior primary ramus (PPR) just as it exits the inter vertebral foramen (which supplies the paraspinal muscles) and then continues as the anterior primary ramus. (APR) The anterior primary rami of C5678T1 are called the roots of the brachial plexus [Figure 1].
Figure 1.
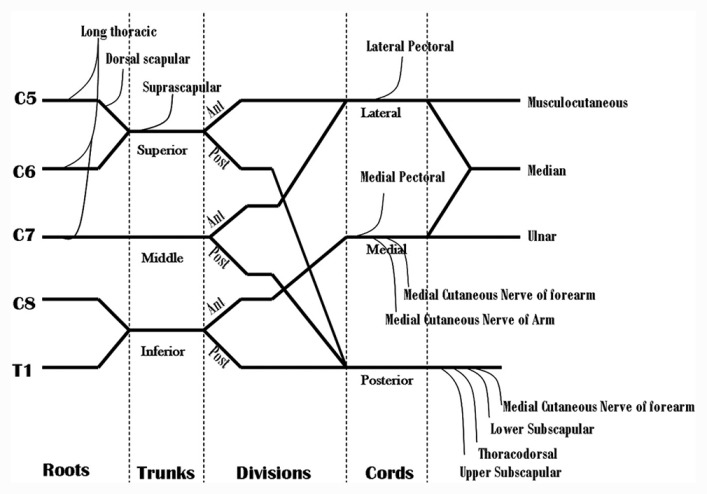
Diagram showing formation and branches of the brachial plexus
The C5 and C6 APR unite to form the upper trunk, C7 APR continues as the middle trunk, and the C8T1 APR join to form the lower trunk. The trunks lie in the neck in the interscalene groove, between the anterior and middle scalene muscles. Behind the clavicle, the trunks again divide into anterior and posterior divisions. The anterior divisions of the upper and middle trunk unite to form the lateral cord, all posterior divisions unit to form the posterior cord, and the anterior division of the lower trunk continues as the medial cord. The cords lie in the axilla.[6]
Branches
The C5 root of the brachial plexus (C5 APR) gives off a branch- the dorsal scapular nerve to the rhomboid and levator scapulae muscles and sometimes a branch to the phrenic nerve. Branches from the C567 roots of brachial plexus unite to form the long thoracic nerve to the serratus anterior muscle. The C5 to C8 APR supply the scalene and longus colli muscles.
There are no branches from the middle and lower trunks. The proximal part of the upper trunk gives off the suprascapular nerve, which innervates the supra and infraspinatus muscles and the nerve to the subclavius muscle. The branches of the lateral cord are lateral pectoral nerve to the pectoralis major muscle, the musculocutaneous nerve to the biceps and brachialis muscles. It terminates as the lateral head of the median nerve. The posterior cord branches are the upper and lower subscapular nerves to the teres major muscle, the thoracodorsal nerve to latissimus dorsi muscle, the axillary nerve to the teres minor and deltoid muscles, and it continues as the radial nerve. The medial cord branches into the medial pectoral nerve to pectoralis major, medial cutaneous nerve of arm and forearm, ulnar nerve and terminates as the medial head of median nerve [Figure 1].[6]
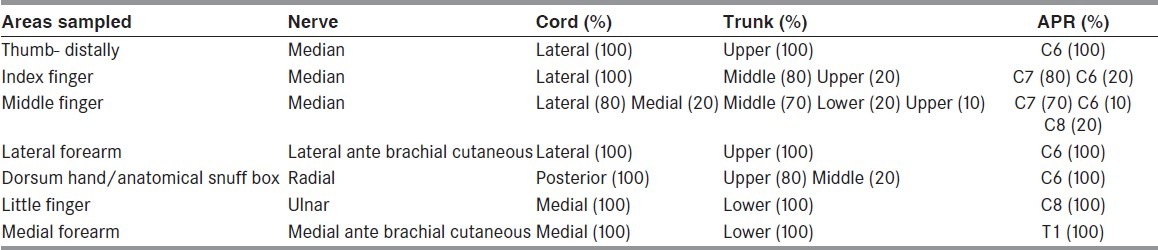
For each segment of the brachial plexus, there is a muscle domain and a sensory domain.[6] The muscle domain includes those muscles which are innervated by that segment, and the sensory domain includes the sensory nerve fibers contained in it, e.g., the lateral cord of the brachial plexus will contain as its muscle domain the muscles innervated by musculocutaneous nerve, lateral pectoral nerve and those muscles innervated by the lateral head of median nerve. Its sensory fibers will be from the lateral cutaneous nerve of forearm and median nerve supplying the thumb and index finger (100%) and middle finger (80%). Detecting this pattern of abnormality helps in the localization of the site of the lesion. Details of sensory and muscle domains are described excellently by Ferrante and Wilbourn.[19]
In our laboratory, we trace the muscle domain from a distal to proximal level i.e., target to source, e.g., the abductor pollicis brevis muscle is innervated by the median nerve, medial cord, lower trunk, and T1 root. [Table 1] This helps to identify the elements of the brachial plexus as the muscles are being sampled, e.g., if the infraspinatus and deltoid are denervated but biceps, serratus anterior and brachioradialis muscle are normal, it immediately suggests that the lesion would be at individual nerve levels. Co-relating with the sensory nerve action potentials, localization of the site of involvement can be ascertained. Similarly, the median index finger has sensory fibers from the median nerve 100% of the time, lateral cord 100% of the time, upper trunk 80% of the time, middle trunk 20% of the time, C6 root 80% of the time, and C7 root 20% of the time[6] [Table 2]. Using this table as the nerve conductions are being done, it is possible to localize the affected brachial plexus element, e.g., if the ulnar, medial ante-brachial cutaneous and lateral ante-brachial cutaneous SNAPs are abnormal, but median and radial SNAPs are normal, it is safe to suspect that the musculocutaneous nerve and the lower trunk are likely to be involved, which can then be confirmed by doing the relevant motor conduction studies and needle electromyographic examination.
Table 1.
Tracing muscle domains from target to source, of commonly used muscles for Needle EMG in traumatic brachial plexus lesions (6,21)
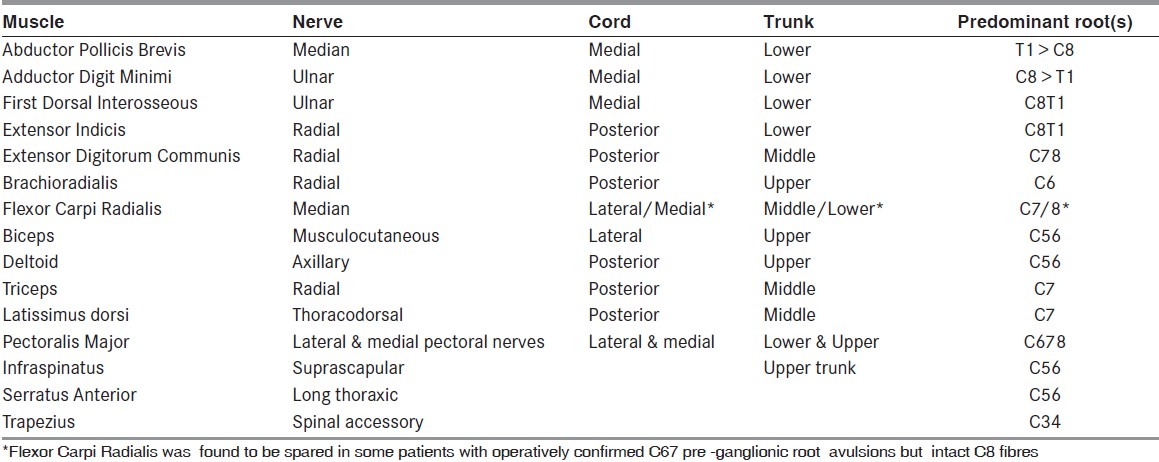
Table 2.
Tracing sensory domains from sites examined while doing the sensory nerve conduction study and frequency of abnormalities at various levels of the elements of the brachial plexus (6,20)
Some comments
The terminology “root” is used differently by anatomists and nerve surgeons. Anatomists call the ventral and dorsal root, which are pre-ganglionic fibers, as the roots. The surgeons include the APR, the PPR, the mixed spinal nerve, and the ventral and dorsal root as “root.”[20] Hence, they include both pre- and post-ganglionic fibers. In short, very proximal lesions, which are not conducive to direct nerve repair, are called as “root” lesion by the brachial plexus surgeons.
The classification of brachial plexus injuries is covered in the article describing treatment options for brachial plexus injuries in this issue and hence is not repeated here in detail. However, lesions proximal to the dorsal root ganglion, that is, those involving the ventral and dorsal roots or rootlets, are called pre-ganglionic and those distal to it, are called post-ganglionic. Involvement of the plexus elements above the clavicle classifies it as a supraclavicular brachial plexopathy (could be pre- or post-ganglionic), and lesions affecting the plexus distal to the clavicle produce infraclavicular brachial plexopathy. Retroclavicular lesions (to the division) are rare and if present, as sometimes seen with clavicular fractures, complicate the pattern of abnormalities.[6,20,21,33]
Supraclavicular brachial plexus injuries present clinically in a “radicular distribution” as the motor fibers contained in them innervate both flexor and extensor groups of muscles. Infraclavicular lesions present as “individual nerve lesions” as the motor fibers in each element innervate either flexors or extensors. The pectoral nerves originate very proximally from the cords, hence the pectoralis major muscle is more often affected in supraclavicular brachial plexus lesions.[21]
Pathophysiology
Most traumatic brachial plexus injuries are axon degenerative and very often severe. Short duration compression of the plexus e.g., due to shoulder dislocation or arm being pulled and pressed between two rollers may produce some amount of demyelination, but there is always significant axon loss.[20]
Pre-Requisites for EDX
Electrodiagnosis is an extension of the clinical examination and not a replacement. Hence, all patients must be examined prior to a study. History forms an important part of the evaluation, as in any neurological consultation. The type of the injury can define the extent, severity, and components of the brachial plexus affected. Violent stretch injuries caused by two-wheeler accidents present as closed traction lesions of the plexus and are usually severe and associated with avulsion injuries. Stretch along with local impact cause brachial plexus plus individual nerve lesions. Minor falls with local impact cause less severe injuries. Injuries associated with shoulder dislocations may cause infraclavicular brachial plexus lesions, which are demyelinating as well as axon-degenerative.[1]
The duration of the injury is important as sensory nerve action potentials drop by day 5 and reach their lowest by day 11 post-injury. Motor amplitudes drop by day 3 and reach lowest by day 7 post-injury, and needle EMG abnormalities are detected by 3 weeks post-injury.[20]
Clinical examination should detect Horners, brisk reflexes of the affected limb (if there is an upper motor neuron lesion) and grade the power.
Finally, the electro-diagnostician should be well-versed in the anatomy of the brachial plexus and the various techniques of electrodiagnosis.
Electrodiagnostic tests
The tests done are: Sensory and motor conductions, needle electromyography, somatosensory-evoked potentials and where indicated, intra-operative evaluation. These are done serially and evaluated collectively.
Sensory nerve conduction
Extensive sensory nerve conduction studies are done for the evaluation of brachial plexus lesions so as to include the entire domain.[6,21] Sensory nerve action potentials (SNAPs) are recorded from the median nerve (index, middle fingers, and the thumb), ulnar, radial, lateral and medial cutaneous nerves of the forearm (lateral ante-brachial cutaneous LABC and medial ante-brachial cutaneous MABC nerves) and are very often compared to those from the unaffected limb. The amplitude and presence or absence of the response is recorded. The SNAP assess the conduction in the post-ganglionic fibers and is a sensitive test to localize the site of the lesion as it remains unaffected in pre-ganglionic lesions (except when the dorsal root ganglion is also affected). The relevant SNAP will be attenuated or absent in a post-ganglionic lesion. In mixed pre- and post-ganglionic lesions, the motor abnormality is more than the relevant sensory amplitude loss, hence a low amplitude SNAP with a relevant absent motor conduction would suggest a pre> post-ganglionic lesion. It is important to note that in brachial plexus lesions, the SNAP starts dropping in amplitude by day 7 post-injury and reaches its lowest value by day 10 or 11. This is the time taken for the distal stump to degenerate. Hence, if the study is timed too early, a post-ganglionic lesion would be misdiagnosed as being pre-ganglionic. The SNAP is not useful for predicting recovery in brachial plexus lesions as once absent, it does not return to normal, even with regeneration.[6,19,20]
Motor nerve conduction
The motor domains sampled are median to abductor pollicis brevis/flexor carpi radialis, ulnar to abductor digiti minini/flexor carpi ulnaris, radial to extensor indicis and extensor digititorum communis, musculocutaneous to biceps, axillary to deltoid, and spinal accessory to trapezius. The amplitude of the compound muscle action potential (CMAP) is recorded from the muscles and compared to the unaffected side. In brachial plexus lesions, the amplitude of the distal CMAP starts to drop by day 3 following the injury, and it reaches its lowest valve by day 7.[20] Hence, the severity of the lesion can be judged after 7 days of the injury. Well-preserved CMAP amplitude from a clinically weak muscle at least 7 days after the injury suggests a neurapraxic lesion. The amplitude of the CMAP correlates well with the severity of the lesion (till re-innervation has occurred), and it can be judged by comparing the CMAP from the affected limb with that of the unaffected limb and using the formula U-A/U X 100= % of axon loss, where U = CMAP amplitude of unaffected side, A = CMAP amplitude of affected side. 50-75% indicates a moderate axon loss, >75% a severe axon loss and absent CMAP indicates no viable axons at the time of the study. The presence or absence of the CMAP also assesses the prognosis. If there is no response, then regeneration has to occur by proximo-distal nerve growth as there are no surviving axons for collateral innervation. If the denervated muscle lies more than 24 inches from the site of a complete nerve injury, recovery is not possible as by the time the nerve (if at all) would reach the muscle, it would have been replaced by fibrous or fatty tissue. (Nerve growth rate is about one inch a month).[6,21] A progressive increase in the amplitude from a muscle on serial studies would signify re-innervation of that muscle. (The amplitudes from the trapezius, flexor carpi radialis, and flexor carpi ulnaris are recorded and compared to the other side as the brachial plexus surgeon at our hospital use those nerves for transfer to re-innervate adjacent muscles when indicated)
Needle electromyography
Needle Electromyography (EMG) examination is required to document and record the axon loss, its proximal extent, and the completeness of the lesion, especially for proximal muscles where CMAP recording is not possible. Needle EMG also documents the earliest sign of recovery in the form of nascent units and unstable polyphasic units.[38] Axon loss is objectively confirmed by the presence of fibrillation potentials, which develop about 3 weeks after the injury (in the most distal muscles), [Figure 2a]. On voluntarily activating the muscle, if motor units are seen, it indicates that there are surviving axons and the lesion is partial. In such cases, the regeneration will take place by co-lateral sprouting. If there are no motor units and no recordable CMAP from the muscle, it indicates a functionally complete lesion, and re-innervation would happen only by nerve growth from the proximal stump, provided the nerve is in anatomical continuity.[6] After adequate time is allowed, regeneration is detected on needle EMG by the presence of unstable polyphasic units, which suggest ongoing re-innervation, [Figure 2b]. Mature, that is electrically complete re-innervation, shows large amplitude and long duration motor units, [Figure 2c].[38] The interference pattern or recruitment co-relates with the power of the muscle. Needle examination also helps to localize the site of the lesion along the brachial plexus as muscles belonging to a domain would be similarly affected with the distal most muscle, showing the most severe involvement. As in other conditions, the most proximal and, if possible, the most distal normal muscles should be sampled. Assessment of the muscles innervated by the branches from the various elements of the brachial plexus is helpful in localization. Denervation of the paraspinal muscles (when detected) places the lesion at the level of the anatomical roots. (pre-ganglionic) (However, in our laboratory, more emphasis is placed on the SNAP amplitude. Even low amplitude SNAPs, recorded from a domain where the relevant CMAP is absent, suggests that the lesion is pre-ganglionic). Denervation detected in the serratus anterior muscle places the lesion at the level of the roots of the plexus (C567). Rhomboid major may not show denervation as it often receives a branch from the C4 spinal nerve. Denervated infraspinatus muscle places the proximal level of involvement at the upper trunk of the plexus. However, it must be remembered that the suprascapular nerve is prone to injuries, especially with local impact lesions and in shoulder dislocations. If other distally innervated upper trunk muscles show no or comparatively less involvement, the lesion is at the suprascapular nerve level. Following the muscle domains as determined by Ferrante and Wilbourn, it is possible to accurately localize the site of the lesion.[6] [Table 2]. A severe and complete lesion is easier to localize than a partial and mild lesion. In partial lesions, with time, the proximal muscles re-innervate well, but the distal muscles continue to show signs of active denervation. Emphasis must not be placed only on spontaneous activity on needle EMG. A careful examination would pick up the changes in the motor unit configuration in the proximal muscles and help to correctly localize the site of the lesion.
Figure 2.
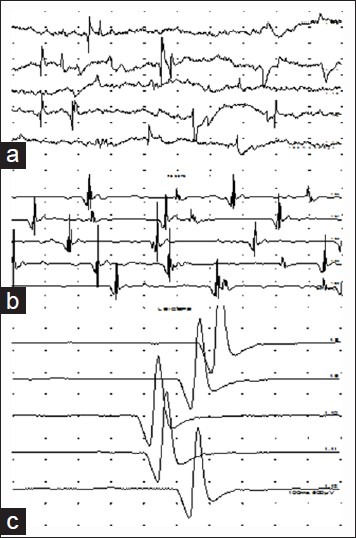
Traces of Needle EMG examination (a) Spontaneous activity, fibrillation potentials at rest in a denervated muscle. (b) Re-innervating polyphasic units (c) Large wide triphasic motor units indicating mature but incomplete re-innervation
Somatosensory-Evoked Potential Study (SSEP)
Upper limb somatosensory-evoked potentials are useful for documenting a complete pre-ganglionic avulsion of the sensory roots. In such cases, only an Erbs point potential is obtained with absent cervical cord and cortical responses. [Figure 3a] As both sensory and motor roots lie in close anatomical proximity, by extrapolation, it may be concluded that the motor fibers are similarly affected, though there are always exceptions to the rule.[24]
Figure 3.
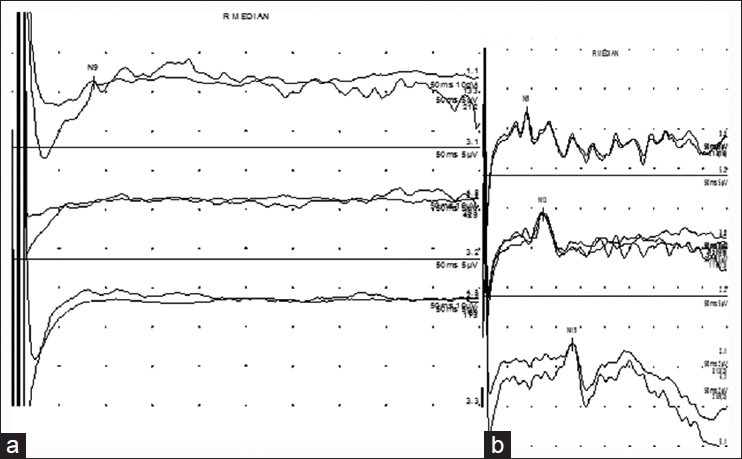
Somatosensory-evoked potential study (a): Trace 1 shows low amplitude but present Erbs point potential. Cervical and cortical responses are not obtained (traces 2 and 3) (b) Normal Erbs point, cervical cord, and cortical responses
Limitations
From the above description, it is evident that there are limitations to the EDX testing. Reliable and complete diagnosis is possible only after sufficient time has elapsed following injury, usually 3 weeks in adults. Prior to that, severity maybe assessed, but extent would require a needle EMG examination. At the time of the first study, if there is a complete lesion, a Sunderland grade 5 injury cannot be differentiated from grade 4 till a follow-up study is done and regeneration is detected on needle EMG examination. Also, routine studies cannot detect regenerating axons till they innervate a distal muscle.[6] If there is a complete proximal lesion, additional distal nerve lesions may be suspected but cannot be confirmed; similarly, when there is a severe complete post-ganglionic lesion, additional pre-ganglionic lesion cannot be localized. This is where the role of intra-operative studies has been emphasized. SSEP test again may not be a true indicator of pre-ganglionic motor function if sensory and motor roots are differentially affected. It should be understood that all meaningful post-operative and intra-operative studies require pre-operative evaluation. EDX studies are not possible if the stimulation and recording sites are not accessible, either due to anatomic reasons or due to plaster casts and external fixators in-situ. Limitations due to lack of good recording techniques and analysis should also be kept in mind, especially if the protocols are not followed. Inadequate study without clinical co-relation and a biased examiner limit the use of the EDX study.
Follow-up
The surgical procedure determines the timing of the follow-up EDX examination. Repeated, frequent studies have no value and at times, dishearten the patient. The nerve growth rate is about an inch a month in ideal circumstances, grafted and lacerated nerves grow slower.[6] On re-evaluation, improvement of the CMAP amplitude should be looked for while doing the motor nerve conduction study. Needle EMG signs of re-innervation should be looked for in a previous denervated muscle. The muscles closest to the site of nerve injury should be examined first and if re-innervation is noted, it should be traced in the more distal muscles to locate the level to which it has progressed. The distal most muscle of that domain should be examined for re-innervation. Similarly, muscles of each affected element of the brachial plexus should be examined proximally and distally. Needle EMG detects re-innervation well before clinical recovery is noted.[39] When nerve transfers have been done, the target muscle should be sampled while activating the primary muscle, e.g., when an intercostal nerve has been transferred to the biceps muscle, needle EMG at the time of the first follow-up should pick up motor units from the biceps with deep inspiration. It goes without saying that the referring doctor must indicate what surgical procedures have been done. In special circumstances, e.g., opposite C7 transfer, a SSEP test can be done to record a potential from the supraclavicular region of the affected side while stimulating the unaffected arm to show presence of potentials contra-laterally. This indicates that the nerve has achieved anatomic continuity at least.
How can the referring doctor help?
A good referral note with clinical findings and details of the injuries cuts down the time taken during the EDX evaluation. If any surgical procedure has been done, the intra-operative findings and list of nerve transfers should be provided. Open or infected wounds over stimulating and recording sites limit the study, and plaster casts/dressings over the limb make the study impossible.
The EDX Report
It is good to follow the principle of “keep it simple.” The nerve conduction data and traces should be included. A summary of the findings of each motor and sensory nerve should be listed. Instead of giving out pages of traces, it is more useful to comment whether the values obtained are normal or abnormal for that patient using appropriate reference data. The final impression should include the side, site, pathophysiology, and extent of involvement. Whether pre- or post-ganglionic or combined. If post-ganglionic, is the lesion in the roots, trunks, cord, or terminal nerves? If combined, where is the predominant lesion? Is it axon degenerative, demyelination or both? The severity and completeness of the lesion when possible must be mentioned, whether partial or complete. If partial, is it mild, moderate, or severe? The state of re-innervation of the muscles must be mentioned (ongoing, mature, or nil) as it helps the surgeon in planning further treatment.
Summary
A well-performed and correctly-timed electrodiagnostic evaluation is extremely helpful in planning the treatment strategy of traumatic brachial plexopathies. It is the only test that co-relates with function, and that is the primary concern of the patient and the surgeon. Incomplete procedures done too early are inconclusive and must be avoided.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil
References
- 1.Stewart JD. Brachial Plexus. In: Stewart J, editor. Focal Peripheral Neuropathies. 3rd ed. Philadelphia: Lippincott Williams and Wilkins; 2000. pp. 117–23. [Google Scholar]
- 2.Wiktionary, The free dictionary. Available from: http://en.wiktionary.org/wiki/plexus .
- 3.Crum BA, Strommen JA. Peripheral nerve stimulation and monitoring during operative procedures. Muscle Nerve. 2007;35:159–70. doi: 10.1002/mus.20707. [DOI] [PubMed] [Google Scholar]
- 4.Kline DG. Nerve surgery as it is now and as it may be. Neurosurgery. 2000;46:1285–93. doi: 10.1097/00006123-200006000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Spinner RJ, Kline DG. Surgery for peripheral nerve and brachial plexus injuries or other nerve lesions. Muscle Nerve. 2000;23:680–95. doi: 10.1002/(sici)1097-4598(200005)23:5<680::aid-mus4>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 6.Ferrante MA, Wilbourn AJ. Electrodiagnostic approach to the patient with suspected brachial plexopathy. Neurol Clin. 2002;20:423–50. doi: 10.1016/s0733-8619(01)00007-x. [DOI] [PubMed] [Google Scholar]
- 7.Murray B, Wilbourn AJ. Brachial Plexus. Arch Neurol. 2002;59:1186–88. doi: 10.1001/archneur.59.7.1186. [DOI] [PubMed] [Google Scholar]
- 8.Kimura J. Historical review. In: Kimura J, editor. Electrodiagnosis in diseases of nerve and muscle: Principles and practice. 3rd ed. New York: Oxford University Press Inc; 2001. pp. 887–92. [Google Scholar]
- 9.Dawson GD. The relative excitability and conduction velocity of sensory and motor nerve fibers in man. J Physiol. 1956;31:436–51. doi: 10.1113/jphysiol.1956.sp005473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilliatt RW, Sears TA. Sensory nerve action potentials in patients with peripheral nerve lesions. J Neurol Neurosurg Psychiatry. 1958;21:109–11. doi: 10.1136/jnnp.21.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonney G. Watson-Jones Lecture 1976 Some lesions of the brachial plexus. Ann R Coll Surg Engl. 1977;59:298–306. [PMC free article] [PubMed] [Google Scholar]
- 12.Yoon TN, Grabois M, Guillen M. Suprascapular nerve injury following trauma to the shoulder. J Trauma. 1981;21:652–5. doi: 10.1097/00005373-198108000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan PE. F-waves as an electrodiagnostic confirmation of brachial plexus neuropathies of the upper trunk. Electromyogr Clin Neurophysiol. 1978;18:527–32. [PubMed] [Google Scholar]
- 14.Daube JR. An electromyographer's view of plexopathy.In Neuromuscular Diseases as Seen by the Electromyographer. Second Annual Continuing Education Course, Americal Association of Electromyography and Electrodiagnosis. 1979 [Google Scholar]
- 15.Streib EW, SunSF , Leibrock L. Brachial plexopathy in patients with breast cancer: unusual electromyographic findings in two patients. Eur Neurol. 1982;21:256–63. doi: 10.1159/000115489. [DOI] [PubMed] [Google Scholar]
- 16.Di Benedetto M, Markey K. Electrodiagnostic localization of traumatic upper trunk brachial plexopathy. Arch Phys Med Rehabil. 1984;65:15–7. [PubMed] [Google Scholar]
- 17.Lederman RJ, Wilbourn AJ. Brachial plexopathy: Recurrent cancer or radiation? Neurology. 1984;34:1331–5. doi: 10.1212/wnl.34.10.1331. [DOI] [PubMed] [Google Scholar]
- 18.Wilbourn AJ. Electrodiagnosis of plexopathies. Neurol Clin. 1985;3:511–29. [PubMed] [Google Scholar]
- 19.Ferrante MA, Wilbourn AJ. The utility of various sensory nerve conduction responses in assessing brachial plexopathies. Muscle Nerve. 1995;18:879–89. doi: 10.1002/mus.880180813. [DOI] [PubMed] [Google Scholar]
- 20.Wilbourn AJ. Assessment of the Brachial Plexus and the Phrenic nerve. In: Pease WS, Johnson EW, editors. Practical Electromyography. 3rd ed. Baltimore: Williams and Wilkins; 1997. pp. 273–310. [Google Scholar]
- 21.Ferrante MA. Electrodiagnostic assessment of the brachial plexus. Neurol Clin. 2012;30:551–80. doi: 10.1016/j.ncl.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 22.Swash M. Diagnosis of brachial root and plexus lesions. J Neurol. 1986;233:131–5. doi: 10.1007/BF00314416. [DOI] [PubMed] [Google Scholar]
- 23.Stewart JD. Electrodiagnostic techniques in the evaluation of nerve compressions and injuries in the upper limb. Hand Clin. 1986;2:677–87. [PubMed] [Google Scholar]
- 24.Aminoff MJ, Olney RK, Parry GJ, Raskin NH. Relative utility of different electrophysiologic techniques in the evaluation of brachial plexopathies. Neurology. 1988;38:546–50. doi: 10.1212/wnl.38.4.546. [DOI] [PubMed] [Google Scholar]
- 25.Rubin M, Lange DJ. Sensory nerve abnormalities in brachial plexopathy. Eur Neurol. 1992;32:245–7. doi: 10.1159/000116834. [DOI] [PubMed] [Google Scholar]
- 26.Parry GJ. Electrodiagnostic studies in the evaluation of peripheral nerve and brachial plexus injuries. Neurol Clin. 1992;10:921–34. [PubMed] [Google Scholar]
- 27.Chuang TY, Chiou-Tan FY, Vennix MJ. Brachial plexopathy in gunshot wounds and motor vehicle accidents: Comparison of electrophysiologic findings. Arch Phys Med Rehabil. 1998;79:201–4. doi: 10.1016/s0003-9993(98)90300-8. [DOI] [PubMed] [Google Scholar]
- 28.Robinson LR. Traumatic injury tp peripheral nerves. Muscle Nerve. 2000;23:863–73. doi: 10.1002/(sici)1097-4598(200006)23:6<863::aid-mus4>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 29.Zaneteas PD. Brachial plexus injuries and the electrodiagnostic examination. Curr Sports Med Rep. 2003;2:7–14. doi: 10.1249/00149619-200302000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Puri V, Chaudhry N, Jain KK, Chowdhury D, Nehru R. Brachial plexopathy: A clinical and electrophysiological study. Electromyogr Clin Neurophysiol. 2004;44:229–35. [PubMed] [Google Scholar]
- 31.Bowles AO, Graves DE, Chio-TanFY Distribution and extent of involvement in brachial plexopathies caused by gunshot wounds, motor vehicle crashes, and other etiologies: A 10-year electromyography study. Arch Phys Med Rehabil. 2004;85:1708–10. doi: 10.1016/j.apmr.2004.01.039. [DOI] [PubMed] [Google Scholar]
- 32.Ferrante MA. Brachial plexopathies: Classification, causes and consequences. Muscle Nerve. 2004;30:547–68. doi: 10.1002/mus.20131. [DOI] [PubMed] [Google Scholar]
- 33.Insola A, Caliandro P, Pirrone R, Aprile I, Pazzagli C, Padua L. Usefulness of a comprehensive neurophysiological assessment for early diagnosis and prognosis of traumatic brachial plexus injuries. Electromyogr Clin Neurophysiol. 2005;45:209–17. [PubMed] [Google Scholar]
- 34.Harper CM. Preoperative and intraoperative electrophysiologic assessment of brachial plexus injuries. Hand Clin. 2005;21:39–46. doi: 10.1016/j.hcl.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 35.Chanlalit C, Vipulakorn , Jiraruttanapochai K, Mairiang E, Chowcheun P. Value of clinical findings, electrodiagnosis and magnetic resonance imaging in the diagnosis of root lesions in traumatic brachial plexus injuries. J Med Assoc Thai. 2005;88:66–70. [PubMed] [Google Scholar]
- 36.Moghekar AR, Moghekar AR, Karli N, Chaudhry V. Brachial plexopathies:Etiology, frequency, and electrodiagnostic localization. J Clin Neuromuscul Dis. 2007;9:243–7. doi: 10.1097/CND.0b013e3181450f7a. [DOI] [PubMed] [Google Scholar]
- 37.O'shea K, Feinberg JH, Wolfe SW. Imaging and electrodiagnostic work-up of acute adult brachial plexus injuries. J Hand Surg Eur Vol. 2011;36:747–59. doi: 10.1177/1753193411422313. [DOI] [PubMed] [Google Scholar]
- 38.Nandedkar SD. “Objective EMG”: Quantitation and Documentation In The Routine Needle Electromyographic Examination. In: Pease WS, Johnson EW, editors. Practical Electromyography. 3rd ed. Baltimore: Williams and Wilkins; 1997. pp. 41–61. [Google Scholar]
- 39.Daube JR, Rubin DI. Needle electromyography. Muscle Nerve. 2009;39:244–70. doi: 10.1002/mus.21180. [DOI] [PubMed] [Google Scholar]


