Abstract
Drug resistance is a major impediment to the effective treatment of parasitic diseases. The role of multidrug resistance (mdr) genes and their products in this drug resistance phenomenon, however, remains controversial. In order to determine whether mdr gene amplification and overexpression can be connected to a multidrug resistance phenotype in parasitic protozoa, a mutant strain of Leishmania donovani was generated by virtue of its ability to proliferate in medium containing increasing concentrations of vinblastine. The vinblastine-resistant strain, VINB1000, displayed a cross-resistance to puromycin and the anthracyclines, a growth phenotype that could be attributed to an impaired ability to accumulate the toxic drugs. By using the polymerase chain reaction, two different DNA fragments, LEMDR06 and LEMDRF2, were amplified from leishmanial genomic DNA, and each amplified fragment encoded a product that was significantly homologous to parts of the mammalian P-glycoprotein. In the VINB1000 strain, the mdr gene recognized by the LEMDR06 probe was amplified approximately 50-fold in copy number, whereas the mdr genes that hybridized to LEMDRF2 or to a fragment of the previously characterized ltpgpA gene were not amplified. Moreover, the VINB1000 cell line expressed a LEMDR06 gene transcript of 12.5 kb in size that was not detected in the parental wild-type strain. To furnish a functional test for mdr gene amplification and expression in L. donovani, the L. donovani gene recognized by the LEMDR06 polymerase chain reaction product, ldmdr1, was isolated from a genomic library, transfected into wild-type cells, and amplified over 500-fold by selection in 0.5 mg of G418 per ml. The resulting transfectants were resistant to all drugs to which VINB1000 cells were resistant and sensitive to all drugs to which VINB1000 cells were sensitive. These studies demonstrate that amplification of the ldmdr1 gene either by direct selection or subsequent to transfection can confer a drug-resistant phenotype in parasitic protozoa similar to that observed for MDR mammalian cells.
Full text
PDF

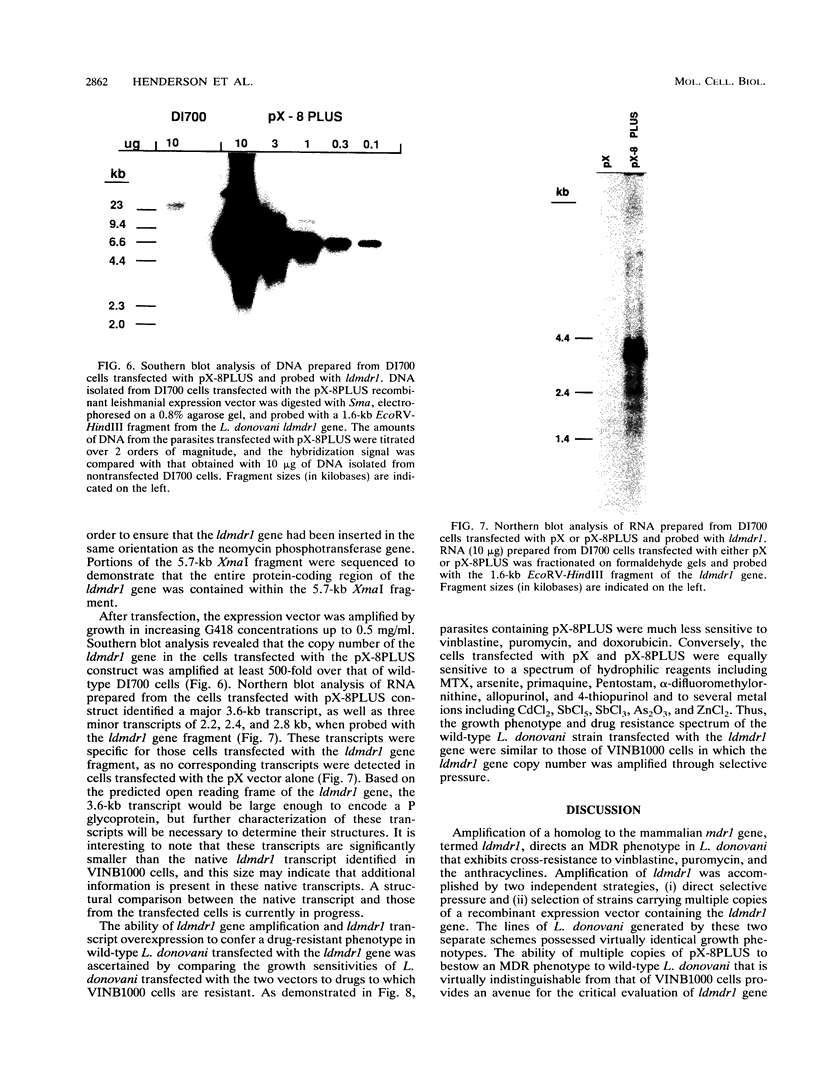
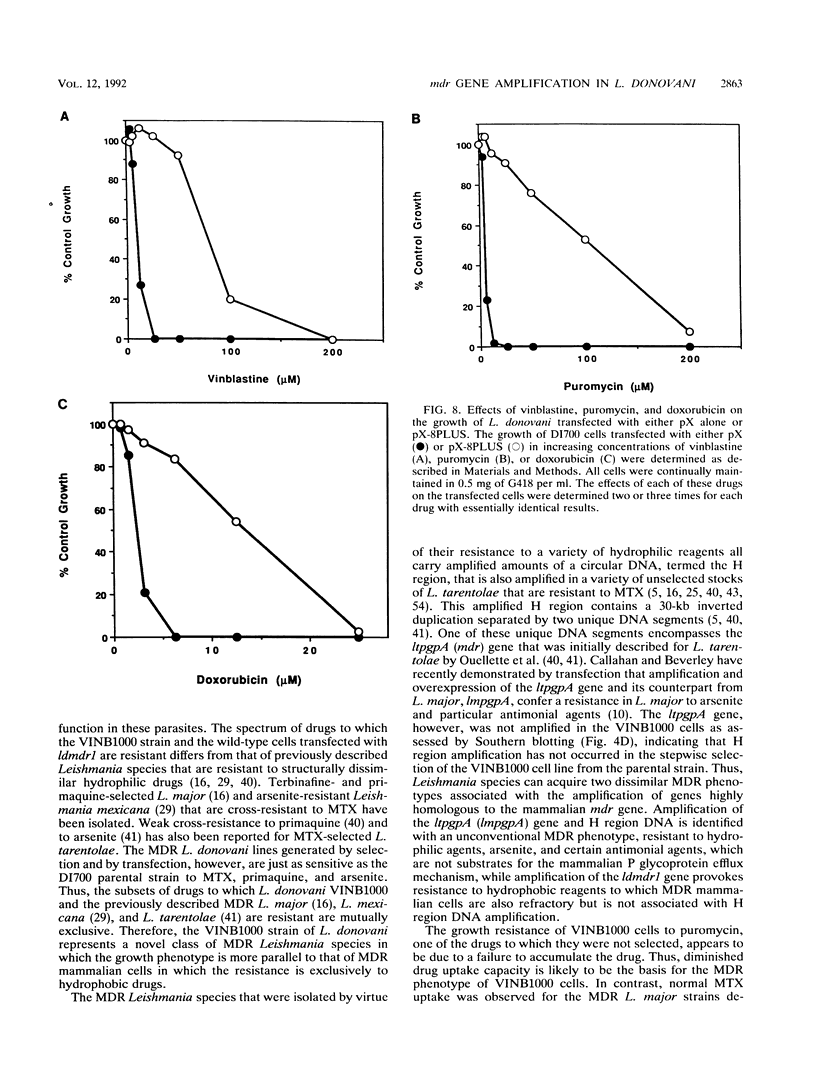


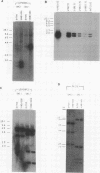
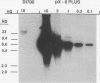
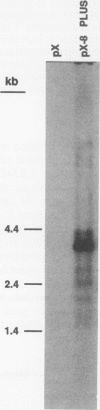
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronow B., Kaur K., McCartan K., Ullman B. Two high affinity nucleoside transporters in Leishmania donovani. Mol Biochem Parasitol. 1987 Jan 2;22(1):29–37. doi: 10.1016/0166-6851(87)90066-1. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverley S. M., Coderre J. A., Santi D. V., Schimke R. T. Unstable DNA amplifications in methotrexate-resistant Leishmania consist of extrachromosomal circles which relocalize during stabilization. Cell. 1984 Sep;38(2):431–439. doi: 10.1016/0092-8674(84)90498-7. [DOI] [PubMed] [Google Scholar]
- Beverley S. M., Ellenberger T. E., Cordingley J. S. Primary structure of the gene encoding the bifunctional dihydrofolate reductase-thymidylate synthase of Leishmania major. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2584–2588. doi: 10.1073/pnas.83.8.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitonti A. J., Sjoerdsma A., McCann P. P., Kyle D. E., Oduola A. M., Rossan R. N., Milhous W. K., Davidson D. E., Jr Reversal of chloroquine resistance in malaria parasite Plasmodium falciparum by desipramine. Science. 1988 Dec 2;242(4883):1301–1303. doi: 10.1126/science.3057629. [DOI] [PubMed] [Google Scholar]
- Boudreau E. F., Webster H. K., Pavanand K., Thosingha L. Type II mefloquine resistance in Thailand. Lancet. 1982 Dec 11;2(8311):1335–1335. doi: 10.1016/s0140-6736(82)91532-x. [DOI] [PubMed] [Google Scholar]
- Cairns B. R., Collard M. W., Landfear S. M. Developmentally regulated gene from Leishmania encodes a putative membrane transport protein. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7682–7686. doi: 10.1073/pnas.86.20.7682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan H. L., Beverley S. M. Heavy metal resistance: a new role for P-glycoproteins in Leishmania. J Biol Chem. 1991 Oct 5;266(28):18427–18430. [PubMed] [Google Scholar]
- Chen C. J., Chin J. E., Ueda K., Clark D. P., Pastan I., Gottesman M. M., Roninson I. B. Internal duplication and homology with bacterial transport proteins in the mdr1 (P-glycoprotein) gene from multidrug-resistant human cells. Cell. 1986 Nov 7;47(3):381–389. doi: 10.1016/0092-8674(86)90595-7. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Doberstyn E. B. Resistance of Plasmodium falciparum. Experientia. 1984 Dec 15;40(12):1311–1317. doi: 10.1007/BF01951884. [DOI] [PubMed] [Google Scholar]
- Ellenberger T. E., Beverley S. M. Multiple drug resistance and conservative amplification of the H region in Leishmania major. J Biol Chem. 1989 Sep 5;264(25):15094–15103. [PubMed] [Google Scholar]
- Endicott J. A., Ling V. The biochemistry of P-glycoprotein-mediated multidrug resistance. Annu Rev Biochem. 1989;58:137–171. doi: 10.1146/annurev.bi.58.070189.001033. [DOI] [PubMed] [Google Scholar]
- Ferro-Luzzi Ames G. The basis of multidrug resistance in mammalian cells: homology with bacterial transport. Cell. 1986 Nov 7;47(3):323–324. doi: 10.1016/0092-8674(86)90585-4. [DOI] [PubMed] [Google Scholar]
- Fitch C. D. Plasmodium falciparum in owl monkeys: drug resistance and chloroquine binding capacity. Science. 1970 Jul 17;169(3942):289–290. doi: 10.1126/science.169.3942.289. [DOI] [PubMed] [Google Scholar]
- Fong D., Lee B. Beta tubulin gene of the parasitic protozoan Leishmania mexicana. Mol Biochem Parasitol. 1988 Oct;31(1):97–106. doi: 10.1016/0166-6851(88)90149-1. [DOI] [PubMed] [Google Scholar]
- Foote S. J., Thompson J. K., Cowman A. F., Kemp D. J. Amplification of the multidrug resistance gene in some chloroquine-resistant isolates of P. falciparum. Cell. 1989 Jun 16;57(6):921–930. doi: 10.1016/0092-8674(89)90330-9. [DOI] [PubMed] [Google Scholar]
- Gottesman M. M., Pastan I. The multidrug transporter, a double-edged sword. J Biol Chem. 1988 Sep 5;263(25):12163–12166. [PubMed] [Google Scholar]
- Gros P., Croop J., Housman D. Mammalian multidrug resistance gene: complete cDNA sequence indicates strong homology to bacterial transport proteins. Cell. 1986 Nov 7;47(3):371–380. doi: 10.1016/0092-8674(86)90594-5. [DOI] [PubMed] [Google Scholar]
- Hanson S., Adelman J., Ullman B. Amplification and molecular cloning of the ornithine decarboxylase gene of Leishmania donovani. J Biol Chem. 1992 Feb 5;267(4):2350–2359. [PubMed] [Google Scholar]
- Hanson W. L., Waits V. B., Hendricks L. D., Hockmeyer W. T., Davidson D. E., Jr, Chapman W. L., Jr Relative insensitivity of a Kenyan strain of Leishmania donovani to pentavalent antimony therapy in hamsters. J Parasitol. 1983 Apr;69(2):446–447. [PubMed] [Google Scholar]
- Hightower R. C., Ruiz-Perez L. M., Wong M. L., Santi D. V. Extrachromosomal elements in the lower eukaryote Leishmania. J Biol Chem. 1988 Nov 15;263(32):16970–16976. [PubMed] [Google Scholar]
- Iovannisci D. M., Kaur K., Young L., Ullman B. Genetic analysis of nucleoside transport in Leishmania donovani. Mol Cell Biol. 1984 Jun;4(6):1013–1019. doi: 10.1128/mcb.4.6.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iovannisci D. M., Ullman B. High efficiency plating method for Leishmania promastigotes in semidefined or completely-defined medium. J Parasitol. 1983 Aug;69(4):633–636. [PubMed] [Google Scholar]
- Kapler G. M., Coburn C. M., Beverley S. M. Stable transfection of the human parasite Leishmania major delineates a 30-kilobase region sufficient for extrachromosomal replication and expression. Mol Cell Biol. 1990 Mar;10(3):1084–1094. doi: 10.1128/mcb.10.3.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katakura K., Chang K. P. H DNA amplification in Leishmania resistant to both arsenite and methotrexate. Mol Biochem Parasitol. 1989 May 1;34(2):189–191. doi: 10.1016/0166-6851(89)90010-8. [DOI] [PubMed] [Google Scholar]
- Kaur K., Coons T., Emmett K., Ullman B. Methotrexate-resistant Leishmania donovani genetically deficient in the folate-methotrexate transporter. J Biol Chem. 1988 May 25;263(15):7020–7028. [PubMed] [Google Scholar]
- Krogstad D. J., Gluzman I. Y., Kyle D. E., Oduola A. M., Martin S. K., Milhous W. K., Schlesinger P. H. Efflux of chloroquine from Plasmodium falciparum: mechanism of chloroquine resistance. Science. 1987 Nov 27;238(4831):1283–1285. doi: 10.1126/science.3317830. [DOI] [PubMed] [Google Scholar]
- Krogstad D. J., Schlesinger P. H. The basis of antimalarial action: non-weak base effects of chloroquine on acid vesicle pH. Am J Trop Med Hyg. 1987 Mar;36(2):213–220. doi: 10.4269/ajtmh.1987.36.213. [DOI] [PubMed] [Google Scholar]
- Landfear S. M., McMahon-Pratt D., Wirth D. F. Tandem arrangement of tubulin genes in the protozoan parasite Leishmania enriettii. Mol Cell Biol. 1983 Jun;3(6):1070–1076. doi: 10.1128/mcb.3.6.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landfear S. M., Wirth D. F. Structure of mRNA encoded by tubulin genes in Leishmania enriettii. Mol Biochem Parasitol. 1985 Apr;15(1):61–82. doi: 10.1016/0166-6851(85)90029-5. [DOI] [PubMed] [Google Scholar]
- LeBowitz J. H., Coburn C. M., McMahon-Pratt D., Beverley S. M. Development of a stable Leishmania expression vector and application to the study of parasite surface antigen genes. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9736–9740. doi: 10.1073/pnas.87.24.9736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S. K., Oduola A. M., Milhous W. K. Reversal of chloroquine resistance in Plasmodium falciparum by verapamil. Science. 1987 Feb 20;235(4791):899–901. doi: 10.1126/science.3544220. [DOI] [PubMed] [Google Scholar]
- McGrath J. P., Varshavsky A. The yeast STE6 gene encodes a homologue of the mammalian multidrug resistance P-glycoprotein. Nature. 1989 Aug 3;340(6232):400–404. doi: 10.1038/340400a0. [DOI] [PubMed] [Google Scholar]
- Meade J. C., Shaw J., Lemaster S., Gallagher G., Stringer J. R. Structure and expression of a tandem gene pair in Leishmania donovani that encodes a protein structurally homologous to eucaryotic cation-transporting ATPases. Mol Cell Biol. 1987 Nov;7(11):3937–3946. doi: 10.1128/mcb.7.11.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oduola A. M., Milhous W. K., Weatherly N. F., Bowdre J. H., Desjardins R. E. Plasmodium falciparum: induction of resistance to mefloquine in cloned strains by continuous drug exposure in vitro. Exp Parasitol. 1988 Dec;67(2):354–360. doi: 10.1016/0014-4894(88)90082-3. [DOI] [PubMed] [Google Scholar]
- Ouellette M., Fase-Fowler F., Borst P. The amplified H circle of methotrexate-resistant leishmania tarentolae contains a novel P-glycoprotein gene. EMBO J. 1990 Apr;9(4):1027–1033. doi: 10.1002/j.1460-2075.1990.tb08206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellette M., Hettema E., Wüst D., Fase-Fowler F., Borst P. Direct and inverted DNA repeats associated with P-glycoprotein gene amplification in drug resistant Leishmania. EMBO J. 1991 Apr;10(4):1009–1016. doi: 10.1002/j.1460-2075.1991.tb08035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters W. The problem of drug resistance in malaria. Parasitology. 1985 Apr;90(Pt 4):705–715. doi: 10.1017/s003118200005232x. [DOI] [PubMed] [Google Scholar]
- Petrillo-Peixoto M. L., Beverley S. M. Amplified DNAs in laboratory stocks of Leishmania tarentolae: extrachromosomal circles structurally and functionally similar to the inverted-H-region amplification of methotrexate-resistant Leishmania major. Mol Cell Biol. 1988 Dec;8(12):5188–5199. doi: 10.1128/mcb.8.12.5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Bugawan T. L., Horn G. T., Mullis K. B., Erlich H. A. Analysis of enzymatically amplified beta-globin and HLA-DQ alpha DNA with allele-specific oligonucleotide probes. Nature. 1986 Nov 13;324(6093):163–166. doi: 10.1038/324163a0. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Samuelson J., Ayala P., Orozco E., Wirth D. Emetine-resistant mutants of Entamoeba histolytica overexpress mRNAs for multidrug resistance. Mol Biochem Parasitol. 1990 Jan 15;38(2):281–290. doi: 10.1016/0166-6851(90)90031-g. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Verdier F., Le Bras J., Clavier F., Hatin I., Blayo M. C. Chloroquine uptake by Plasmodium falciparum-infected human erythrocytes during in vitro culture and its relationship to chloroquine resistance. Antimicrob Agents Chemother. 1985 Apr;27(4):561–564. doi: 10.1128/aac.27.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster H. K., Thaithong S., Pavanand K., Yongvanitchit K., Pinswasdi C., Boudreau E. F. Cloning and characterization of mefloquine-resistant Plasmodium falciparum from Thailand. Am J Trop Med Hyg. 1985 Nov;34(6):1022–1027. doi: 10.4269/ajtmh.1985.34.1022. [DOI] [PubMed] [Google Scholar]
- Wellems T. E., Panton L. J., Gluzman I. Y., do Rosario V. E., Gwadz R. W., Walker-Jonah A., Krogstad D. J. Chloroquine resistance not linked to mdr-like genes in a Plasmodium falciparum cross. Nature. 1990 May 17;345(6272):253–255. doi: 10.1038/345253a0. [DOI] [PubMed] [Google Scholar]
- White T. C., Fase-Fowler F., van Luenen H., Calafat J., Borst P. The H circles of Leishmania tarentolae are a unique amplifiable system of oligomeric DNAs associated with drug resistance. J Biol Chem. 1988 Nov 15;263(32):16977–16983. [PubMed] [Google Scholar]
- Wilson C. M., Serrano A. E., Wasley A., Bogenschutz M. P., Shankar A. H., Wirth D. F. Amplification of a gene related to mammalian mdr genes in drug-resistant Plasmodium falciparum. Science. 1989 Jun 9;244(4909):1184–1186. doi: 10.1126/science.2658061. [DOI] [PubMed] [Google Scholar]
- Wilson K., Collart F. R., Huberman E., Stringer J. R., Ullman B. Amplification and molecular cloning of the IMP dehydrogenase gene of Leishmania donovani. J Biol Chem. 1991 Jan 25;266(3):1665–1671. [PubMed] [Google Scholar]