Abstract
Adult post traumatic Brachial plexus injury is unfortunately a rather common injury in young adults. In India the most common scenario is of a young man injured in a motorcycle accident. Exact incidence figures are not available but of the injuries presenting to us about 90% invole the above combination This article reviews peer-reviewed publications including clinical papers, review articles and Meta analysis of the subject. In addition, the authors′ experience of several hundred cases over the last 15 years has been added and has influenced the ultimate text. Results have been discussed and analysed to get an idea of factors influencing final recovery. It appears that time from injury and number of roots involved are most crucial.
Keywords: Brachial plexus injury, adult, surgical strategy
Introduction
Brachial plexus injury (BPI) is one of the most devastating injuries from the point of view of the patient. It effectively cripples function in one and rarely two upper limbs, causing significant loss of function and ability to perform tasks of daily living as well as delivering in his/her workplace. Potentially this can lead to unemployment, economic hardship, depression and in rare instances even suicidal urges. The typical patient is a young male who has had an accident while riding a two wheeler where he has been thrown off the vehicle and suffered traction between neck and shoulder damaging his plexus to varying degrees.
It is therefore vital that this very valuable segment of our population is functionally restored as early as possible to the best of our ability. With modern techniques in hand and microsurgery, this is very much feasible provided the patient is treated in time. There are techniques available for late referrals too, but early commencement of treatment makes a huge difference to the eventual outcome.
This article is a review of the various issues in diagnosis and management for injuries to the brachial plexus. It is based on an extensive survey of published peer reviewed literature as well as insights gained by the author in treating several hundred cases of adult brachial plexus injuries.
History
One of the earliest descriptions of injuries to the brachial plexus can be found in Homer's Iliad,[1] but it was not until this past century that attempts at reconstruction were reported. The first known documentation of obstetric brachial plexus injury was by Smellie in 1764,[2] and Duchenne in 1872[3] surmised that traction was the cause of the palsy. Erb described a similar palsy in adults in 1874[4] and suggested that traction or compression of the C5 and C6 roots could produce the injury. Thorburn was the first to publish an article describing direct repair of the components of the brachial plexus in 1900[5] and the first neurotizations were reported in 1903 by Harris and Low.[6] In 1920, Vulpius and Stoffel[7] rerouted some of the available fascicles of the pectoral nerves onto the musculocutaneous and the axillary nerves. In 1947, Seddon published his proposed method of the surgical correction of traction injuries with application of long interpositional nerve grafts.[8] At the Paris meeting of the International Society for Orthopaedic Surgery and Traumatology (SICOT) in 1966, it was concluded that surgical repair of the lesions was almost impossible and, even when performed, did not guarantee a useful result.[1]
Although cases of brachial plexus injury due to traction had been reported by Flaubert (1827) and Malgaigne (1847) and the traction theory of injury had been advanced by Gerdy and Horsely, Stevens was the first to carefully analyze the mechanical vectors created by the anatomy and to estimate the actual forces involved.[9] In fact Stevens′ treatise is a forgotten classic which accurately shows the bio mechanics of traction in the upper limb and neck and the resultant injuries of the brachial plexus.
The introduction of microsurgical techniques, microsutures and new understanding in nerve repair and regeneration started a renaissance in the surgical repair of brachial plexus injuries led by pioneers like Narakas, Millesi, Allieu, Brunelli, Gu, Terzis, Doi, and others.[10–18]
Etiology and pathophysiology
In the majority of cases treated by the author as also elsewhere in the world, the main etiology remains vehicular accident typically on a two wheeler. A list of common etiologies is given below:
Vehicular accident (majority two wheelers) accounts for >90% of cases
Industrial trauma—weight falling on shoulder from a height, being dragged inside a machine by the arm
Heavy fall with stretching of neck
Assault with a sharp object
Bullet injury—rare in India
Iatrogenic injury, either deliberate as in tumor surgery involving nerve roots or accidental while operating in the posterior triangle of the neck.
The pathophysiology of the common cause i.e. numbers 1.2 and 3 mainly involves traction on the plexus caused by an abnormal neck shoulder angle while the person is being thrown from the vehicle after the impact. If the shoulder is in adduction at the time then the upper plexus is affected involving the C5C6+/-C7, with simultaneous abduction of the shoulder and the stress is directed to the lower plexus i.e., C8T1 roots. If the transfer of momentum is massive due to the high combined velocity of the two vehicles involved, then all roots can be damaged resulting in a flail upper limb. The latter is unfortunately far too common. In the author's personal series, it amounts to approx. 50% of cases.
Pathophysiology of pre and post ganglionic lesions
This is perhaps the most important distinction in the pathology of brachial plexus injury. The nature of the lesion is very important for deciding the treatment. Lesions proximal to the dorsal root ganglion (DRG) on the sensory side and at the level of the rootlets from the anterior horn cells (AHC) on the motor side are Pre Ganglionic and those distal to these structures i.e., in the mixed spinal nerve emerging out of the foramina of the cervical spine are post Ganglionic. Pre Ganglionic lesions essentially signal a permanent loss of that root and the axons within it. Post Ganglionic lesions are amenable to repair from the root stump since they represent axons distal to the cell body which can regenerate.
A pull or a stretch on the plexus results in a spectrum of lesions. Sunderland's[19] well-known classification is useful to understand the nature of the injury. Broadly speaking for the surgeon, there are three different kinds of lesions:
Neuropraxia—reversible rapidly in weeks, rarely reaches the surgeon
Externally intact looking nerves (Sunderland type two or three injury — axonotomesis) —not to be resected in the neck but distal transfers may be needed if progress is poor
Neuroma in continuity—represents a post ganglionic lesion (Sunderland Type III and IV axonotomessis) and requires surgical repair after excision of the neuroma. Rarely is the neuroma conductive, if it is a neurolysis may suffice
Rupture—Post Ganglionic lesion (neurotomessis sunderland typeV), amenable to intra plexal nerve repair
Avulsion—Pre Ganglionic lesion, typically that root has to be abandoned as a source of regenerating axon.s
Classification as per site
Brachial Plexus injuries can be classified in various ways:
-
As per site
Root
Cord
Trunk
or Nerve level injury
Often a mixture of all
-
Which roots
Upper plexus i.e. C5C6+/-C7 or
Lower plexus C8T1
Global C5C6C7C8T1
-
Relation to clavicle
Supra clavicular
Retro clavicular
Infra clavicular.
Patient evaluation
Consists of:
A detailed history and noting the date of injury
- Complete clinical examination
- Muscle charting and note muscle wasting
- Sensory charting—note dry skin
- Noting associated trauma like fracture clavicle
- Checking radial pulse for subclavian artery injury
- Horner's sign
Detailed electrophysiology report
Imaging.
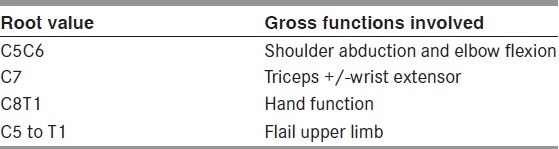
Muscle charting
As a simple thumb rule C5C6 represent the shoulder and elbow function, mainly of the deltoid and the biceps. If abduction and elbow flexion is missing, then C5C6 can be considered. C7 is an interesting root, in that, it is not specific to any particular muscle or muscle group and in fact can be harvested from the contralateral healthy side in case of a total palsy with very minor deficit resulting in the donor limb as shown by Gu et al.[16] and subsequently validated around the world. However the addition of C7 to a C5C6 injury results in triceps loss and sometimes in loss of wrist extension (this is variable as C8 too supplies the wrist extensors). Hand function is mainly represented by C8T1.
Thus if a patient is missing shoulder and elbow function, it is likely to be C5C6+/- C7 lesion depending on triceps function. This is an upper plexus injury. On the other hand, if patient has good shoulder and biceps but hand function is missing, then it is a C8T1 lesion or a lower plexus injury. If patient has a flail upper limb then all roots are involved. Table 1 summarizes this description.
Table 1.
Summary of root wise motor function
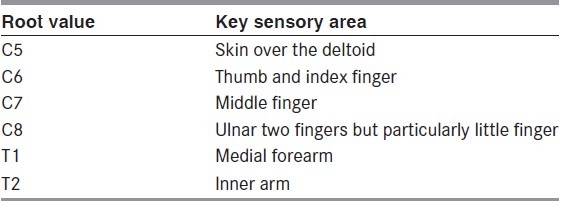
Sensory evaluation
It is important to note loss of sensation and often what patient perceives as altered sensation. However on rigorous sensory testing these areas too are often anesthetic. Dry skin is a give away for affected dermatomes due to loss of sudomotor function. Table 2 summarizes some easy rules of the thumb.
Table 2.
Summary of sensory innervation
Other clinical findings
It is important to note the presence of any associated trauma as it has a bearing on patients′ ability to get operated early. In case of fracture clavicle it is likely to result in a post ganglionic injury and also may be associated with a subclavian artery injury which will impact future free functional muscle transfers (FFMT) especially in a flail upper limb as the donor vessels may be compromised or limited. Multiple limb fractures and head injury will affect the outcome only if they delay plexus surgery beyond 3 to 6 months. In case of humerus fractures, the clinician should be alerted about the existence of an additional radial nerve injury which is then difficult to diagnose.
Horner's sign indicates a very proximal (usually Pre Ganglionic) type of lesion and signals the need for aggressive early management of the plexus injury with multi staged reconstruction including FFMT amongst other things.
Electrophysiology/Electro diagnostics (Edx)
In the authors′ opinion, this is perhaps the single most important investigation for a plexus injury and far more valuable to make surgical and therapeutic decisions than any imaging technique. This of course pre supposes the existence of an excellent electro diagnostic department in your center which unfortunately is not always the case. The following things can be determined by Edx:
Type of lesion, i.e., pre or post ganglionic
Localization of lesion to roots trunks cords and nerves
Extent of the lesion
Status of individual muscles—denervated, reinnervating etc
Sequential Edx can point to recovery and help post op monitoring of results
Compound motor action potential (CMAP) of important nerves like the ulnar and median which are potential donor nerves in upper plexus injuries.
Imaging
Imaging gives valuable information about the lesion as also about the associated injuries. Some modalities are listed below and this can be a subject of a review article in itself.
Plain X-rays for fractures and raised diaphragm (phrenic nerve injury)
CT myelography to determine the root status—not really done now but was the gold standard a few years ago
MR neurography—currently the most valuable tool for visualizing the plexus.
There are several papers discussing these modalities.[20–30] At one time CT myelography was the gold standard to decide on root avulsion in cases of adult palsy. Currently MRI is considered very useful, at least in the adults. Although there are reports of the use of MR scans the author does not routinely perform MR scans every time he sees a patient, although patients often already have one when seen by a hand and plastic surgeon. Clinical exam and electro physiology can give very adequate evidence of the status of the plexus and the indication for surgery.
Surgery for brachial plexus injury
Surgery especially for obstetric plexus injuries was pioneered by Kennedy 1903,[31] Sever 1916[32] and Wyeth and Sharpe in 1917.[33] Kennedy in fact reported very encouraging results. However Sever's results and paper describing 1000 cases were a damper for future work for almost 50 years. Herbert Seddon revived interest in the field after world war II.[34] It was only later that Gilbert,[35] Narakas,[36] Kawabata[37] and Millesi[38] and others started the surgical treatment for both children and adults again in the 80's with remarkably impressive results.
Indications for surgery and timing
Any brachial plexus injury which has not shown substantial spontaneous recovery in 3 months deserves to be explored. Timing is crucial due to the eventual loss of neuro muscular end plates at 20 to 24 months after denervation.[39] If there is global palsy with MRI proven pseudomeningocoeles showing pre ganglionic avulsion type of injury then no delay is justified. Operation can be performed in days or weeks to get the maximum out of any possible nerve transfers. In partial injuries especially of the upper plexus, a maximum period of 3 months is worthwhile to look for improved CMAP's of donor nerves and resolve the neuropraxia part in functioning roots. The best window is in the first three months and the next in the subsequent 3 months. After that results of proximal nerve repairs are less than satisfactory, although distal nerve transfers and free functional muscle transfers are possible. Indian data from the Post Graduate thesis of two of my students[40,41] clearly demonstrate that the first three months are the best period followed by 3 to 6 months after injury. Age too impacts results. Young patients at or around 20 years show rapid recovery with higher gain of strength. People over 40 are thought to show reduced results; however, they still show adequately good results to justify surgery at any age unless medical factors make the person unfit for reconstruction. Bhatia AG[42] has shown documented consistent good results of nerve reconstruction in people over 50 in a sample size of 38 cases. Age range was 45-59 and median age 50. Pre-op delay was few days to 12 months. The results showed a similar percentage of greater than M3 power as in younger people.
Surgical Exposure
Typically the exposure is both above and below the clavicle to get at the entire plexus and its nerve [Figure 1]. A detailed paper[43] is available discussing the approach and its technical details. In obstetric plexus cases, it is often necessary to ostetomize the clavicle to get a good exposure;[43] however, in adult cases except for the truly retro clavicular injury we do not always osteotomize the clavicle.
Figure 1.
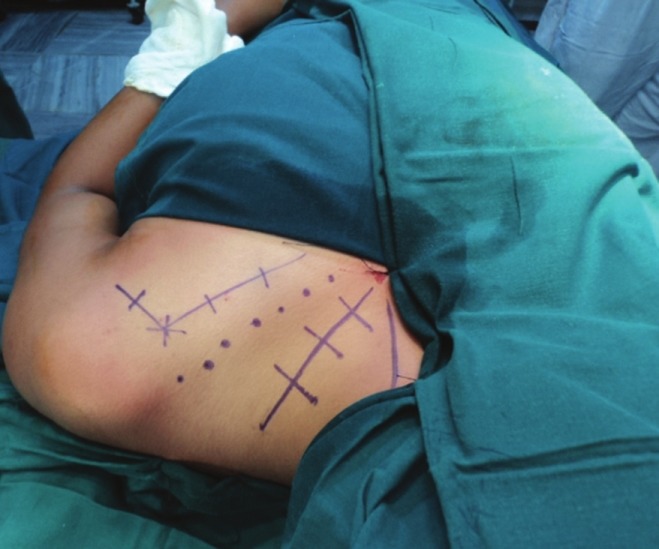
Marking for typical exposure of supra and infra clavicular plexus
Treatment Strategy
Broadly surgery for these is divided in two broad categories:
Surgery for nerve repair
Secondary procedures
Whenever feasible depending on timing, surgery for nerve repair takes precedence over all other procedures since time is of the essence. As soon as other injuries are dealt with primarily the patient should have the earliest possible nerve repair.
Secondary procedures are done after nerve repairs or in very late cases as a substitute to restore function. Either way all patients can be offered some treatment at all stages.
Surgery of the nerves
Broadly divided into:
Intra plexal repair
Extra plexal repair
Distal nerve transfers
Contralateral C7 transfer.
Quite often a combination of these may be offered.
Intra plexal repair: In cases of post ganglionic injury where donor roots are available, the root stumps are joined to distal targets which may be trunks, cords or individual nerves with the help of autologous nerve grafts. These may be free grafts or vascularized grafts. In general short grafts do better than long grafts; however, such a choice is not always available.
Extra plexal repair: Nerves not arising from the plexus are used as donor nerves. Classic example as being, intercostal nerves (ICN) to musculo cutaneous nerve (MCN) for biceps and spinal accessory nerve (SAN) to supra scapular nerve (SSN) for rotator cuff reinnervation. Some authors use phrenic nerve.[44,45] Though they claim that almost all parameters gradually recover to preoperative status levels within 1 year; the loss of diaphragm function deters many others from using it. Bhandari et al.[46] have shown that though the phrenic transfer does produce the desired motor result, the long term follow up of the patients showed a persistent pulmonary function deficit even at the end of several years in very fit young adults. Their data and conclusions are reproduced below. Their series is of on 16 patients with brachial palsy (15 total and 1 partial, where it was used for axillary nerve). The observations have been as under
None of the patient manifested respiratory problems after unilateral phrenic nerve transfer
Three patients with weak or nonfunctional spinal accessory nerve underwent simultaneous unilateral transfer of phrenic nerve to the suprascapular nerve and three intercostal nerves to the musculocutaneous nerve. These patients also remained symptom free in the post operative period
Pulmonary function tests in postoperative period exhibited a significant reduction in vital capacity, total lung capacity, forced vital capacity, and forced expiratory volume in 1 s
These patients were followed up for a period of 28 to 36 months. All remained asymptomatic on running short distances (500 meter), but became more breathless on long runs when compared with control group (healthy individuals of identical age group without brachial plexus palsy)
Pulmonary functions remained suboptimal even 3 years after the surgery
The diaphragm remained raised in all patients, though the range of elevation was not universal.
Based on these observations, in his opinion, phrenic nerve is not an expendable nerve. During young age, a majority of them may remain asymptomatic. However with advancing age and in high demand situations (pulmonary infections), they will be more prone to develop respiratory complications.
Distal Nerve Transfers
The work of Oberlin, Somsak, McKinnon[47–51] and others have opened exciting new options on treating BPI. Essentially the concept is to use the fascicles or branches of a functioning distal nerve to re innervate a denervated muscle or group of muscles. The donor nerve typically suffers very little functional deficit but the recepient muscle being nearby (compared to intra plexal neurotization in neck) gets quickly innervated and functional. Typically this is a dream win–win situation, some examples:
Ulnar nerve fascicle to MCN for biceps function[47]
Branch to long head of triceps to posterior division of axillary nerve for deltoid[48]
Branch of MCN to brachialis muscle given to median nerve for finger flexion.[50]
Contralateral C7 root transfer.
Pioneered by Gu et al.[16] from Shanghai. This is a paradigm changing procedure where either the full or half contralateral C7 root from the normal side is harvested and connected to the target nerves on the affected side. In situations where there is global avulsion on the affected side this is a vital new technique which offers promise. Gu et al. have even used it to regain hand function despite the long distance involved but other authors including this author have not been able to replicate that except in obstetric cases or in very young adults. For older adults we prefer to use the contralateral C7 to get either the lateral cord or the posterior cord innervated for more proximal muscle function and the results are reliable.
Strategies for reconstruction
Strategies for adults and children (Birth Brachial Plexus Injury BBPI) differ considerably. The author has discussed the BBPI strategies elsewhere in detail.[52] Children have a far greater regeneration capacity and distance to travel for the regenerating axons is far smaller. Thus potentially total reconstruction up to and including the hand intrinsic muscles is feasible even in an all 5 root injury if operated in time. This is rarely feasible in an adult total palsy.
For all injuries we will discuss strategies for intra plexal repair and other nerve transfers simultaneously. The surgeon's judgement will depend upon his/her experience, expertise, training and the peculiar circumstances of each patient.
Strategy for C5C6 injury
In a post ganglionic injury treated early in a young patient:
C5 to lateral cord/upper trunk
C6 to posterior cord
with XIth to SSN transfer.
Secondary procedures
In a pre ganglionic Injury or Older Patient or late repair:
Ulnar+/- Median fascicle to MCN for Biceps and Brachialis[47] (Oberlin)
Nerve to Long Head of Triceps to Anterior division of Axillar Nerve for Deltoid[48] (Somsak)
XIth Nerve to SSN for Supra Infra Spinatus.
Currently the Oberlin and Somsak transfers are gaining popularity even in post ganglionic injuries amongst many surgeons due to the much higher chances of success especially in older patients and delayed repairs.
If C7 loss is added to the upper plexus injury
For post ganglionic injuries it will remain similar to C5C6 injury in cases of early surgery on a young patient. Except that, C7 stump will be attached to middle trunk/posterior cord. For cases with pre ganglionic injury or older patient or late repair the strategy is:
Ulnar to MCN
XIth to SSN
ICN's to Axillary[49] (Somsak)
Median to Triceps (long head branch).
Cases of C5C6C7 injury where the C8T1 too are not strong and CMAP on the Ulnar/Median is not good.
In that case ICN's are reserved for MCN to get a strong elbow flexion.
Figures 2 and 3 show some results. Videos of results are available in the online version.
Figure 2.
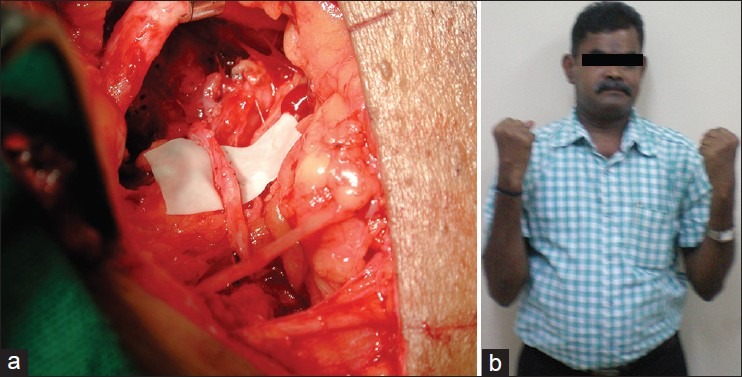
Elbow flexion restored using Oberlin's Technique (a) Ulnar nerve fascicle coapted to musculocutaneous nerve (b) Result showing elbow flexion restored using Oberlin's Technique (b) Clinical result
Figure 3.

Restoration of shoulder abduction using Somsak's technique
C8T1 or lower plexus injury
In a truly lower plexus injury the ipsilateral or contralateral C7 can be used to innervate the Lower trunk/Medial cord. If patient is young and operated early. In late cases or if C7 is not available then distal nerve transfers are possible:
Figure 4 shows a result of authors′ case of Nerve to Brachialis transfer to Median Nerve in a late presentation of a lower plexus injury.
Figure 4.
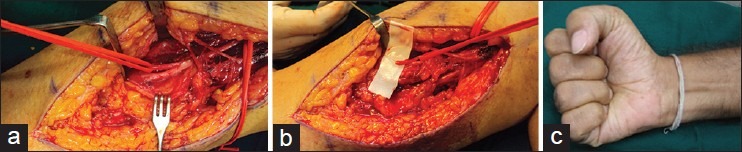
(a) Nerve to Brachialis isolated to co apt to the median nerve (b) Nerve to brachialis cut (c) Restoration of finger flexion following the repair
Strategy for flail upper limb C5-T1 injury
In a post ganglionic injury and on an early referral, total intraplexal reconstruction is possible from roots to trunks and/or cords. Figure 5 shows results in such a case.
Figure 5.

Various functions restored following intraplexal repair in a total palsy
The commoner injury is a pre ganglionic total avulsion. In these cases a multi staged strategy is employed by the author. Doi et al.[57] have shown interesting results with double Gracilis functional muscle transfers. The author uses a different strategy as follows:
Stage I: Explore Plexus, and neurotise what is feasible,
XI th to SSN
Contralateral C7 to lateral or Posterior cord to get either biceps plus Pectoralis Major (if Lateral Cord is the target of the C7) or to get Deltoid, Triceps and ECRL for Wrist extension (If Posterior cord is the target).
Stage II (3 months following stage I): Free Functional Gracilis transfer using thoracodorsal vessels and ICN's as the motors routed volarly across the elbow and sutured to the Flexor Digitorum Profundus and Flexor Pollicis Longus (FDP's and FPL). Simultaneously use one ICN for triceps if C7 is on lateral cord.
Stage III (one year after stage II): Wrist fusion if no ECRL recovery.
Stage IV: Tendon transfers to improve hand function for grasping, shoulder fusion if shoulder is unstable.
Upto 50% functional recovery is possible with restoration of activities of daily living, ability to drive, go shopping and lifting up to 5 Kg, if all goes as per plan. Using computer mouse is also possible. Fine function like buttoning a shirt, writing, typing etc., is not restored. Figures 6 shows a result of secondary procedures:
Figure 6.
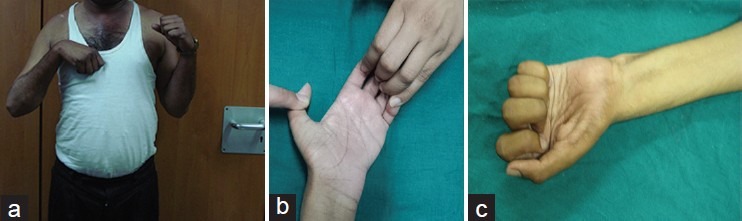
Functional restoration after reconstruction of flail upper limb (a) Elbow flexion (b) Functional restoration after reconstruction of flail upper limb (b) Fingers flexing against resistance using Gracilis (c) Functional restoration after reconstruction of flail upper limb (c) Finger flexion after Opp C7 transfer to median
These are done in late cases when nothing else is feasible
Tendon transfers using available muscles
Trapezius transfer to stabilise shoulder
Shoulder and wrist arthrodesis to improve posture
Free Functional muscle transfer—can always be attempted as the donor muscle is uninjured and has never been denervated.
Discussion and Results
Results vary depending on multiple parameters:
Age of patient-younger patients get better results
Time between injury and surgery-earlier the better-first 3 months is the best period
Extent of injury-partial plexus injuries have superior results, especially upper plexus injuries
Rehab facility-people on good rehab programmes show greater functionality and weight tolerance.
Babhulkar and Thatte[41] analyzed a small subset of the data in Bombay Hospital over a 4 year period where at least 2 years (approx.) follow up was available. This was a prospective study of adult patients with either total or partial traumatic brachial plexopathy between August 2005 and May 2009 studied at Bombay Hospital Institute of Medical Sciences, Mumbai. The aim was to evaluate the outcome of surgical management of brachial plexus injury patients with a follow up of minimum two years along with social and emotional impact over patients of brachial plexus injury.
Patients were treated with a combination of neurolysis (79 patients), neurotization (84 patients) or nerve grafting (29 patients) according to intra-operative findings. The youngest patient was of 18 years with mean of 27.4 years. The most common mode of injury was motor bike accidents (86%). The mean time interval between injury and surgery was 5.13 months. The average post operative follow up was 22.8 months. Patients subsequently may have got free functional muscle transfers in severe cases but this was not factored into the assessment. Pure nerve repair results are analysed.
Summary of findings:
When the delay for operation was more than 6 months, it affected the outcome significantly.
Patients with upper trunk injury showed maximum number of good results (70%) while those with global plexopathy showed good outcome in only 20%, fair in 36% and poor in 44%.
In global plexopathy, those having preganglionic injury had the worst outcome in the group. Outcome was inversely proportional to number of avulsed roots.
Pre-ganglionic injuries showed significantly poorer outcome than post-ganglionic injuries
Outcome with primary coaptation without nerve graft had significantly better result than the patients where the nerve graft was used.
All the factors studied in our series (delay in operation, number of avulsed roots, type and level of injury and use of nerve graft) except age of the patient affected the outcome on univariate analysis. On multimodal regression analysis, delay in operation (P value 0.049) and number of avulsed roots (P value 0.003) significantly affect the outcome of surgery, whereas age of the patient (P value 0.252), type of injury (P value 0.664), level of injury (P value 0.192) and use of nerve graft (P value 0.487) fail to show any significant association with the outcome of surgery.
We conclude that these complex injuries require tailored approach for improved prognosis. Multiple factors impart important influence on the outcome of brachial plexus surgery, only factors like delay in operation and number of avulsed roots significantly affected the outcome of surgery in our series.
FFMT using Gracilis has added a very valuable tool to enhance results in cases of flail upper limb. In the past these patients had no hope of really getting a usable upper limb following pure nerve repair. This has changed drastically with the use of Gracilis FFMT. It is therefore important to offer this option to the global avulsion patients right at the outset and outline a comprehensive program of sequential surgery and rehab to avoid depression.
Though social and emotional assessment was not done using any standardized index, it showed that poor outcome was associated with dissatisfaction, depression and impact on the career.
Recent advances
The main frustration of brachial plexus surgeons is the patient with avulsed roots—no usable proximal donor axons. Some groups[58–65] are now trying to reimplant the avulsed roots into the spinal cord with the hope of reconnecting with the tracts coming from and going to the CNS. They have had partial success but they seem to work (that too partially) only if done very early, like in the first few weeks after injury. This is a big limitation as patients are often seen quite late by the hand surgeon/plastic surgeon who is regularly treating plexus injuries.
Workers in basic biology are reporting something more fascinating in non mammalian animals; two groups working on the sea cucumber (an echinoderm)[66] and the Zebra fish[67] have shown amazing regeneration of the nervous system. The main cell responsible is the equivalent of the mammalian radial glial cell which manages to help the organism in regeneration and bridging the gap. In mammals too the glia come in at the site of an injury but currently appear to remain static there and in fact hinder regeneration to some extent. The key will lie in up regulation of genes like her-4.1 responsible for making it behave differently and cause regeneration of neurons.
Conclusions
All patients with brachial plexus injury need early referral to a person specializing in treating it
Patients get better results with earlier referral
All patients can be offered some modality of treatment irrespective of time of referral
No patient must be abandoned without offering treatment and rehabilitation.
See video on www.annalsofian.org
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.Robotti E, Longhi P, Verna G, Bocchiotti G. Brachial plexus surgery: An historical perspective. Hand Clin. 1995;4:517–33. [PubMed] [Google Scholar]
- 2.Smellie W. Vol. 3. London: Wilson and Durham; 1764. Collection of Preternatural Cases and Observations in Midwifery. [Google Scholar]
- 3.Duchenne GB. 3rd ed. Paris: Bailliere; 1872. De l′Electrisation Localisee et de son Application a la Pathologie et a la Therapeutique. [Google Scholar]
- 4.Erb WH. Ueber eine eigenthumliche localisation von lahmungen in plexus brachialis. Verh Dtsch Natur Med. 1874;2:130. [Google Scholar]
- 5.Thorburn W. A Clinical Lecture on Secondary Suture of the Brachial Plexus. Br Med J. 1900;1:1073–5. doi: 10.1136/bmj.1.2053.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harris W, Low VW. On the importance of accurate muscular analysis in lesions of the brachial plexus and the treatment of Erb's palsy and infantile paralysis of the upper extremity by cross-union of nerve roots. Br Med J. 1903;2:1035. [Google Scholar]
- 7.Vulpius O, Stoffel A. 2nd ed. Stuttgart: Enke; 1920. Orthopadische Operationslehre. [Google Scholar]
- 8.Seddon HJ. The use of autogenous grafts for the repair of large gaps in peripheral nerves. Br J Surg. 1947;35:151–67. doi: 10.1002/bjs.18003513808. [DOI] [PubMed] [Google Scholar]
- 9.Stevens JH. Brachial plexus paralysis. In: Codman EA, editor. The shoulder. Boston: Privately Published; 1934. pp. 344–50. [Google Scholar]
- 10.Narakas A. Surgical treatment of traction injuries of the brachial plexus. Clin Orthop. 1978;133:71–90. [PubMed] [Google Scholar]
- 11.Narakas A. Brachial plexus surgery. Orthop Clin North Am. 1981;12:303–23. [PubMed] [Google Scholar]
- 12.Millesi H. Surgical management of brachial plexus injuries. J Hand Surg. 1977;2:367–78. doi: 10.1016/s0363-5023(77)80046-4. [DOI] [PubMed] [Google Scholar]
- 13.Allieu Y, Privat JM, Bonnel F. Paralysis in root avulsion of the brachial plexus neurotization by the spinal accessory nerve. Clin Plast Surg. 1984;11:133–6. [PubMed] [Google Scholar]
- 14.Brunelli G, Brunelli F. Use of anterior nerves of cervical plexus to partially neurotize the avulsed brachial plexus. In: Brunelli G, editor. Textbook of Microsurgery. Milano: Masson; 1988. pp. 803–7. [Google Scholar]
- 15.Gu YD, Wu MM, Zhen YL, Zhao JA, Zhang GM, Chen DS, et al. Phrenic nerve transfer for brachial plexus motor neurotization. Microsurgery. 1989;10:287–9. doi: 10.1002/micr.1920100407. [DOI] [PubMed] [Google Scholar]
- 16.Gu YD, Zhang GM, Chen DS, Yan JG, Cheng XM, Chen L. Seventh cervical nerve root transfer from the contralateral healthy side for treatment of brachial plexus root avulsion. J Hand Surg. 1992;17:518–21. doi: 10.1016/s0266-7681(05)80235-9. [DOI] [PubMed] [Google Scholar]
- 17.Terzis JK, Papakonstantinou KC. The surgical treatment of brachial plexus injuries in adults. Plast Reconstr Surg. 2000;106:1097–122. doi: 10.1097/00006534-200010000-00022. [DOI] [PubMed] [Google Scholar]
- 18.Doi K, Muramatsu K, Hattori Y, Otsuka K, Tan SH, Nanda V, et al. Restoration of prehension with the double free muscle technique following complete avulsion of the brachial plexus: Indications and long-term results. J. Bone Joint Surg. 2000;82:652–66. [PubMed] [Google Scholar]
- 19.Sunderland S. London: Churchill Livingstone; 1978. Nerves and Nerve Injuries. [Google Scholar]
- 20.Nagano A, Ochiai N, Sugioka H, Hara T, Tsuyama N. Usefulness of myelography in brachial plexus injuries. J Hand Surg. 1989;14:59–64. doi: 10.1016/0266-7681(89)90017-x. [DOI] [PubMed] [Google Scholar]
- 21.Petras AF, Sobel DF, Mani JR, Lucas PR. CT myelography in cervical nerve root avulsion. J Comput Assist Tomogr. 1985;9:275–9. doi: 10.1097/00004728-198503000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Piatt JH, Jr, Hudson AR, Hoffman HJ. Preliminary experiences with brachial plexus exploration in children: Birth injury and vehicular trauma. Neurosurgery. 1988;22:715–23. doi: 10.1227/00006123-198804000-00016. [DOI] [PubMed] [Google Scholar]
- 23.Popovich MJ, Taylor FC, Helmer E. MR imaging of birth-related brachial plexus avulsion. AJNR Am J Neuroradiol. 1989;10:S98. [PMC free article] [PubMed] [Google Scholar]
- 24.Sherrier RH, Sostman HD. Magnetic resonance imaging of the brachial plexus. J Thorac Imag. 1993;8:27–33. [PubMed] [Google Scholar]
- 25.Urabe F, Matsuishi T, Kojima K, Abe T, Utsunomiya H, Okudera T. MR imaging of birth brachial palsy in a two-month-old infant. Brain Dev. 1991;13:130–1. doi: 10.1016/s0387-7604(12)80121-5. [DOI] [PubMed] [Google Scholar]
- 26.Vielvoye GJ, Hoffmann CF. Neuroradiological investigations in cervical root avulsion. Clin Neurol Neurosurg. 1993;95:S36–8. doi: 10.1016/0303-8467(93)90033-d. [DOI] [PubMed] [Google Scholar]
- 27.Wehrli FW. Fast-scan magnetic resonance: Principles and applications. Magn Reson Q. 1990;6:165–236. [PubMed] [Google Scholar]
- 28.Gupta RK, Mehta VS, Banerji AK, Jain RK. MR evaluation of brachial plexus injuries. Neuroradiology. 1989;31:377–81. doi: 10.1007/BF00343859. [DOI] [PubMed] [Google Scholar]
- 29.Doi K, Otsuka K, Okamoto Y, Fujii H, Hattori Y, Baliarsing AS. Cervical nerve root avulsion in brachial plexus injuries: Magnetic resonance imaging classification and comparison with myelography and computerized tomography myelography. J Neurosurg. 2002;96:277–84. doi: 10.3171/spi.2002.96.3.0277. [DOI] [PubMed] [Google Scholar]
- 30.Amrami KK, Port JD. Imaging the brachial plexus. Hand Clin. 2005;21:25–37. doi: 10.1016/j.hcl.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 31.Yeoman PM, Seddon HJ. Brachial plexus injuries: Treatment of the flail arm. J Bone Joint Surg. 1961;43:493. [Google Scholar]
- 32.Kennedy R. Suture of the brachial plexus in birth paralysis of the upper extremity. Br Med J. 1903;1:298–301. doi: 10.1136/bmj.1.2197.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sever JW. Obstetric paralysis: Its etiology, clinical aspects and treatment, with a report of four hundred and seventy cases. Arch Pediatr Adolesc Med. 1916;12:541–7. [Google Scholar]
- 34.Wyeth JA, Sharpe W. The field of neurological surgery in a general hospital. Surg Gynecol Obstet. 1917;24:29–36. [Google Scholar]
- 35.Gilbert A, Tassin JL. Reparation chirurgicale du plexus brachial dans la paralysie bstetricale. Chirurgie. 1984;110:70–5. [PubMed] [Google Scholar]
- 36.Narakas AO. Obstetrical brachial plexus injuries. In: Lamb DW, editor. The Paralysed Hand. Edinburgh: Churchill Livingstone; 1987. pp. 116–35. [Google Scholar]
- 37.Kawabata H, Masada K, Tsuyuguchi Y. Early microsurgical reconstruction in birth palsy. Clin Orthop. 1987;215:233–42. [PubMed] [Google Scholar]
- 38.Millessi H. Brachial plexus injuries: Nerve grafting. Clin Orthop. 1988;237:43–56. [PubMed] [Google Scholar]
- 39.Ferrante MA. Electrodiagnostic Assessment of the Brachial Plexus. Neurol Clin. 2012;30:551–80. doi: 10.1016/j.ncl.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 40.Bhandari R. (Guided by and using data of Thatte MR) Thesis submitted to the University of Bombay for MS Orth. 2004 [Google Scholar]
- 41.Babhulkar S. (Guided by and using data of Thatte MR) Thesis submitted to the National Board for DNB Plastic. 2010 [Google Scholar]
- 42.Bhatia AG. How old is “TOO OLD” for nerve reconstruction? Presentation at the meeting of the European Federation of Societies for Microsurgery held at Genova in Italy in May. 2010 [Google Scholar]
- 43.Thatte MR, Agashe M, Rathod C, Lad P, Mehta R. An approach to the supraclavicular and infraclavicular aspects of the brachial plexus. Tech Hand Up Extrem Surg. 2011;15:188–97. doi: 10.1097/BTH.0b013e3182164b15. [DOI] [PubMed] [Google Scholar]
- 44.Gu Y, Meng K. Use of the Phrenic Nerve for Brachial Plexus Reconstruction. Clin Orthop Relat Res. 1996;323:119–21. doi: 10.1097/00003086-199602000-00016. [DOI] [PubMed] [Google Scholar]
- 45.Xu WD, Gu YD, Lu JB, Yu C, Zhang CG, Xu JG. Pulmonary function after complete unilateral phrenic nerve transection. J Neurosurg. 2005;103:464–7. doi: 10.3171/jns.2005.103.3.0464. [DOI] [PubMed] [Google Scholar]
- 46.Bhandari PS. Paper presented at APSICON 2010, Annual Conference of Associayion of Plastic Surgeons of Undia in Goa India. [Google Scholar]
- 47.Oberlin C, Beal D, Leechavengvongs S, Salon A, Dauge MC, Sarcy JJ. Nerve transfer to biceps muscle using a part of ulnar nerve for C5/C6 avulsion of the brachial plexus. Anatomical study and report of cases. J Hand Surg. 1994;19:232–7. doi: 10.1016/0363-5023(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 48.Leechavengvongs S, Witoonchart K, Uerpairojkit C, Thuvasethakul P. Nerve transfer to deltoid muscle using the nerve to the long head of the triceps, part II: A report of 7 cases. J Hand Surg Am. 2003;28:633–8. doi: 10.1016/s0363-5023(03)00199-0. [DOI] [PubMed] [Google Scholar]
- 49.Malungpaishrope K, Leechavengvongs S, Uerpairojkit C, Witoonchart K, Jitprapaikulsarn S, Chongthammakun S. Nerve transfer to deltoid muscle using the intercostal nerves through the posterior approach: An anatomic study and two case reports. J Hand Surg Am. 2007;32:218–24. doi: 10.1016/j.jhsa.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 50.Colbert SH, Mackinnon SE. Nerve transfers for brachial plexus reconstruction. Hand Clin. 2008;24:341–61. doi: 10.1016/j.hcl.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 51.Mackinnon SE, Dellon AL. New York: Thieme Medical Publishers; 1988. Surgery of the Peripheral Nerve. [Google Scholar]
- 52.Thatte MR, Mehta R. Obstetric brachial plexus injury. Indian J Plast Surg. 2011;44:380–9. doi: 10.4103/0970-0358.90805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gu Y, Wang H, Zhang L, Zhang G, Zhao X, Chen L. Transfer of brachialis branch of musculocutaneous nerve for finger flexion: Anatomic study and case report. Microsurgery. 2004;24:358–62. doi: 10.1002/micr.20053. [DOI] [PubMed] [Google Scholar]
- 54.Zhao X, Lao J, Hung LK, Zhang GM, Zhang LY, Gu YD. Selective neurotization of the median nerve in the arm to treat brachial plexus palsy. An anatomic study and case report. J Bone Joint Surg Am. 2004;86-A:736–42. doi: 10.2106/00004623-200404000-00010. [DOI] [PubMed] [Google Scholar]
- 55.Dong Z, Gu YD, Zhang CG, Zhang L. Clinical use of supinator motor branch transfer to the posterior interosseous nerve in C7-T1 brachial plexus palsies. J Neurosurg. 2010;113:113–7. doi: 10.3171/2010.1.JNS091441. [DOI] [PubMed] [Google Scholar]
- 56.Bertelli JA, Ghizoni MF, Tacca CP. Transfer of the supinator muscle to the extensor pollicis brevis for thumb extension reconstruction in C7-T1 brachial plexus palsy. J Hand Surg Eur. 2010;35:29–31. doi: 10.1177/1753193409350251. [DOI] [PubMed] [Google Scholar]
- 57.Doi K, Sakai K, Kuwata N, Ihara K, Kawai S. Double-muscle technique for reconstruction of prehension after complete avulsion of brachial plexus. J Hand Surg. 1995;20:408–14. doi: 10.1016/S0363-5023(05)80097-8. [DOI] [PubMed] [Google Scholar]
- 58.Carlstedt T, Grane P, Hallin RG, Noren G. Return of function after spinal cord implantation of avulsed spinal nerve roots. Lancet. 1995;346:1323–5. doi: 10.1016/s0140-6736(95)92342-x. [DOI] [PubMed] [Google Scholar]
- 59.Carlstedt TP, Anand P, Hallin R, Misra PV, Noren G, Seferlis T. spinal nerve root repair and reimplantation of avuled ventral roots into the spinal cord after brachial plexus injury. J Neurosurg. 2000;93:237–42. doi: 10.3171/spi.2000.93.2.0237. [DOI] [PubMed] [Google Scholar]
- 60.Bertelli JA, Mira JC. Brachial plexus repair by peripheral nerve grafts directly into the spinal cords in rats. Behavioral and anatomical evidence of functional recovery. J Neurosurg. 1994;81:107–14. doi: 10.3171/jns.1994.81.1.0107. [DOI] [PubMed] [Google Scholar]
- 61.Bertelli JA, Orsal D, Mira JC. Median nerve neurotization by peripheral nerve grafts directly implanted into the spinal cord: Anatomical, behavioural and electrophysiological evidence of sensorimotor recovery. Brain Res. 1994;644:150–9. doi: 10.1016/0006-8993(94)90358-1. [DOI] [PubMed] [Google Scholar]
- 62.Bertelli JA, Taleb M, Mira JC, Calixto JB, Kassar L. Brachial plexus repair by peripheral nerve grafts directly implanted into the contralateral spinal cord. Restor Neurol Neurosci. 1997;11:189–94. [Google Scholar]
- 63.Bertelli JA, Taleb M, Mira JC, Kassar L. Selective restoration of sensation by PNG directly implanted into contralateral C7 DRG. An experimental study in the rat brachial plexus. Neurosurgery. 1998;42:125–9. doi: 10.1097/00006123-199801000-00025. [DOI] [PubMed] [Google Scholar]
- 64.Bertelli JA, Ghizoni MF. Brachial plexus avulsion injury repairs with nerve transfers and nerve grafts directly implanted into the spinal cord yield partial recovery of shoulder and elbow movements. Neurosurgery. 2003;52:1385–90. doi: 10.1227/01.neu.0000065134.21334.d7. [DOI] [PubMed] [Google Scholar]
- 65.Fournier HD, Mercier P, Menei P. Repair of Avulsed Ventral Nerve Roots by Direct Ventral Intraspinal Implantation after Brachial Plexus Injury. Hand Clin. 2005;21:109–18. doi: 10.1016/j.hcl.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 66.Mashanov VS, Zueva OR, Heinzeller T. Regeneration of the radial nerve cord in a holothurian: A promising new model system for studying post-traumatic recovery in the adult nervous system. Tissue Cell. 2008;40:351–72. doi: 10.1016/j.tice.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 67.Kroehne V, Freudenreich D, Hans S, Kaslin J, Brand M. Regeneration of the adult zebrafish brain from neurogenic radial glia-type progenitors. Development. 2011;138:4831–41. doi: 10.1242/dev.072587. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


