Abstract
Objectives:
Electrocardiographic (ECG) changes are reported frequently after acute strokes. It seems that cardiovascular effects of strokes are modulated by concomitant or pre-existent cardiac diseases, and are also related to the type of cerebrovascular disease and its localization. We aimed to determine the pattern of ECG changes associated with pathophysiologic categories of acute stroke among patients with/without cardiovascular disease and to determine if specific ECG changes are related to the location of the lesion.
Materials and Methods
The electrocardiographic records of 361 patients with acute stroke were studied to assess the relative frequencies of ECG abnormalities among the pathophysiologic categories of stroke.
Results:
In the present study, the most common ECG abnormalities associated with stroke were T-wave abnormalities, prolonged QTc interval and arrhythmias, which were respectively found in 39.9%, 32.4%, and 27.1% of the stroke patients and 28.9%, 30.7%, and 16.2 of the patients with no primary cardiac disease. We observed that other ECG changes comprising pathologic Q- wave, ST-segment depression, ST-segment elevation, and prominent U wave may also occur in selected or non-selected stroke patients; thereby simulate an acute myocardial injury. We observed an increased number of patients with abnormal T-wave and posterior fossa bleedings and more rhythm disturbances for ischemic lesions, localized in the anterior fossa.
Conclusion:
Ischemia-like ECG changes and arrhythmias are frequently seen in stroke patients, even in those with no history or signs of primary heart disease, which support a central nervous system origin of these ECG abnormalities. Further study is necessary to better define the brain-heart interaction.
Keywords: Cardiac, cerebrovascular attack, electrocardiography
Introduction
Every year, more than half a million people in the world suffer from acute cerebrovascular events, including ischemic stroke, intracerebral and subarachnoid hemorrhage, giving a mortality of nearly 20%.[1] Acute strokes, especially subarachnoid hemorrhage is frequently accompanied by a variety of electrocardiographic (ECG) abnormalities,[2–7] some of which may be indistinguishable from those seen in association with an episode of severe myocardial ischemia and/or infarction. In addition, patients often have simultaneous hypertension or coronary atherosclerosis, leading to ECG abnormalities. In addition, many primary cardiac disorders, like myxoma, mural thrombus, endocarditis, and atrial septal defect with deep venous thrombosis, can lead to cerebral emboli; arrhythmias, heart block, and decreased cardiac output, which may precipitate cerebral ischemia. Then, healthcare professionals presented with this clinical/electrocardiographic picture are confronted with special challenges, and it is crucial to distinguish stroke-induced ECG changes from ECG changes due to concomitant ischemic heart disease.
There is a very considerable heterogeneity in how ECG changes in stroke patients are presented in the literature.[2–7] This electrocardiographic spectrum seems to be related to the type of cerebrovascular disease and its localization. The autonomic and cardiovascular effects of stroke; however, are modulated by concomitant factors such as pre-existent cardiac diseases and electrolyte disorders. Although many subsequent reports have described ECG abnormalities and rhythm disturbances in stroke, especially subarachnoid hemorrhage, few have included an adequate number of patients to statistically assess the relative frequencies of these abnormalities among the pathophysiologic categories of stroke. Furthermore, few previous studies have evaluated ECG changes and rhythm disturbances in ischemic stroke patients without primary heart disease to distinguish abnormalities specifically associated with acute stroke. In view of the varied explanations for the ECG abnormalities in acute CVA, the present study was undertaken to review the pattern of ECG changes associated with pathophysiologic categories of acute stroke among patients with/without cardiovascular disease and to determine if specific ECG changes are related to the location of the lesion.
Materials and Methods
Study design and setting
The study was conducted in an Iranian tertiary center (Sina hospital) affiliated to Tehran University of Medical Sciences (TUMS). This was a retrospective review of a cohort of patients admitted with a diagnosis of acute cerebrovascular events including ischemic stroke, intracerebral and subarachnoid hemorrhage in our center. Those with: 1) unavailable ECG within 24 hours; 2) a head trauma within 1 week prior to the stroke; 3) a documented history of subdural hematoma; 4) stroke in the setting of dissecting aortic aneurysm; 5) a functioning artificial pacemaker; or 6) alternative diagnoses such as cerebral tumors, electrolyte disturbances, or left ventricular hypertrophy on ECG, and bundle branch block were excluded. The study was conducted in accordance with Helsinki declaration and was performed in line with considerations, recommended by TUMS ethics committee.
Ischemic stroke was defined as a rapidly developing clinical sign of focal or global disturbances of cerebral function, lasting at least 24 h or leading to death with no apparent cause other than vascular origin, and it was verified by cranial computerized tomography (CT).
Subarachnoid hemorrhage was diagnosed if 1) the patient suffered from the sudden onset of headache and stiff neck without lateralizing neurologic signs, and had grossly bloody cerebrospinal fluid; or 2) angiography or CT findings were diagnostic.
Intracerebral hemorrhage was diagnosed if the patient suffered from the sudden onset of headache, lateralizing signs, or loss of consciousness approved by CT scanning.
Clinical characteristics and ECG changes were compared in different categories of acute stroke. Hypertension was defined as systolic blood pressure ≥140 mmHg and diastolic blood pressure ≥90 mmHg or receiving anti-hypertensive medications. Dyslipidemia was defined as receiving lipid-lowering agents or total serum cholesterol ≥240 mg/dl or serum triglyceride ≥200 mg/dl. A positive smoking history was defined as a person smoking at least 10 cigarettes a day for at least 5 years.[8] Individuals with the continuous consumption of 100-150 gr ethanol for at least 2 years were considered alcohol consumers. Ischemic heart disease was defined as the presence of 1: history of angina pectoris or angina equivalent symptoms and coronary care unit admission, 2: myocardial infarction, 3: coronary revascularization and/or stenting, 4: positive myocardial spect scan, 5: positive stress testing.
Electrocardiography
A 12-lead ECG was recorded with a sensitivity of 10 mm/mv and a paper speed of 25 mm/s from all patients within 24 h after admission. ECG measurements were made manually by a single experienced cardiologist blinded to the patients’ information. The ECGs were analyzed for QTc- (heart rate–corrected QT)[7] interval duration, ST-segment depression or elevation, T-wave abnormalities, U-wave presence, and arrhythmias. ECG abnormalities were defined according to the following criteria:
Down-sloping ST-segment depression or its flat depression of 1 mm and the elevation of 1.0 mm in extremity and precordial leads (but elevation of 2.0 mm for V1 and V2) were considered as significant.[5]
Q-wave and T-wave abnormalities were assessed in leads I, II, aVL, aVF, and V2–6. T-wave abnormalities included T-waves that are of low voltage or are flat or inverted in leads that are normally upright or that are abnormally tall and peaked; a T-wave less than 0.1 mV in depth was defined as inverted.[5]
The Q-waves were considered significant (unless confined to lead III) if they were greater than 0.04 seconds in duration or more than 25% of the height of the R-wave for that lead.[5]
Abnormal U-wave was defined as negative U-wave with more than 0.1 mV depth or positive U wave higher than 25% of T-wave.[5] U-waves were considered significant if they were visible in more than 2 leads.
The QT interval was measured from the beginning of QRS complex to the end of T-wave, defined as a return to TP baseline.[7] If U-wave was present, the QT interval was measured to the nadir of the curve between T- and U-waves. The corrected QT (QTc) interval was defined as the QT interval divided by the square root of the RR interval (Bazett's formula) from an average of 3 complexes in lead II and was considered abnormal if it was longer than 0.44 s.[7]
Rhythm disturbances were stratified as sinus bradycardia or tachycardia, atrial or ventricular premature beat, atrial fibrillation (AF), atrioventricular block, supraventricular and ventricular tachycardia.
Statistics
The data were entered into Microsoft Excel and transported into SPSS for Windows for analysis. Categorical variables are presented as frequency of occurrence and were analyzed by χ2 or Fisher's exact test, if necessary. All tests were two-tailed with alpha set at 0.05.
Results
A total of 361 patients were included in this study; 303 patients with ischemic stroke, 41 patients with SAH, and 17 patients with ICH. Clinical data from the three patient groups are presented in Table 1. The sex distribution was similar in the SAH (70.6% men) and ICH groups (70.7% men) with the predominance of male gender, while the ischemic stroke group had a similar proportion of male and female. The history of cardiovascular disease, previous stroke, diabetes mellitus, hypertension, and dyslipidemia was the same in all groups.
Table 1.
Clinical characteristics of stroke patients
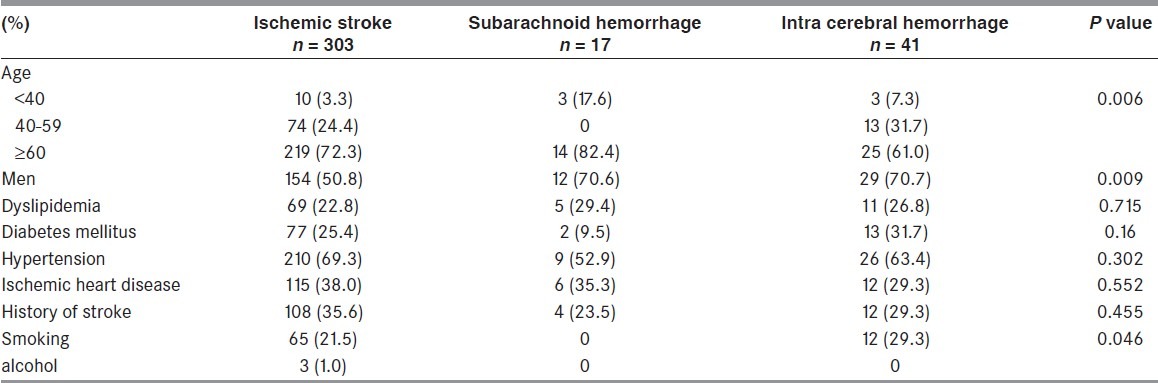
Table 2 compares various ECG findings between patients of the study groups. T-wave abnormalities were observed in 39.9% of the patients with stroke and prolonged QTc intervals in 32.4%, constituting the most frequent single ECG abnormalities in stroke. The most frequent ECG abnormality observed in both ischemic stroke and ICH groups was abnormal T-wave, which was present in 39.9% and 43.9% of the patients, respectively. The most pronounced difference between the groups was observed for the occurrence of the Q-T interval of more than 0.44 s and Pathologic Q-wave (each P value = 0.001). QT prolongation occurred more frequently in patients with SAH (53%) than in other types of stroke (31%; P = 0.001), as did Pathologic Q (47.1% vs. 15.8%; P = 0.001). No relationship was obtained between the frequency of other ECG abnormalities including ST-segment, T- and U-wave abnormalities, and arrhythmias and type of stroke.
Table 2.
Comparison of electrocardiographic findings in different categories of stroke in Patients without cardiovascular diseases or hypertension
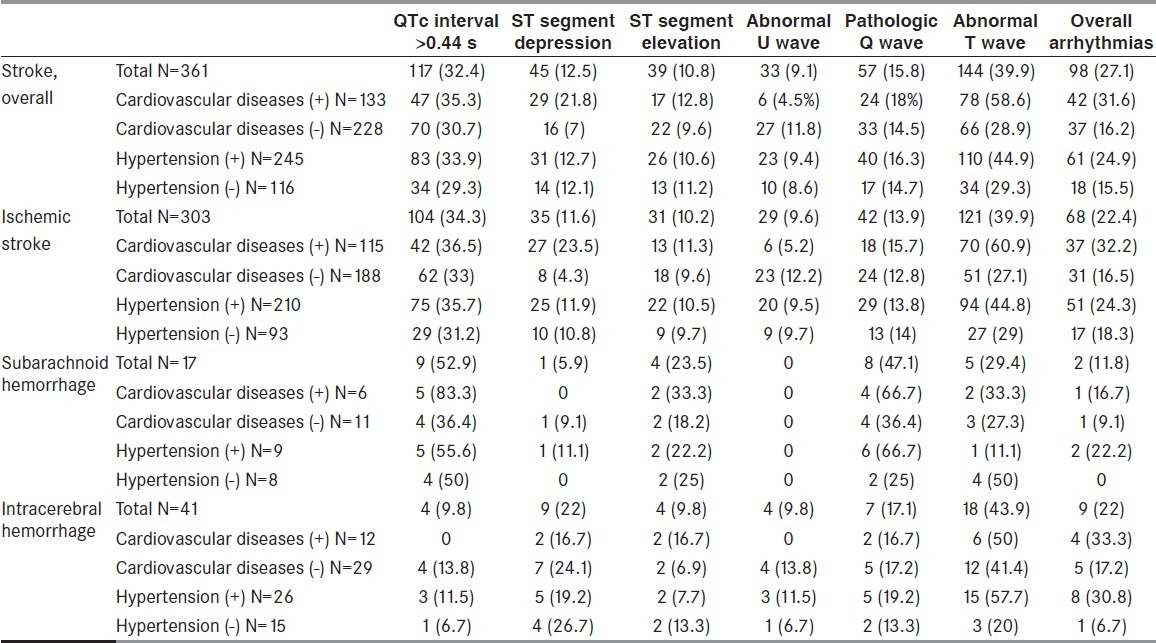
Among patients without cardiovascular disease (n = 228), 30.7% had prolonged QTc interval, 14.5% had Pathologic Q-wave, and 16.2% had arrhythmias of any type. Among ischemic ECG changes, ST-segment depression was observed in 7%, ST-segment elevation in 9.6%, T-wave abnormalities in 28.9%, and abnormal U-wave in 11.8% of them. In order to analyze the importance of the influence of the non-cerebral disorders in ECG, patients with ECG changes in each group were compared between those with/without cardiovascular diseases and hypertension [Table 2]. By comparing these groups, among ischemic stroke patients, all ECG alteration were more common in groups with history of cardiovascular diseases and hypertension, except for abnormal U-wave.
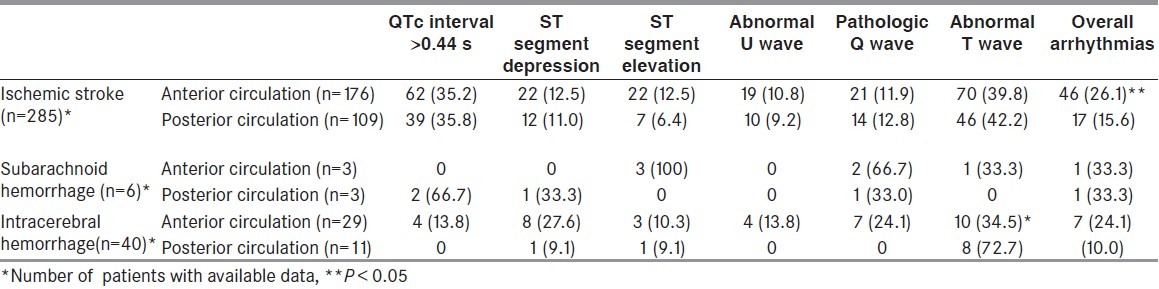
The frequency of ECG abnormalities according to lesion location is reported in Table 3. The study groups were broken down according to the location of the lesion, and ECG findings in patients with the lesions localized in the anterior fossa in each group were compared with the corresponding figures of the remaining patients in posterior fossa. Except for a higher frequency of an abnormal T-wave in the posteriorly localized intracerebral bleedings and more rhythm disturbances for ischemic lesions, localized in the anterior fossa (P = 0.03 and P = 0.02, respectively), no major differences could be demonstrated.
Table 3.
The frequency (%) of electrocardiographic abnormalities according to lesion locations in stroke patients
Discussion
The mechanisms by which acute cerebrovascular events cause ECG changes are unsettled. It has been suggested that changes in the autonomic nervous system activity can be primarily responsible for these ischemic, arrhythmic, and repolarization changes.[9] Sustained sympathetic stimulation results in structural damages to the myocardium, which may be mediated by a sudden increase in intracranial pressure,[10] hypothalamic,[11] and cardiac nerve stimulation or through an arrhythmogenic center in the insular cortex.[12] Moreover, direct damage to the cardiac innervations or imbalance between the left and right sympathetic outflows to the heart, underlying atherosclerotic or hypertensive cardiovascular disease, or asymptomatic/undetectable primary heart disease are among the suggested causes.[13,14]
In the present study, the most common ECG stroke-associated abnormalities were T-wave abnormalities, prolonged QTc interval, and arrhythmias found respectively in 39.9%, 32.4%, and 27.1% of the stroke patients and 28.9%, 30.7%, and 16.2 of patients with no primary heart disease. We observed that other ECG changes comprising pathologic Q-wave, ST-segment depression, ST-segment elevation, and prominent-U wave may also occur in selected or non-selected stroke patients, thereby simulating an acute myocardial injury. The high frequency of ECG abnormalities in our study population is in line with the frequency reported in the literature.[2–7,11,15–17] According to the results of these studies, the ischemia-like ECG abnormalities and QT interval prolongation can occur in more than 90% of the patients with ischemic or hemorrhagic stroke, and this rate will decrease by 8–40% if patients with a history of known heart disease and treatment by cardiac drugs are excluded. In an early study of 150 patients with acute stroke, Golstein[5] detected QT prolongation in 45%, ischemic changes in 39%, arrhythmias in 27%, and U-waves in 28% of their patients. In a study on patients with ischemic stroke but without history of primary heart disease, Dogan et al.[4] found ischemia-like ECG changes in 65% of patients, QTc interval prolongation in 26%, and arrhythmias in 44% of them. In the study of Lindgren et al.,[18] transient ST-T changes were found in 54% of patients with ischemic stroke with no primary heart disease. Prominent U-wave, QT interval prolongation, and arrhythmia were observed in 17%, 13%, and 4% of them, respectively. Compared with our study, however, that study included only 24 patients with cerebral infarction. On the other hand, in the study of McDermott et al.,[19] ST-segment depression was noted in only 8% of the selected patients without a history of coronary heart disease, whereas it was observed in 60% of the patients. However, the number of study patients was also limited to 51 cases.
Most of the ECG abnormalities described after an acute cerebral event were linked to SAH. In SAH, the incidence of ECG abnormalities ranges from 49% to 100%, including changes in ST segment (15% to 51% of patients), T-waves (12% to 92%), prominent U-waves (4% to 47%), QT prolongation (11% to 66%), pathological Q waves, and sinus dysrhythmias.[2,6,15–17]
In conformity with previous reports on SAH patients,[2,6,15–17] the most frequent ECG abnormality observed in the present study was prolonged QTc interval, which represents disorders of the repolarization process, and has been suggested to be associated with an increased susceptibility to cardiac arrhythmias. The cause of the initial prolongation of the QTc interval after SAH remains unclear. First, the interval prolongation may be due to a disturbed regional myocardial sympathetic activity.[20] Second, SAH causes a decrease in serum magnesium mediated possibly by free fatty acids, sympathetic stimulation, and coronary vasospasm (decreasing the serum magnesium also causes vasospasm).[6] Although widely reported, the prolonged length of the QTc interval was debated by Shuster.[21] He cautioned against inadvertently including the U-wave in the QTc interval. The U-wave, which is often 1 mm or greater in amplitude in patients with SAH, can be mistakenly interpreted as a part of a notched T-wave if the U wave occurs early during repolarization.
Abnormal T-wave was the most abnormal ECG findings in patients with ischemic stroke. However, the higher incidence of abnormal T-wave in ischemic stroke patients with cardiovascular diseases implies the influences of other factors on the T-wave than the intracranial disorder in this group. The accurate interpretation of T-wave changes can assist the clinician toward an accurate diagnosis. Low-amplitude and abnormally inverted T-waves may be the result of non-cardiac pathophysiology; while T-wave inversions produced by MI are classically narrow and symmetric. ST segment depression is another ECG change often reported in SAH patients.[3] It has been reported that advanced age itself, which is the time of occurrence of stroke,[5,18,19] is associated with the presence of ST-segment change. In the population based-study, ischemia-like ECG changes were observed in 27% of men and 31% of women in the population aged 65–74 years. New pathologic Q-waves, in contrast to ischemic changes, rarely occur in stroke. Only 2 cases in which acute strokes produced Q-waves without an autopsy evidence of infarction have been reported, yet.[5]
Attempts to correlate the ECG abnormalities with the location of the brain lesion have been made previously by several authors, but the reported results are divergent. The direct stimulation of many areas of the CNS is known to result in abnormal ECG patterns; while no relationship of the ECG changes, to the site of the bleeding aneurysm, was found by Cropp and Manning,[22] Shuster[21] and Hunt et al.[23] In studies focused on the intracranial vascular spasm after SAH and the appearance of ECG abnormalities, Wilkins et al.[24] showed no relationship, while Stober and Kunze[25] were able to find a correlation between cerebral arteries spasms of the left hemisphere, T-wave inversion, and QT prolongation. A correlation between arrhythmias and T-wave abnormalities and hemorrhages in the anterior cerebral circulation has been reported in patients with cerebral hemorrhages.[26] Dogan et al.[4] observed that lesion of insular cortex, which lies beneath frontoparietal and superior temporal opercula, leads to several cardiac abnormalities such as ischemic ECG changes, arrhythmias, and myocytolysis.[27] Our major finding was an increased number of patients with abnormal T-wave, posterior fossa bleedings, and more rhythm disturbances for ischemic lesions, localized in the anterior fossa. Thus, from these various observations, it has not been possible to establish a clear correlation between the location of the intracranial disorder and a specific ECG abnormality.
We believe that our study, although retrospective, adds to the current knowledge regarding the utility of an ECG in the setting of acute stroke. We also believe that our study group was less selected than the aforementioned studies, and our population represents the majority of stroke patients who would have been hospitalized at our center during the study period. Since we did not routinely perform thoracic echocardiography and other investigations such as stress echocardiography, myocardial scintigraphy, and scanning techniques for coronary artery territory, asymptomatic coronary heart disease may have remained undetectable.
In conclusion, ischemia-like ECG changes and arrhythmias are frequently seen in stroke patients, even in those with no history or signs of primary heart disease, which support a central nervous system origin of these ECG abnormalities. In addition, specific ECG abnormality correlated with a localized intracranial pathological change could not be established. Further studies are required to more precisely clarify the causal connection between these abnormalities and the intracranial lesion.
Acknowledgments
The authors would like to thank Research Development Center of Sina Hospital for technical assistance.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil
References
- 1.Alter M, Zhang ZX, Sobel E, Fisher M, Davanipour Z, Friday G. Standardized incidence ratios of stroke: A worldwide review. Neuroepidemiology. 1986;5:148–58. doi: 10.1159/000110824. [DOI] [PubMed] [Google Scholar]
- 2.Sommargren CE. Electrocardiographic abnormalities in patients with subarachnoid hemorrhage. Am J Crit Care. 2002;11:48–56. [PubMed] [Google Scholar]
- 3.Catanzaro JN, Meraj PM, Zheng S, Bloom G, Roethel M, Makaryus AN. Electrocardiographic T-wave changes underlying acute cardiac and cerebral events. Am J Emerg Med. 2008;26:716–20. doi: 10.1016/j.ajem.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 4.Dogan A, Tunc E, Ozturk M, Kerman M, Akhan G. Electrocardiographic changes in patients with ischaemic stroke and their prognostic importance. Int J Clin Pract. 2004;58:436–40. doi: 10.1111/j.1368-5031.2004.00010.x. [DOI] [PubMed] [Google Scholar]
- 5.Goldstein DS. The electrocardiogram in stroke. Relationship to pathophysiological type and comparison with prior tracings. Stroke. 1979;10:253–9. doi: 10.1161/01.str.10.3.253. [DOI] [PubMed] [Google Scholar]
- 6.van den Bergh WM, Algra A, Rinkel GJ. Electrocardiographic abnormalities and serum magnesium in patients with subarachnoid hemorrhage. Stroke. 2004;35:644–8. doi: 10.1161/01.STR.0000117092.38460.4F. [DOI] [PubMed] [Google Scholar]
- 7.Villa A, Bacchetta A, Milani O, Omboni E. QT interval prolongation as a predictor of early mortality in acute ischemic stroke patients. Am J Emerg Med. 2001;19:332–3. doi: 10.1053/ajem.2001.24450. [DOI] [PubMed] [Google Scholar]
- 8.Ridkev PM, Cushman M, Stamker MJ, Stampfer MJ, Tracy RP, Hennekens CH. Plasma concentration of C-reactive protein and risk of developing peripheral vascular disease. Circulation. 1998;97:125–8. doi: 10.1161/01.cir.97.5.425. [DOI] [PubMed] [Google Scholar]
- 9.Myers MG, Norris JW, Hachniski VC, Sole MJ. Plasma norepinephrine in stroke. Stroke. 1981;12:200–4. doi: 10.1161/01.str.12.2.200. [DOI] [PubMed] [Google Scholar]
- 10.Shanlin RJ, Sole MJ, Rahimifar M, Tator CH, Factor SM. Increased intracranial pressure elicits hypertension, increased sympathetic activity, electrocardiographic abnormalities and myocardial damage in rats. J Am Coll Cardiol. 1988;12:726–36. doi: 10.1016/s0735-1097(88)80065-2. [DOI] [PubMed] [Google Scholar]
- 11.Melville KI, Blum B, Shister HE, Silver MD. Cardiac ischemic changes and arrhythmias induced by hypothalamic stimulation. Am J Cardiol. 1963;12:781–91. doi: 10.1016/0002-9149(63)90281-9. [DOI] [PubMed] [Google Scholar]
- 12.Hirashima Y, Takashima S, Matsumura N, Kurimoto M, Origasa H, Endo S. Right sylvian fissure subarachnoid hemorrhage has electrocardiographic consequences. Stroke. 2001;32:2278–81. doi: 10.1161/hs1001.096620. [DOI] [PubMed] [Google Scholar]
- 13.Natelson BH. Neurocardiology: An interdisciplinary area for the 80s. Arch Neurol. 1985;42:178–84. doi: 10.1001/archneur.1985.04060020096022. [DOI] [PubMed] [Google Scholar]
- 14.Greenhoot JH, Reichenbach DD. Cardiac injury and subarachnoid hemorrhage: A clinical, pathological and physiological correlation. J Neurosurg. 1969;30:521–31. doi: 10.3171/jns.1969.30.5.0521. [DOI] [PubMed] [Google Scholar]
- 15.Frontera JA, Parra A, Shimbo D, Fernandez A, Schmidt JM, Peter P, et al. Cardiac Arrhythmias after Subarachnoid Hemorrhage: Risk Factors and Impact on Outcome. Cerebrovasc Dis. 2008;26:71–8. doi: 10.1159/000135711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liman T, Endres M. Elevated troponin and ECG alterations in acute ischemic stroke and subarachnoid hemorrhage. Nervenarzt. 2008;79:1388–90. doi: 10.1007/s00115-008-2541-z. [DOI] [PubMed] [Google Scholar]
- 17.Mayer SA, Lin J, Homma S, Solomon RA, Lennihan L, Sherman D, et al. Myocardial injury and left ventricular performance after subarachnoid hemorrhage. Stroke. 1999;30:780–6. doi: 10.1161/01.str.30.4.780. [DOI] [PubMed] [Google Scholar]
- 18.Lindgren A, Wohlfart A, Pahlm O, Johansson BB. Electrocardiographic changes in stroke patients without primary heart disease. Clin Physiol. 1994;14:223–31. doi: 10.1111/j.1475-097x.1994.tb00508.x. [DOI] [PubMed] [Google Scholar]
- 19.McDermott MM, Lefevre F, Arron M, Martin GJ, Biller J. ST segment depression detected by continuous electrocardiography in patients with acute ischemic stroke or transient ischemic attack. Stoke. 1994;25:1820–4. doi: 10.1161/01.str.25.9.1820. [DOI] [PubMed] [Google Scholar]
- 20.Abildskov JA. Adrenergic effects on the QT interval of the electrocardiogram. Am Heart J. 1976;92:210–6. doi: 10.1016/s0002-8703(76)80256-6. [DOI] [PubMed] [Google Scholar]
- 21.Shuster S. The electrocardiogram in subarachnoid haemorrhage. Br Heart J. 1960;22:316–20. doi: 10.1136/hrt.22.3.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cropp GJ, Manning GW. Electrocardiographic changes simulating myocardial ischemia and infarction associated with spontaneous intracranial hemorrhage. Circulation. 1960;22:25–38. doi: 10.1161/01.cir.22.1.25. [DOI] [PubMed] [Google Scholar]
- 23.Hunt D, McRae C, Zapf P. Electrocardiographic and serum enzyme changes in subarachnoid hemorrhage. Am Heart J. 1969;77:479–88. doi: 10.1016/0002-8703(69)90157-4. [DOI] [PubMed] [Google Scholar]
- 24.Wilkins RH, Alexander JA, Odom GL. Intracranial arterial spasm: A clinical analysis. J Neurosurg. 1968;29:121–34. doi: 10.3171/jns.1968.29.2.0121. [DOI] [PubMed] [Google Scholar]
- 25.Stober T, Kunze K. Electrocardiographic alterations in subarachnoid haemorrhage. J Neurol. 1982;227:99–113. doi: 10.1007/BF00313776. [DOI] [PubMed] [Google Scholar]
- 26.Yamour BJ, Sridharan MR, Rice JF, Flowers NC. Electrocardiographic changes in cerebrovascular hemorrhage. Am Heart J. 1980;99:294–300. doi: 10.1016/0002-8703(80)90343-9. [DOI] [PubMed] [Google Scholar]
- 27.Oppenheimer SM, Gelb AW, Girvin JP, Hachinski VC. Cardiovascular effects of human insular cortex stimulation. Neurology. 1992;42:1727–32. doi: 10.1212/wnl.42.9.1727. [DOI] [PubMed] [Google Scholar]


