Abstract
Evaluating external and internal stimuli is critical to survival. Potentially tissue-damaging conditions generate sensory experiences that the organism must respond to in an appropriate, adaptive manner (e.g., withdrawal from the noxious stimulus, if possible, or seeking relief from pain and discomfort). The importance we assign to a signal generated by a noxious state, its salience, reflects our belief as to how likely the underlying situation is to impact our chance of survival. Importantly, it has been hypothesized that aberrant functioning of the brain circuits which assign salience values to stimuli may contribute to chronic pain. We describe examples of this phenomenon, including ‘feeling pain’ in the absence of a painful stimulus, reporting minimal pain in the setting of major trauma, having an ‘analgesic’ response in the absence of an active treatment, or reporting no pain relief after administration of a potent analgesic medication, which may provide critical insights into the role that salience circuits play in contributing to numerous conditions characterized by persistent pain. Collectively, a refined understanding of abnormal activity or connectivity of elements within the salience network may allow us to more effectively target interventions to relevant components of this network in patients with chronic pain.
1. Introduction: Context and Pain
Escape from pain and its attendant risk of bodily harm is critical for survival. However, pain is not a purely sensory experience. Pain produced in the absence of tissue injury (e.g., emotional pain) and pain relief in the absence of drugs (e.g., placebo analgesia) provide compelling evidence that salience – how we interpret the importance of a given physiological state – is, alone, able to produce similar experiences to those produced by overt tissue injury or potent analgesic medications. What remains enigmatic is the nature of the brain's processing of salience-related information about pain, as well as how our emerging understanding of salience should guide the treatment of pain. It has become clear that some of the brain circuitry involved in processing pain-related information can be engaged by social and emotional experiences such as experiencing personal rejection (Eisenberger, 2012; Eisenberger et al., 2003; Kross et al., 2011), or viewing another individual in pain (Danziger et al., 2009; Hein and Singer, 2008), and these experiences appear to selectively involve neurocircuitry related to emotional rather than sensory aspects of pain (Singer et al., 2004). Indeed, brain regions involved in empathetic pain (anterior insula (AI), rostral anterior cingulate cortex (ACC), brainstem) map onto brain sites implicated in salience (see below). Moreover, even patients with congenital insensitivity to pain appear able to evaluate others’ feelings of pain, highlighting the potential to experience pain-related affect, even in the absence of sensory pain experiences (Danziger et al., 2009). Globally, a common theme underlying these disparate findings is that at least a subset of the neural circuits that instantiate the experience of ‘physical pain’ may be involved in processing salience.
Both placebo and nocebo effects appear to result from changes in response expectancies that are shaped by the salience of situational or environmental factors (Bingel et al., 2011; Levine and Gordon, 1984) through endogenous inhibitory or facilitatory neural systems (Porreca et al., 2001); (Burgess et al., 2002); (Benedetti et al., 2005; Carlino et al., 2011; Colloca and Benedetti, 2007; Scott et al., 2008). These effects can make extremely powerful contributions to individuals’ experiences of pain and analgesia. For example, when identical concentrations of the same putatively analgesic drug are administered under “hidden” conditions (in which the patient is unaware that medication have been administered) compared to “open” conditions, opioid and anti-inflammatory medications appear to lose a considerable portion of their analgesic effects (Colloca et al., 2004; Levine and Gordon, 1984). Recent fMRI studies reveal that the analgesic effects of our most potent opioidergic medications can be either completely abolished, or roughly doubled, by verbally shaping participants’ pre-treatment expectations for the effects of the administered medication (Bingel et al., 2011). Taken together, these behavioral experiences implicate salience as a major determinant of pain and analgesia, and imply that the neural networks evaluating the non-sensory aspects of pain must play a significant role in shaping the assignment of survival value to stimuli in the external and internal milieu.
2. Definitions and Concepts
2.1. Salience
In the conventional view, salience “refers to the physical distinctiveness or conspicuity of a stimulus, a relative property that depends on its relationship to the other surrounding stimuli” (Legrain et al., 2011). The rank ordering of the importance of various environmental stimuli, focusing on their salience, is clearly important for survival. During normal brain function, the integration of multiple sensory inputs (e.g., nociceptive, auditory, visual, gustatory, etc.) allows for a biologically adaptive response to those sensory experience (Stein and Stanford, 2008). These inputs allow for the segregation of signals, and the amplification of neural processing to a particular input (to the exclusion of other inputs), as that signal becomes more salient. Multisensory neurons are localized throughout the brain in regions including, but not limited to, the superior colliculus (Meredith and Stein, 1986), thalamus (Burstein et al., 2010) and cortex (Alvarado et al., 2007; Kayser et al., 2009). Salience attribution occurs by ‘gating’ of stimulus representations in the prefrontal cortex and it is one of the key functions attributed to the mesolimbic dopaminergic pathways, encoded via interactions between tonic (baseline) and phasic spikes in dopaminegic neurons (McClure et al., 2003). In general, neuronal responses to salience appear to be dependent on the confluence of contextual cues and expectancies (i.e., neuronal activity is enhanced by events that differ from what is predicted).
2.2. Stimulus Salience (SS) vs. Responsivity Salience (RS)
The individual response to a given stimulus differs between normal/physiological (e.g., a healthy adult undergoing experimental pain stimulation) and pathological (e.g., chronic/ongoing pain) conditions. In the latter, the salience value of a particular sensory input may be aberrant because of underlying alterations in brain structure and function. While an external stimulus triggers an evaluation of its salience, interoceptive stimuli and brain-related motivational states shape the salience of this information. Responsivity Salience (RS), defined here as the innate state of brain function (on which external signals act) at a particular time, reflects the interactions of multiple brain-state factors (emotional, motivational and cognitive). Thus, responsivity Salience may not only be associated with physical factors related to an external stimulus (i.e., intensity, clarity or size), but also with ongoing interoceptive brain processes.
How does an individual's brain evaluate and interpret salience in the context of pain and analgesia? In the following sections we provide a brief review of the Brain's Salience networks as a basis for further understanding, for example, how we can perceive emotional pain without physical pain, and how placebo treatments can produce robust analgesia (see Figure 1). Such insights provide a basis for understanding individual variability in the experience of chronic pain and in responses to analgesic interventions. We hope that an improved characterization of this variability may offer new insights into potential therapeutic approaches to managing persistent pain.
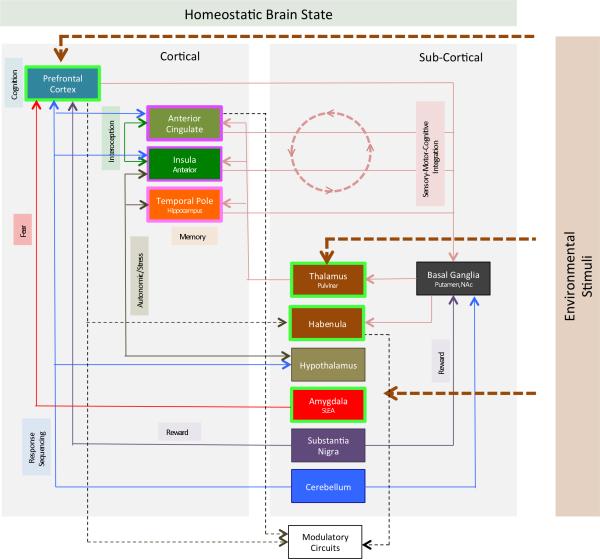
Figure 1. Interconnections in the Salience Network.
The figure provides a map of connectivity between different brain regions of the salience network. In addition the behavioral context of some of these pathways are also noted (viz. fear, reward, autonomic responses, interoception, memory etc.). Cortical and subcortical areas are differentiated in the figure. Salient stimuli include cognitive (e.g., to prefrontal cortex), sensory (e.g., to thalamus) and emotional (e.g., to amygdala). The Salience Network senses, integrates and produces actions in systems to stabilize the homeostatic challenge (e.g., activation of modulatory circuits in the brainstem through connections for a number of regions that include the cingulate cortex (Stein et al., 2012; Zhang et al., 2005). Some aspects of these networks have been better described than others. For example, the interactions between the anterior insula and the anterior cingulate cortex form a “salience network” that acts to determine hierarchical behavioral responses to stimuli (internal or external). The insula is involved in this function in a number of ways including detection of salient events (e.g., the posterior insula structure is involved in encoding pain intensity (Baumgartner et al., 2006); accessing other networks such as those involved in attention or working memory and also motor networks following detection of a salient stimulus and autonomic modulation in response to salient stimuli (Menon and Uddin, 2010). Although not detailed in this figure, most of the structures interconnect functionally with different components of the salience network (see Seeley and colleagues (Seeley et al., 2007)). For example, the cerebellum (Crus VI) in functional imaging connectivity studies shows connectivity with the caudate, the anterior cingulate, the insula, the red nucleus, and the thalamus (Habas et al., 2009). The regions that are outlined in bright green, and purple represent examples of potential hierarchical organization of the salience network (see Figure 2). Specific known functions of these different regions are noted in Table 1. Brain function at rest and in action trends to a homeostatic baseline that is perturbed by environmental stimuli.
3. CNS Salience Networks
Brain imaging studies implicate a baseline neural network considered to be involved in salience - the salience network - first defined by Grecius and colleagues at Stanford (Seeley et al., 2007). The salience network itself comprises brain regions that include the cingulate, medial prefrontal cortex, anterior insula, cerebellum, and pulvinar (see Figure 1 and details below). Other areas such as the parietal lobe (Arcizet et al., 2011), amygdalo-hippocampal network (Albrecht et al., 2010) and habenula (Bromberg-Martin et al., 2010) have also been implicated in salience-related functions. Figure 1 summarizes the main interactions between these brain regions that form the putative salience network. When considering abnormal functioning of the brain's processing of salience-related information, it is likely that dysfunction in some of these areas (e.g., amygdalo-frontal cortex processing) may then alter overall functional connectivity, and thus behavioral outputs, for the system as a whole. Resting-state functional connectivity, which reflects structural connectivity and shared activation patterns, has been extremely useful in enriching our understanding of these canonical brain networks (Greicius et al., 2009). There is an enhanced functional connectivity between ACC, insula, basal ganglia (including pallidum), temporal lobe, and medial prefrontal cortices forming this network. As an example of this, Figure 2 shows functional connectivity between the cerebellum (one region in the putative salience network) and other regions in the network.
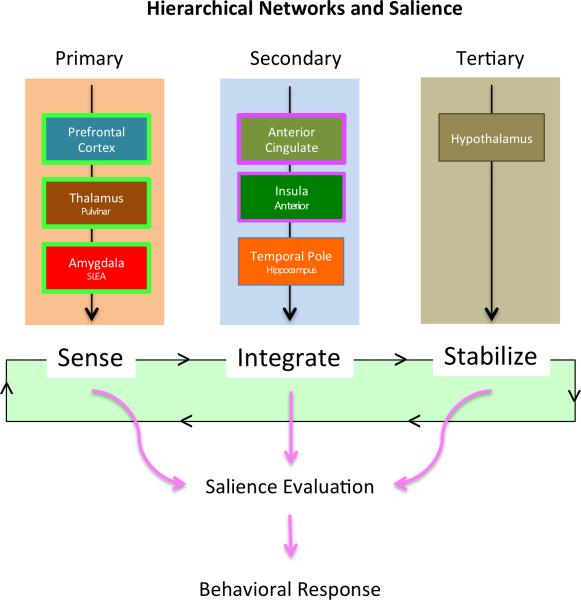
Figure 2. Model of Hierarchical Function in Salience Network Regions.
Primary regions include those involved in “sensing” sensory, cognitive and emotional stimuli. Secondary regions are involved in integration of such stimuli while Tertiary regions are involved in homeostatic normalization or stabilization.
In general, brain structures associated based on their spatio-temporal characteristics; the underlying assumption is that interconnected structures will affect each others’ activity and that this will be reflected as synchronous modulation in the BOLD fMRI signal. Furthermore, within networks, regions that serve as hubs of predominant information traffic can be identified. Such structures tend to be dominant in the networks and appear as the principal substrates (Hwang et al., 2012; van den Heuvel et al., 2012). The putative definitions of network functions are based on observation s of cognitive/sensory function associated with brain activity as well as alterations in mental states with defined behavioral outcomes (Smith et al., 2009). While there is some conceptual and neuroanatomic overlap between networks, the salience network seems to appear in many studies as a well-defined, unique, network with specific well-detailed functions. As further network analysis is carried out in the salience network, it is likely that the ACC and Insula will rise in dominance as hubs of the network. The other identified brain regions (as noted in Table 1) contribute additional value as contextual and emotional modulators of sensory inputs (Guitart-Masip et al., 2010)). For example, midbrain dopaminergic neurons and their terminal fields (striatum, amygdala, and prefrontal cortex) appear to be the common neural structures engaged by salience attributed to a range of stimuli (Berridge, 2007; Friston et al., 2012). Globally, experiences such as pain require the coordinated activity of numerous widespread brain networks (Heine et al., 2012). The salience network as a whole appears to play a central role in dynamically coordinating the function of other networks (e.g., facilitating deactivation of the default mode network in response to a salient stimulus or task (Bonnelle et al., 2012; Heine et al., 2012). While the salience network presumably interacts with attentional networks, they also contain distinct brain regions and several studies have shown that the salience network, executive attention network, and dorsal attention network are differentially affected as exemplified by conditions such as schizophrenia and various types of dementia (Filippi et al., 2012; Woodward et al., 2011).
Table 1.
Brain Salience Networks: Reqions and Putative Functions
| Brain Region | Sub Regions | Putative Role in Salience Evaluation/Integration | Reference |
|---|---|---|---|
| Cortical | |||
| Frontal | Anterior Cingulate | Detection of Salient Stimuli Planning of responses to Salient events |
(Devinsky et al., 1995; Maddock, 1999). (Downar et al., 2002) |
| Medial Prefrontal | Goal directed behaviors Attention demanding tasks |
(Gusnard et al., 2001) (D'Argembeau et al., 2007) |
|
| Insula | Interoceptive awareness, empathic processing Task level control and focal attention Homeostatic relevance to internal and external inputs Preconscious processing of subliminal stimuli |
(Menon and Uddin, 2010) (Nelson et al., 2010) (Craig, 2009) (Sabatini et al., 2009) |
|
| Temporal | Hippocampus | Conscious Memory including past experience Response to Stress Stimulus Association |
(Squire, 2004) (Bourne and Harris, 2008) (Moore and Stickney, 1980) |
| Parietal | Precuneus | Experience of Agency | (Cavanna and Trimble, 2006) |
| Subcortical | |||
| Basal Ganglia | Accumbens | Signal Valence Aversive, novel, and behaviorally relevant events, especially when unexpected |
(Cooper and Knutson, 2008) (Zink et al., 2003) |
| Putamen | Effort investment related to future decisions | (Zink et al., 2003) (Kurniawan et al., 2010) | |
| Amygdala | Contextual Fear | (Albrecht et al., 2010) | |
| Thalamus | Pulvinar | Generates signals related to salience of visual objects Information that precedes perception and action Higher order relay from one cortical area to anther |
(Robinson and Petersen, 1992) (Robinson, 1993) |
| Habenula | Motivates Anticipation | (Bromberg-Martin et al., 2010) | |
| Hypothalamus | Stress Hormones (e.g., Oxytocin) related to social clues |
(Thomason et al., 2011) (Averbeck, 2010) |
|
| Brainstem | Red Nuclei | Detection of ‘unexpected’ events | (Habas et al., 2010) |
| Substantia Nigra VTA |
Timing of Actions Coding of Stimulus Novelty |
(Fan et al., 2012) (Bunzeck and Duzel, 2006)) |
|
| Cerebellum | Crus VI | Connectivity to other structures in Salience Network Involved in aversive sensory and emotional stimuli |
(Habas et al., 2009) (Moulton et al., 2011) |
In disease states, the ability of the brain to integrate and interpret salient signals may be tonically dysregulated as a consequence of altered patterns of connectivity and activation within the brain's salience network. Such changes have been most extensively documented in Parkinson's Disease (Nagy et al., 2012; Serranova et al., 2011; Shine et al., 2011), obesity (Egecioglu et al., 2011), schizophrenia (Nielsen et al., 2012), and addiction (Ma et al., 2010), with aberrant salience processing forming a core part of the pathophysiology of such conditions (Berridge, 2007; Blum et al., 2012).
4. Salience and Salience Network Function in Chronic Pain
4.1. Physiological State (Acute Pain) vs. Pathological State (Chronic Pain)
Recently, a number of authors have argued, quite cogently, that the “network” of brain regions involved in a pain response is far less pain-specific than the neuroimaging community had assumed. Some authors even suggest that “pain neuromatrix” may best be described as a salience network (Legrain et al., 2011; Mouraux et al., 2011). While this may be the case for acute pain, chronic pain offers the additional complexity of an altered “baseline” brain state, and there are good reasons to believe that responsive salience networks in the brain are disrupted or dysregulated. Indeed, dysregulated brain salience systems may be overly responsive to certain types of stimuli because they cannot properly filter information. For example, in a recent study of low back pain (LBP) patients, subjects were exposed to pictures depicting painful events (Shimo et al., 2011). The LBP group reported an increase in back pain during the viewing of the images, and showed significantly more visual stimulation-related activation in salience-relevant regions such as inferior temporal gyrus, medial prefrontal cortex, precuneus, posterior cingulate cortex, and anterior insula. Similarly, compared to pain-free controls, chronic migraine patients (who were not experiencing pain at the time of the scan) showed enhanced activations of anterior insula and orbitofrontal cortex when viewing pain-related words, suggesting the possibility that chronic pain syndromes may share a common predisposition to aberrant activation of salience networks by stimuli that are pain-related, but are not themselves painful (Eck et al., 2011).
Salience also plays a central role in the memory of painful events. Stone and colleagues (Stone et al., 2005) reported that the variability of real-time (peak) pain in patients with chronic rheumatological conditions is a prime determinant of their later recall of pain intensity. This effect is also reported in patients recalling pain experienced during colonoscopy – where real-time pain was a predictor of accuracy of recall (Gavaruzzi et al., 2010), which builds on the classic article of the salience of the last stimulus in patients undergoing colonoscopy (Redelmeier and Kahneman, 1996). Collectively, these findings suggest that the salience of short-term peaks in pain intensity guides subsequent recall of “typical’ or “average” pain intensity among patients experiencing both chronic and acute pain. Finally, patients demonstrating the greatest variability of real-time pain are those who are most likely to show the largest placebo analgesic responses, suggesting that these individuals may have the most sensitive and tonically active pain-related salience systems (Harris et al., 2005).
It is also important to consider that ongoing spontaneous pain and exaggerated responses to stimuli evoke behavioral responses that are critically dependent on an individual's expectations (see, for example, the large and growing literature on placebo (Atlas et al., 2012; Geuter et al., 2013; Kotsis et al., 2012; Petrovic et al., 2010; Scott et al., 2007) an individual's emotional state (Berna et al., 2010), and cognitive processes such as catastrophizing, a pain-specific psychosocial construct comprised of cognitive and emotional processes such as helplessness, pessimism, rumination about pain-related symptoms, and magnification of pain complaints (Edwards et al., 2011). These processes (e.g., enhanced catastrophizing, a propensity to experience negative emotions) likely contribute to the broad inter-individual differences between chronic pain patients. For example, patients with chronic widespread pain (who are, on average, high in catastrophizing) demonstrate elevated anticipatory activation of a variety of cortical and sub-cortical brain regions prior to administration of a noxious stimulus, presumably due to a heightened intrinsic salience state (Burgmer et al., 2011). In addition, studies in both controls (Seminowicz and Davis, 2006) and fibromyalgia patients (Gracely et al., 2004) have reported that higher levels of pain catastrophizing are related to elevated pain-related activations in regions such as dorsolateral and medial prefrontal cortex, anterior cingulate cortex, anterior insula, and portions of the striatum (Campbell and Edwards, 2009). Salience processing in the context of chronic pain, therefore, depends on a large number of interactions between psychosocial processes, environmental factors, stimulus characteristics, and qualities and functioning of brain networks of the perceiving individual. As we describe below, these interactions are filtered through two inter-connected brain systems (ES and IS) that instantiate the processing of spontaneous and evoked pain; disruptions in the function of these systems appear to constitute a relatively under-appreciated part of the pathophysiology of chronic pain. Each of these brain regions noted above have known or putative functions inferred from imaging studies on salience in chronic pain (see Table 2).
Table 2.
Putative Regions Activation in Chronic Pain and Measures that relate to “Salience”
| Region | Salience Related Measures | Reference | |
|---|---|---|---|
| Cortical | |||
| Hippocampus | Activation in Visualization of painful experiences in CBP | (Shimo et al., 2011) | |
| Precuneus | Gender differences in migraine | ||
| Insula | Activation in Visualization of painful experiences in CBP | (Shimo et al., 2011) | |
| Stronger spectral power in patients with chronic pain; altered spatial connectivity between insula and ACC | (Malinen et al., 2010) | ||
| Cingulate (ACC) | Decreased connectivity in Diabetic Neuropathic Pain |
(Cauda et al., 2010) | |
| Medial Prefrontal Cortex | Decreased Gray Matter in IBS | (Seminowicz et al., 2010) | |
| Temporal Lobe | Altered in Migraine | (Moulton et al., 2011) | |
| Subcortical | |||
| Putamen (BG) | Decreased gray matter in Endometriosis + Pain | (As-Sanie et al., 2012) | |
| Activation in Trigeminal Neuropathic Pain | (Becerra et al., 2006) | ||
| Pulvinar | Activation in Visualization of painful experiences in CBP | (Shimo et al., 2011) | |
| Activated in Migraine | |||
| Destruction for pain relief | (Yoshii and Fukuda, 1979) | ||
| Disruption of Thalamic feedback in Diabetic Neuropathic Pain |
(Cauda et al., 2009a) | ||
| Red Nucleus | Increased Iron deposition Chronic Migraine | (Kruit et al., 2009) | |
| Cerebellum (VI) | Activation in Visualization of painful experiences in CBP | (Shimo et al., 2011) | |
| Windup in Chronic post-herniotomy pain | (Kupers et al., 2011) | ||
| Brush and Heat in Neuropathic pain | (Borsook et al., 2008) |
4.2. Salience Networks and Chronic Pain Conditions
Salience depends on context, and on the state of the perceiving organism, as well as on the characteristics of the stimulus. We suggest that the brain, in patients with chronic pain, has different ‘salient states’, or baselines, that allow for different response profiles for the same stimulus (see Figure 3). Those differences in brain state then subsequently define the relevant salience status of an evoked stimulus. Melloni and colleagues implicate two processes: “bottom-up saliency” and “top down control”, which create saliency maps in the brain (Melloni et al., 2012). Descriptions of “bottom-up saliency” tend to highlight the automatic nature of assigning salience, and some authors have suggested “Painful stimuli tend to have sustained salience even without explicit behavioral relevance or voluntary attention” (Downar et al., 2003). Interestingly, however, recent findings suggest that the consciously accessible salience state does not necessarily differ between painful and non-painful stimuli at least among individuals free from chronic pain (Mouraux et al., 2011; Mouraux and Iannetti, 2009). In these studies, the average ratings of the salience of acute, painful stimuli were similar to the salience ratings for non-painful somatosensory stimuli, visual stimuli, or auditory stimuli. However, other studies have reported differences in the activation of saliency networks in response to short or prolonged pain stimuli in healthy subjects; these researchers identified a frontal-parietal-cingulate network of regions that responded transiently to non-painful sensory events (a network sensitive to the task relevance and novelty of sensory events) and showed a sustained response in these areas for duration of painful stimulation. These regions therefore “show tonic responses to stimuli with tonic salience, supporting a general role for these areas in representing stimulus salience” (Downar et al., 2003) including in chronic pain (Malinen et al., 2010). Specific differences were also noted in the thalamus and putamen, which responded tonically throughout painful but not non-painful stimulation. Thus, regions that include the basal ganglia may play a more general role in supporting sustained salience to noxious stimuli.
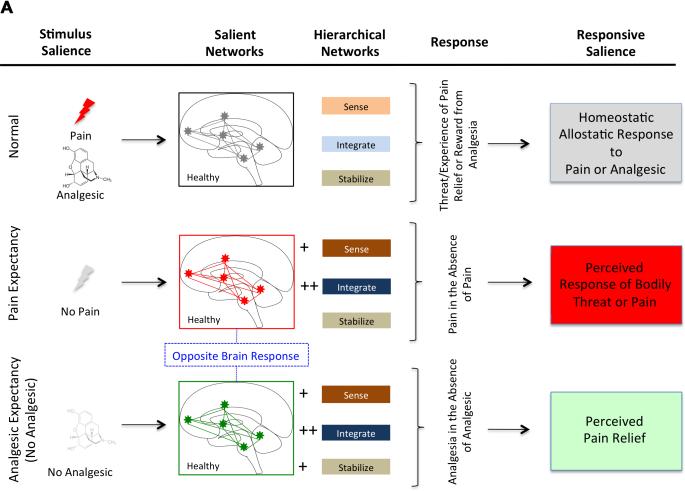
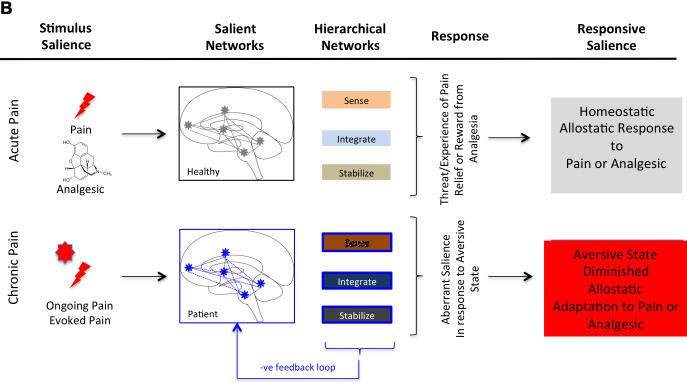
Figure 3. Salience Processing in Acute (Experimental) and Chronic Pain.
3A: Salience Systems in Healthy Subjects: Three states are shown.
Top – normal where a salience response to pain (e.g., heat, sharp object, etc.) results in adaptive changes that sense, evaluate and respond to the stimulus in an adaptive manner (e.g., sense the stimulus as painful or potentially painful and may avoid the stimulus).
Middle - Salience Systems in the condition of Perceived Pain but no actual pain. Examples include social or personal empathy for an observed process such as watching a loved-one having pain, or nocebo (increased pain to a normally analgesic drug).
Bottom - Salience Systems in the condition of Perceived Pain Relief in the absence of an analgesic medication: Examples include the placebo response where an inert substance produces profound analgesia based on expectancy (placebo effect).
3B: Salience Systems in Chronic Pain Patient: Compared with healthy subjects, salient systems in chronic pain patients may be aberrant to both pain (ongoing or evoked). Abnormal processing in their sense (e.g., cognitive processing (Apkarian et al., 2004), integration (e.g., basal ganglia function ((Borsook et al., 2010);(Maleki et al., 2011); (Starr et al., 2011); (Baliki et al., 2012) and modulatory processing (e.g., periaqueductal gray ((Behbehani, 1995); (Linnman et al., 2012)) is postulated to contribute to such aberrant function of normal homeostatic salience processing. Ongoing background pain and evoked pain with use (particularly in conditions such as osteoarthritis) sets up a process of fear of future events. Such changes, that may be mediated through medial prefrontal -> amygdala circuits may result in “system failure” since it becomes a feed-forward loop that exacerbates the condition. The salience network is now damaged at two levels – its innate state is sensitized to pain-relevant input, and its response to external/salient cues or stimuli in the environment is compromised. Thus avoidance of physical activity for example may lead to further disability and worsening brain state. See Table 2.
Salience-related responses to sustained painful stimulation appear to differ in patients with chronic pain relative to controls, as we have behavioral evidence for deficits in habituation to tonic noxious stimulation among patients with a variety of chronic pain syndromes (Becker et al., 2011; Lowenstein et al., 2009; Smith et al., 2008). That is, sustained painful stimulation becomes less painful over time in controls, but this effect is either blunted or absent among groups of patients with persistent pain. As noted below, alterations in the salience network in chronic pain have been reported across a number of neuroimaging studies, and dysregulation of salience processing may partially account for such deficits in habituation in the context of chronic pain. This salience network, anchored by dorsal anterior cingulate (dACC) and orbital frontoinsular cortices, and with robust connectivity to subcortical and limbic structures, as been suggested by Seeley and colleagues (Seeley et al., 2007), may thus play a significant role in understanding behavioral conditions such as chronic pain.
Diabetic Neuropathy
In one of the few fMRI studies using functional connectivity to directly assess the salience network in chronic pain, Cauda and colleagues reported a generalized reduction in the length of functional connections in a diabetic neuropathic pain group (Cauda et al., 2010). Anti-correlated patterns were observed in two different networks in the patient with chronic pain. The functional network most closely approximating the salience network included the following structures: left precuneus, dorsolateral prefrontal cortex, frontopolar cortex (both bilaterally), right superior frontal gyrus, left inferior frontal gyrus, thalami, and both insula (Cauda et al., 2009b). Cauda notes “similar alterations of resting state network dynamics have been demonstrated in . . . clinical conditions characterized by impaired attention . . .” (including Alzheimer's, ADD, neglect, etc.)”, suggesting that chronic pain patients have impaired attention processes.
Headache
Compared with controls, chronic headache patients showed decreased grey matter volume in the right thalamus, head of the right caudate nucleus, right precentral gyrus, right posterior cingulate cortex, bilateral middle frontal gyrus, right middle temporal gyrus, left inferior parietal lobule, and left insula (Absinta et al., 2012), all of which are regions involved in the salience network. As noted above, recent findings suggest that in response to pain-related cues (i.e., adjectives describing pain), chronic migraine patients demonstrated enhanced activations of key salience regions such as anterior insula and orbitofrontal cortex. It is important to note that these patients were not in pain during the scan, and were exposed only to visual (not to somatosensory) stimuli, highlighting the multisensory aspects of dysregulated salience processing in chronic pain (Eck et al., 2011). In a recent evaluation of gender differences in migraine patients, different circuits were involved in men and women (matched for age, disease duration of headache, and treatment) suggesting that such changes may alter the functioning of their salience networks. Indeed, alterations were found in women that included salience-related regions such as the insula and precuneus (Maleki et al., 2012).
Irritable bowel syndrome (IBS)
IBS is a functional bowel disorder characterized by recurrent abdominal pain and discomfort that is associated with altered bowel habits (Malone, 2011). IBS affects up to 1/5 of the population and is among the most common pain-related clinical problems encountered in gastroenterological practice. Visceral sensory abnormalities, including reduced pain thresholds for intestinal distension, are a hallmark of the condition, and functional neuroimaging findings strongly suggest alterations in the central nervous system's processing of pain-related information. Imaging studies of patients with IBS demonstrate decreased activation of the thalamus, striatal regions and dorsolateral prefrontal cortex compared with control subjects, suggesting diminished salience-driven sustained attention reactions. In addition IBS patients showed diminished modulation of affective responses to discomfort and pain (Hall et al., 2010), and amplified stress-induced increases in pain-related activation of salience network regions such as anterior insula, mid-cingulate cortex, and areas of prefrontal cortex (Elsenbruch et al., 2010).
Temporomandibular Disorders (TMD)
TMD disorders are comprised of a group of conditions characterized by orofacial joint and muscle pain. Recent neuroimaging studies suggest that altered behavioral responses (including altered cognition, reduced pain inhibition, etc.) in TMD may be due to attenuated, slower, and/or unsynchronized recruitment of attention/cognition processing areas (Weissman-Fogel et al., 2011). Compared to matched controls, TMD patients exposed to a difficult and stressful cognitive task had slower reaction times, reported increases in spontaneous clinical pain and showed altered task-related activation the functional connectivity of various salience-network-related brain regions such as anterior and posterior cingulate cortex and prefrontal cortex (Weissman-Fogel et al., 2011). These authors note that: “These abnormalities may be due to the salience of chronic pain, which inherently requires attention”. Although no specific behavioral data on salience were provided in this study, the results suggest that due to ongoing pain, other stimuli are less salient to chronic pain patients, which results in slower reaction times and reduced performance on various cognitive tasks, a conclusion consistent with a broad literature on dysregulated cognitive processing in chronic pain (see (Eccleston and Crombez, 1999).
How may these insights be applied to chronic pain? First, altered salience may contribute to or be part of the state of suffering in chronic pain. One reason for this is the continuous reverberation of the aversive internal state – ongoing or spontaneous pain. Both conscious and unconscious processing may be involved in suffering. A major component of chronic pain is its altered emotional/affective state and suffering (“To feel pain or distress; sustain loss, injury, harm, or punishment”) that may lead to destruction of self-identity and transformed responses to markers of salience. Interestingly, chronic pain is associated with impairments in general measures of cognitive function, including memory, and these deficits are correlated with structural findings of reduced brain volume in key salience-processing regions such as prefrontal and cingulate cortex (Jongsma et al., 2011; Luerding et al., 2008). Jongsma and colleagues (Jongsma et al., 2011) explored numerous factors, including comorbid conditions such as depression, opioid use, and substance abuse, and found that the strongest factor predicting cognitive decline among patients with chronic pain was pain duration. However, despite chronic pain's apparent detrimental effect on general measures of memory performance, pain patients are more adept at recalling suffering-related autobiographical events such as painful injuries, traumatic abuse, and hospitalizations (McBeth et al., 2001; Wright and Morley, 1995), potentially as a consequence of tonically altered shifts in pain-related salience networks.
Second, lesions of brain regions (e.g., cingulate or insular cortices) involved in salience processing provide useful insights into the importance of salience in chronic pain. For example, some reports suggest that chronic pain patients who have undergone a cingulotomy suggest that their sensory experience of pain is the same but they ‘don't care’ (see (Cohen et al., 2001)). Cingulotomy patients also show deficits of focused and sustained attention (Cohen et al., 1999; Yen et al., 2009). The anterior cingulate cortex (ACC), a part of the limbic system, is involved in a number of functions (Bush et al., 2000), including the detection of emotionally salient stimuli (Devinsky et al., 1995; Maddock, 1999). Both the anterior and posterior cingulate are implicated in the salience network. The participation of the cingulate in salience networks is observed in detecting and/or planning responses to salient events (Downar et al., 2002) and the cingulate is involved in: reward-related activation of mesolimbic circuits (Hickey et al., 2010), commission errors (Ham et al., 2012), and fluid reasoning (Yuan et al., 2012). Learning paradigms that involve reducing ACC activity appear to diminish the intensity (and potentially the salience) of both acute and chronic pain (deCharms et al., 2005). In addition to lesions of the anterior cingulate noted above, lesions of the anterior insula have been implicated in halting or altering addiction (Bienkowski et al., 2010) and pain (Veldhuizen et al., 2010; (Starr et al., 2009). In the report by Starr and colleagues (Starr et al., 2009), they suggest that such insula lesions may be involved in “tuning cortical regions to appropriately use previous cognitive information during afferent processing” (e.g., salience processing).
Third, co-morbid disease (addiction and depression) may further alter salience processing in chronic pain patients. Patients with major depression show abnormal responses of the amygdala, cingulate cortex, and insular cortex when undergoing painful or negative-emotion-related tasks (Mutschler et al., 2012; Strigo et al., 2008). In the addiction literature, the term incentive salience has been used to define motivational “wanting” in response to reward-predicting stimuli. Wanting is different from liking (a pleasure immediately gained from consumption or other contact with stimuli), while “wanting” of incentive salience is a motivational value of a stimulus that makes it a potential targeted goal (see (Berridge, 2009)); in other words, in this context, it is something that is sought out. Chronic pain on the other hand has opposite attributes (i.e., “non-wanting” and “not-liking”) because of the ongoing potential for stimuli to produce evoked pain or increase spontaneous pain. That is, chronic pain patients may suffer from deficits in appetitive “approach” behavior and may experience avoidance of pain as a reward. In this sense pain may be as salient as withdrawal symptomatology or drug cues. Conversely, reversal of ongoing pain (analgesia) is clearly parallel to incentive salience (Elman et al., 2011). Chronic pain includes a reward deficit state (Comings and Blum, 2000), disincentive salience where the aversive state is translated into abnormal behaviors including and the development of habits that include restricted physical activity and participation in activities of daily life.
Fourth, group differences in salience processing may underlie some of the well-categorized group differences in the report of pain experiences. For example, there are gender differences in the brain regions subserving emotional responses to painful stimuli, as recently reported in female vs. male migraine patients (Maleki et al., 2012).
5. Salience and Analgesia
The major classes of drugs targeting the CNS that are used in the treatment of chronic pain include opioids (e.g., oxycodone, methadone, fentanyl), membrane stabilizers (e.g., pregabalin), antidepressants (e.g., amitriptylline, duloxetine), and excitatory neurotransmitter modulators (e.g., lamotrigine, ketamine). Do these drugs act to diminish the salience of chronic pain? That is to say, do these drugs have effects on responsive salience of how chronic pain is ‘attended to’ or perceived? The issues related to analgesics and salience include (i) drug related cues if they are beneficial and produce analgesia; (ii) hedonic blunting; (iii) sensory blunting; and (iv) targeting of salience networks.
Nalbuphine, an agonist-antagonist kappa-opioid, produces brief analgesia followed by enhanced pain in male postsurgical patients. The drug produces profound analgesia without pain enhancement when combined with low dose naloxone. Using pharmacological magnetic resonance imaging (phMRI) in a double blind crossover in healthy male volunteers, Nalbuphine (compared to saline) produced significantly increased activity in 60 brain regions and decreased activity in 9; in contrast, naloxone activated only 14 regions and deactivated only 3 regions. Nalbuphine-induced changes in brain activity possess characteristics of both analgesia and algesia; naloxone selectively blocks activity in areas associated with algesia (Gear et al., 2012). These findings suggest that nalbuphine interacts with a pain salience system, which can modulate perceived pain intensity. Collectively, aberrant functioning of the salience system may result in altered awareness that may be disease-based or drug (analgesic)-induced. Ideally, medications should alter the representation of relevant and diminish responses to irrelevant/aversive stimuli. Analgesic strategies focused on normalizing salience network responsivity may provide a new direction for treatment of chronic pain. Clearly these treatments are not limited to pharmacological approaches and may include behavioral and psychosocial techniques as well.
6. Emotional Pain without physical pain and Pain Relief without Analgesics: Implications for our understanding of Chronic Pain
Brain circuits are the basis for behavioral processes, including pain and analgesia. What pain- and analgesia-related phenomena best exemplify the idea of pain and analgesia as salience? As noted in the introduction, the powerful determinants of emotional pain (pain without tissue or nerve injury) and placebo analgesia (analgesia without analgesics) provide a compelling basis for the role of salience in pain and analgesia. In this section we elaborate on these issues, with a particular focus on the relevant brain networks (see Figure 1), and provide a foundation on which to understand salience networks in the context of pain and analgesia.
6.1. Salience, Conscious Processing and Pain
6.1.1. Pain with no physical cause
Convergent data implicates activation in brain networks in patients who perceive others pain and in patients who have never experienced pain. These networks include a number of structures involved in the salience network, strongly suggesting an underlying process that is involved in pain salience in acute and chronic pain. This background process may be innate (part of subconscious processing that includes interoception; and important in survival instincts and processes – see below) or affected by experience, including cognition, reward, fear, and memory of prior experiences. As such, these areas that contribute to the emotional brain processes, “reflect functions and circuits related to survival” that integrate “motivation, reinforcement, and arousal” (LeDoux, 2012). Three examples, discussed below, support the notion of pain evaluation in the absence of pain itself (1) congenital insensitivity to pain; (2) dreams of pain in healthy subjects; and (3) pain circuit activation from anticipation of pain.
Individuals can have emotional responses to pain through empathetic feelings for a sensation that these patients have never experienced. Empathy has been defined as “feeling or expressing emotion for another and thus the ability to understand the experience of another individual via cognitive and affective processing.” In imaging studies, empathy for pain may effectively produce activation in the pain-related neural circuitry of an individual observing another person's pain (Singer et al., 2004). Using fMRI, patients with congenital insensitivity to pain, and who therefore have no prior experience of pain sensation, showed similar empathy-related activations to painful pictures (Danzinger et al., 2009). This finding suggests that the circuitry that subserves the emotional or affective components of neural processing of pain or aversive stimuli may form part of an interpretive network that does not require specific sensory input.
In related processing of empathetic pain, dreaming about pain may have some parallel aspects (Nielsen et al., 1993). First, it is compatible with the representational code of dreaming – a semi-conscious state; second, some patients report feeling pain in their dreams including traumatic self injury, but not having a prior or current clinical state or stimulating the brain to produce a painful behavior (in the absence of ongoing pain). Similarly, some patients with psychosis describe specific pain (feeling and location) during their psychotic episodes in the absence of any obvious pathology (Veilleux and Melzack, 1976). It should be noted that psychotic patients are considered to be relatively pain-insensitive (and/or to exhibit deficits in pain expression) (Bonnot et al., 2009; Singh et al., 2006). Abnormal bodily experiences that meet the criteria for delusions have been described in early schizophrenia (Stanghellini et al., 2012). The responses point to the ability of the brain to have a ‘pain experience’ in these patients without obvious physical pain. This is of particular interest since schizophrenic patients have a decreased sensitivity to experimental pain (Boettger et al., 2012) and expression of pain (Martins et al., 2011). The basis for such delusions may relate to alterations in dopaminergic signaling involved in salience function (Heinz and Schlagenhauf, 2010).
Finally, studies on pain anticipation (as opposed to pain perception) may shed some light on neural processes related to pain salience. In a recent study, fMRI was used to evaluate the effects of anticipation on the perception of pain under low- and high-threat conditions (Wiech et al., 2010). In this healthy sample, stimuli were rated as more painful in the high threat condition; and the pre-stimulus functional connectivity between the anterior insula and the mid-cingulate cortex (MCC) predicted pain responses. As noted, the insula is a brain structure implicated in a wide variety of processes that include disparate cognitive, affective, and regulatory functions, including interoceptive awareness, emotional responses, as well as empathic processing (Menon and Uddin, 2010). Functionally, the anterior insula has been considered to mediate interactions between other large-scale brain networks that are involved in “externally oriented attention and internally oriented or self-related cognition” (Menon and Uddin, 2010). Within the anterior insula, the dorsal regions support processes potentially related to both task-level control and focal attention (Nelson et al., 2010). The salience network, related to the ventral anterior insula, displays stronger connections with the anterior cingulate cortex on the right side, and with the frontal cortex on the left side (Cauda et al., 2011). This connectivity has been shown to be robust across a number of studies (Taylor et al., 2009). Specifically, as noted by Mennon and Uddin, the interactions between the anterior insula and the anterior cingulate cortex form the core of a “salience network” that acts to determine hierarchical behavioral responses to stimuli (internal or external) (Menon and Uddin, 2010). These authors propose that insula is involved in this function in a number of ways including detection of salient events (e.g., the posterior insula structure is involved in encoding pain intensity (Baumgartner et al., 2006 16899640); accessing other networks such as those involved in attention or working memory and also motor networks following detection of a salient stimulus and autonomic modulation in response to salient stimuli (Menon and Uddin, 2010). Collectively, the anterior insula appears to assign homeostatic relevance to both internal and external sensory inputs to the brain, and is a key region of affective and attentional processing of pain stimuli (Craig, 2009). It even appears to mediate the preconscious processing of subliminally-presented salient stimuli (Sabatini et al., 2009) indicating that intrinsic salience networks are likely able to direct attention-processing resources outside of conscious awareness.
6.1.2. Analgesia with no analgesics
Placebo analgesia is a process whereby analgesia results from the expectation of pain relief, a multifactorially-determined cognitive construct. Interestingly, studies have reported that the effects of opioids on brain activation patterns are similar to those observed for placebo (Petrovic et al., 2002) implicating brain regions involved in salience. Placebo analgesia is also accompanied by significant decreases in activity in pain related areas of brain function including cingulate, insula and thalamus, all involved in pain and salience evaluation (Hashmi et al., 2012).
Of a number of putative brain regions involved in the placebo effect, many are also involved in the salience network; of prominent importance are the forebrain, basal ganglia, the nucleus accumbens, and putamen. The striatum has also been implicated in various functions, including those related to both motor and cognitive and emotional processing (Kreitzer and Malenka, 2008). Within the basal ganglia, the accumbens (NAc) and putamen have been strongly implicated in salience processing. Both valence and salience contribute to NAc activation (Cooper and Knutson, 2008). Activity within the striatum (including its major dopaminergic inputs) codes all salient events, including and extending beyond reward. Regions such as the putamen may be involved in the process of investing effort to influence our future decisions and behaviors (Kurniawan et al., 2010). Both dorsal (Rolls et al., 1983; Takikawa et al., 2002) and ventral (Setlow et al., 2003) striatal neurons respond to such salient stimuli, including arousing, aversive, novel, and behaviorally relevant events, especially when the events are unexpected (Zink et al., 2003). Reduced responses have been observed during anhedonia in the right ventral striatum and left putamen, both of which showed reduced responses to positive/rewarding stimuli in anhedonic individuals (Dowd and Barch, 2010).
6.2. Salience and Subconscious Processing and Pain
In 1908 Edward Titchener postulated that “the object of attention comes to consciousness more quickly than the objects which we are not attending to” (see in (Spence and Parise, 2010)) which implies unconscious processing of objects in our visual fields. Such observations imply a dissociation between “bottom-up attention” and consciousness (Mulckhuyse and Theeuwes, 2010). In a classic paper on “Feeling” before “Thinking” (Zajonic, 1980) suggesting that awareness precedes evaluation of the attributes of the stimulus. While the underlying neurocircuitry of preconscious perception is not well defined, some have suggested a network that includes the superior colliculus, pulvinar and amygdala (Mulckhuyse and Theeuwes, 2010). Such a network may explain responses to subliminal cues. Recent reviews of the attentional literature have highlighted the presence of a rapid, unconscious or preconscious, bottom-up attentional orienting response that takes place within very short time frames (e.g., less than 100 msec) and that serves to automatically direct attention toward novel, changing, potentially important (i.e., salient) stimuli (Mulckhuyse and Theeuwes, 2010). While not often studied in the context of pain, some research has suggested that subliminal priming with relevant cues can alter both behavioral and brain responses to pain-related stimuli. For example, healthy subjects presented with briefly displayed words such as “wound” and “headache” were unaware of the presence of these words, but nevertheless demonstrated reduced pain tolerance during a cold pressor task (Meerman et al., 2011). In addition, recent fMRI studies have implicated a specific role for the anterior insula in this type of pain-relevant unconscious priming. Presentation of distressing visual stimuli (i.e., an angry face) was paired with noxious somatosensory stimulation and was delivered either overtly or subliminally (Sabatini et al., 2009). The authors described a dissociation of responses within the anterior and posterior insula. Anterior insula activation was the same during overt and subliminal priming, while the posterior insula responded only to the presence of the overt priming stimulus, suggesting a specific role for the anterior insula in processing salience outside of conscious awareness.
7. Conclusions
Based on the evidence presented, the notion that pain may be a disease of salience needs to be evaluated differently for acute pain and chronic pain. Acute pain models have provided a great deal of support for how salience may be manipulated by suggestion, distraction, placebo responses etc. In addition, salience in the context of acutely painful medical procedure (e.g., colonoscopy) is an important determinant of pain recall following the event. As such, acute pain may be a condition characterized by altered responses to stimulus salience. However, the case of salience in chronic pain needs to be viewed in a different way. The condition itself, or a co-morbid condition, may alter the process of salience just as the case of psychosis, where delusions and hallucinations provide clear evidence for altered salience processing. In the discussion above we provide support for the contentions that: (1) in chronic pain there are altered salience networks, at least as defined by fMRI; (2) lesions of some components of the salience network (e.g., anterior cingulate) in chronic pain patients may reduce pain's significance (salience); (3) comorbid changes such as depression with pain may alter reward processing (through lowering of dopamine levels); and (4) the psychosocial status of chronic pain patients may shape pain-related outcomes in part by influencing the salience assigned to pain- or treatment-related cues. As such, chronic pain may be considered, at least in part, as a condition of altered responsive salience.
We argue that individuals may have different pain experiences and different responses to treatments based on the level of integrated salience network functioning. This involves a constellation of networks and endogenous chemicals including endorphins, cholecystokinin and dopamine (Lemoine, 2011). Given that salience systems can be measured, directed therapeutic interventions that target normalization or rectification of these networks may provide a more useful guide to discovering novel treatments than current approaches that seem to focus, at least in the realm of pharmacotherapeutic development, on specific receptor systems of drugs with a specific mechanism of action. This approach may also have implications for long-term enhancement of the placebo response to various pain treatment interventions. Perhaps most important, there is an appealing opportunity for evaluation of “personalized” therapy for chronic pain even in the context of unknown genetic or pharmacogenomic measures in an individual patient. With respect to the latter, alterations in specific genotype (e.g., 5HTTLPR (Morey et al., 2011; (Drabant et al., 2012) confer risk of pathology, and neuroimaging markers of alteration in salience networks can be demonstrated in patients with particular genotypes (see (Drabant et al., 2012). Alteration of different components of salience processing may contribute to positive (spontaneous and evoked pain) and negative (depression, anhedonia, fear, altered cognition) symptoms. Differential adaptation or maladaptation (vulnerability) of the salience network may define the course and severity of the evolution of these symptoms. Candidate genes and genome-wide studies (GWAS) have yet to identify a set of genetic variations that explain a significant portion of the variance in chronic pain, and brain circuit phenotypes may be useful to achieve a more structured approach to individualized medicine as has been suggested for other disorders (Claus et al., 2011). Salience-related functional brain circuit phenotypes are likely to be at the forefront of these advances in pain science and pain management in the coming years.
✓ Salience networks are brain circuits that interpret our homeostatic condition.
✓ Salience is a major determinant of whether we consider a situation noxious or not (e.g., nocebo, placebo).
✓ Knowledge of effects of drugs on salience networks may impact the design of novel therapies for the treatment of pain.
✓ In chronic pain, integration and interpretation of salient signals may be tonically dysregulated.
Acknowledgements
This work was supported by the Herlands Fund for Pain Research (DB, LB) and grants from NIH (NINDS – K24 NS064050 (NINDS), and NIH Grant R01 NS065051) to DB.
Abbreviation List
- ACC
anterior cingulate cortex
- dACC
dorsal anterior cingulate cortex
- ADD
Attention deficit disorder
- AI
anterior insula
- IBS
irritable bowel syndrome
- NAc
nucleus accumbens
- TMJ
temporomandibular disorder
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Absinta M, Rocca MA, Colombo B, Falini A, Comi G, Filippi M. Selective decreased grey matter volume of the pain-matrix network in cluster headache. Cephalalgia. 2012;32:109–115. doi: 10.1177/0333102411431334. [DOI] [PubMed] [Google Scholar]
- Albrecht A, Bergado-Acosta JR, Pape HC, Stork O. Role of the neural cell adhesion molecule (NCAM) in amygdalo-hippocampal interactions and salience determination of contextual fear memory. Int J Neuropsychopharmacol. 2010;13:661–674. doi: 10.1017/S1461145709991106. [DOI] [PubMed] [Google Scholar]
- Alvarado JC, Stanford TR, Vaughan JW, Stein BE. Cortex mediates multisensory but not unisensory integration in superior colliculus. J Neurosci. 2007;27:12775–12786. doi: 10.1523/JNEUROSCI.3524-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apkarian AV, Sosa Y, Krauss BR, Thomas PS, Fredrickson BE, Levy RE, Harden RN, Chialvo DR. Chronic pain patients are impaired on an emotional decision-making task. Pain. 2004;108:129–136. doi: 10.1016/j.pain.2003.12.015. [DOI] [PubMed] [Google Scholar]
- Arcizet F, Mirpour K, Bisley JW. A pure salience response in posterior parietal cortex. Cereb Cortex. 2011;21:2498–2506. doi: 10.1093/cercor/bhr035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- As-Sanie S, Harris RE, Napadow V, Kim J, Neshewat G, Kairys A, Williams D, Clauw DJ, Schmidt-Wilcke T. Changes in regional gray matter volume in women with chronic pelvic pain: a voxel-based morphometry study. Pain. 2012;153:1006–1014. doi: 10.1016/j.pain.2012.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlas LY, Whittington RA, Lindquist MA, Wielgosz J, Sonty N, Wager TD. Dissociable influences of opiates and expectations on pain. J Neurosci. 2012;32:8053–8064. doi: 10.1523/JNEUROSCI.0383-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averbeck BB. Oxytocin and the salience of social cues. Proc Natl Acad Sci U S A. 2010;107:9033–9034. doi: 10.1073/pnas.1004892107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki MN, Petre B, Torbey S, Herrmann KM, Huang L, Schnitzer TJ, Fields HL, Apkarian AV. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat Neurosci. 2012;15:1117–1119. doi: 10.1038/nn.3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner U, Tiede W, Treede RD, Craig AD. Laser-evoked potentials are graded and somatotopically organized anteroposteriorly in the operculoinsular cortex of anesthetized monkeys. J Neurophysiol. 2006;96:2802–2808. doi: 10.1152/jn.00512.2006. [DOI] [PubMed] [Google Scholar]
- Becerra L, Morris S, Bazes S, Gostic R, Sherman S, Gostic J, Pendse G, Moulton E, Scrivani S, Keith D, Chizh B, Borsook D. Trigeminal neuropathic pain alters responses in CNS circuits to mechanical (brush) and thermal (cold and heat) stimuli. J Neurosci. 2006;26:10646–10657. doi: 10.1523/JNEUROSCI.2305-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker S, Kleinbohl D, Baus D, Holzl R. Operant learning of perceptual sensitization and habituation is impaired in fibromyalgia patients with and without irritable bowel syndrome. Pain. 2011;152:1408–1417. doi: 10.1016/j.pain.2011.02.027. [DOI] [PubMed] [Google Scholar]
- Behbehani MM. Functional characteristics of the midbrain periaqueductal gray. Prog Neurobiol. 1995;46:575–605. doi: 10.1016/0301-0082(95)00009-k. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Mayberg HS, Wager TD, Stohler CS, Zubieta JK. Neurobiological mechanisms of the placebo effect. J Neurosci. 2005;25:10390–10402. doi: 10.1523/JNEUROSCI.3458-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berna C, Leknes S, Holmes EA, Edwards RR, Goodwin GM, Tracey I. Induction of depressed mood disrupts emotion regulation neurocircuitry and enhances pain unpleasantness. Biol Psychiatry. 2010;67:1083–1090. doi: 10.1016/j.biopsych.2010.01.014. [DOI] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine's role in reward: the case for incentive salience. Psychopharmacology (Berl) 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Berridge KC. ‘Liking’ and ‘wanting’ food rewards: brain substrates and roles in eating disorders. Physiol Behav. 2009;97:537–550. doi: 10.1016/j.physbeh.2009.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienkowski P, Zatorski P, Baranowska A, Ryglewicz D, Sienkiewicz-Jarosz H. Insular lesions and smoking cessation after first-ever ischemic stroke: a 3-month follow-up. Neurosci Lett. 2010;478:161–164. doi: 10.1016/j.neulet.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Bingel U, Wanigasekera V, Wiech K, Ni Mhuircheartaigh R, Lee MC, Ploner M, Tracey I. The effect of treatment expectation on drug efficacy: imaging the analgesic benefit of the opioid remifentanil. Sci Transl Med. 2011;3:70ra14. doi: 10.1126/scitranslmed.3001244. [DOI] [PubMed] [Google Scholar]
- Blum K, Gardner E, Oscar-Berman M, Gold M. “Liking” and “wanting” linked to Reward Deficiency Syndrome (RDS): hypothesizing differential responsivity in brain reward circuitry. Curr Pharm Des. 2012;18:113–118. doi: 10.2174/138161212798919110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettger MK, Grossmann D, Bar KJ. Increased cold and heat pain thresholds influence the thermal grill illusion in schizophrenia. Eur J Pain. 2012 doi: 10.1002/j.1532-2149.2012.00188.x. [DOI] [PubMed] [Google Scholar]
- Bonnelle V, Ham TE, Leech R, Kinnunen KM, Mehta MA, Greenwood RJ, Sharp DJ. Salience network integrity predicts default mode network function after traumatic brain injury. Proc Natl Acad Sci U S A. 2012;109:4690–4695. doi: 10.1073/pnas.1113455109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnot O, Anderson GM, Cohen D, Willer JC, Tordjman S. Are patients with schizophrenia insensitive to pain? A reconsideration of the question. Clin J Pain. 2009;25:244–252. doi: 10.1097/AJP.0b013e318192be97. [DOI] [PubMed] [Google Scholar]
- Borsook D, Moulton EA, Tully S, Schmahmann JD, Becerra L. Human cerebellar responses to brush and heat stimuli in healthy and neuropathic pain subjects. Cerebellum. 2008;7:252–272. doi: 10.1007/s12311-008-0011-6. [DOI] [PubMed] [Google Scholar]
- Borsook D, Upadhyay J, Chudler EH, Becerra L. A key role of the basal ganglia in pain and analgesia--insights gained through human functional imaging. Mol Pain. 2010;6:27. doi: 10.1186/1744-8069-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne JN, Harris KM. Balancing structure and function at hippocampal dendritic spines. Annu Rev Neurosci. 2008;31:47–67. doi: 10.1146/annurev.neuro.31.060407.125646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Matsumoto M, Hikosaka O. Distinct tonic and phasic anticipatory activity in lateral habenula and dopamine neurons. Neuron. 2010;67:144–155. doi: 10.1016/j.neuron.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunzeck N, Duzel E. Absolute coding of stimulus novelty in the human substantia nigra/VTA. Neuron. 2006;51:369–379. doi: 10.1016/j.neuron.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Burgess SE, Gardell LR, Ossipov MH, Malan TP, Jr., Vanderah TW, Lai J, Porreca F. Time-dependent descending facilitation from the rostral ventromedial medulla maintains, but does not initiate, neuropathic pain. J Neurosci. 2002;22:5129–5136. doi: 10.1523/JNEUROSCI.22-12-05129.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgmer M, Petzke F, Giesecke T, Gaubitz M, Heuft G, Pfleiderer B. Cerebral activation and catastrophizing during pain anticipation in patients with fibromyalgia. Psychosom Med. 2011;73:751–759. doi: 10.1097/PSY.0b013e318236588a. [DOI] [PubMed] [Google Scholar]
- Burstein R, Jakubowski M, Garcia-Nicas E, Kainz V, Bajwa Z, Hargreaves R, Becerra L, Borsook D. Thalamic sensitization transforms localized pain into widespread allodynia. Ann Neurol. 2010;68:81–91. doi: 10.1002/ana.21994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Campbell CM, Edwards RR. Mind-body interactions in pain: the neurophysiology of anxious and catastrophic pain-related thoughts. Transl Res. 2009;153:97–101. doi: 10.1016/j.trsl.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlino E, Pollo A, Benedetti F. Placebo analgesia and beyond: a melting pot of concepts and ideas for neuroscience. Curr Opin Anaesthesiol. 2011;24:540–544. doi: 10.1097/ACO.0b013e328349d0c2. [DOI] [PubMed] [Google Scholar]
- Cauda F, D'Agata F, Sacco K, Duca S, Cocito D, Paolasso I, Isoardo G, Geminiani G. Altered resting state attentional networks in diabetic neuropathic pain. J Neurol Neurosurg Psychiatry. 2010;81:806–811. doi: 10.1136/jnnp.2009.188631. [DOI] [PubMed] [Google Scholar]
- Cauda F, D'Agata F, Sacco K, Duca S, Geminiani G, Vercelli A. Functional connectivity of the insula in the resting brain. Neuroimage. 2011;55:8–23. doi: 10.1016/j.neuroimage.2010.11.049. [DOI] [PubMed] [Google Scholar]
- Cauda F, Sacco K, D'Agata F, Duca S, Cocito D, Geminiani G, Migliorati F, Isoardo G. Low-frequency BOLD fluctuations demonstrate altered thalamocortical connectivity in diabetic neuropathic pain. BMC Neurosci. 2009a;10:138. doi: 10.1186/1471-2202-10-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauda F, Sacco K, Duca S, Cocito D, D'Agata F, Geminiani GC, Canavero S. Altered resting state in diabetic neuropathic pain. PLoS One. 2009b;4:e4542. doi: 10.1371/journal.pone.0004542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Claus ED, Ewing SW, Filbey FM, Sabbineni A, Hutchison KE. Identifying neurobiological phenotypes associated with alcohol use disorder severity. Neuropsychopharmacology. 2011;36:2086–2096. doi: 10.1038/npp.2011.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RA, Kaplan RF, Moser DJ, Jenkins MA, Wilkinson H. Impairments of attention after cingulotomy. Neurology. 1999;53:819–824. doi: 10.1212/wnl.53.4.819. [DOI] [PubMed] [Google Scholar]
- Cohen RA, Paul R, Zawacki TM, Moser DJ, Sweet L, Wilkinson H. Emotional and personality changes following cingulotomy. Emotion. 2001;1:38–50. doi: 10.1037/1528-3542.1.1.38. [DOI] [PubMed] [Google Scholar]
- Colloca L, Benedetti F. Nocebo hyperalgesia: how anxiety is turned into pain. Curr Opin Anaesthesiol. 2007;20:435–439. doi: 10.1097/ACO.0b013e3282b972fb. [DOI] [PubMed] [Google Scholar]
- Colloca L, Lopiano L, Lanotte M, Benedetti F. Overt versus covert treatment for pain, anxiety, and Parkinson's disease. Lancet Neurol. 2004;3:679–684. doi: 10.1016/S1474-4422(04)00908-1. [DOI] [PubMed] [Google Scholar]
- Comings DE, Blum K. Reward deficiency syndrome: genetic aspects of behavioral disorders. Prog Brain Res. 2000;126:325–341. doi: 10.1016/S0079-6123(00)26022-6. [DOI] [PubMed] [Google Scholar]
- Cooper JC, Knutson B. Valence and salience contribute to nucleus accumbens activation. Neuroimage. 2008;39:538–547. doi: 10.1016/j.neuroimage.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- D'Argembeau A, Ruby P, Collette F, Degueldre C, Balteau E, Luxen A, Maquet P, Salmon E. Distinct regions of the medial prefrontal cortex are associated with self-referential processing and perspective taking. J Cogn Neurosci. 2007;19:935–944. doi: 10.1162/jocn.2007.19.6.935. [DOI] [PubMed] [Google Scholar]
- Danziger N, Faillenot I, Peyron R. Can we share a pain we never felt? Neural correlates of empathy in patients with congenital insensitivity to pain. Neuron. 2009;61:203–212. doi: 10.1016/j.neuron.2008.11.023. [DOI] [PubMed] [Google Scholar]
- deCharms RC, Maeda F, Glover GH, Ludlow D, Pauly JM, Soneji D, Gabrieli JD, Mackey SC. Control over brain activation and pain learned by using real-time functional MRI. Proc Natl Acad Sci U S A. 2005;102:18626–18631. doi: 10.1073/pnas.0505210102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118(Pt 1):279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Dowd EC, Barch DM. Anhedonia and emotional experience in schizophrenia: neural and behavioral indicators. Biol Psychiatry. 2010;67:902–911. doi: 10.1016/j.biopsych.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downar J, Crawley AP, Mikulis DJ, Davis KD. A cortical network sensitive to stimulus salience in a neutral behavioral context across multiple sensory modalities. J Neurophysiol. 2002;87:615–620. doi: 10.1152/jn.00636.2001. [DOI] [PubMed] [Google Scholar]
- Downar J, Mikulis DJ, Davis KD. Neural correlates of the prolonged salience of painful stimulation. Neuroimage. 2003;20:1540–1551. doi: 10.1016/s1053-8119(03)00407-5. [DOI] [PubMed] [Google Scholar]
- Drabant EM, Ramel W, Edge MD, Hyde LW, Kuo JR, Goldin PR, Hariri AR, Gross JJ. Neural mechanisms underlying 5-HTTLPR-related sensitivity to acute stress. Am J Psychiatry. 2012;169:397–405. doi: 10.1176/appi.ajp.2011.10111699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccleston C, Crombez G. Pain demands attention: a cognitive-affective model of the interruptive function of pain. Psychol Bull. 1999;125:356–366. doi: 10.1037/0033-2909.125.3.356. [DOI] [PubMed] [Google Scholar]
- Eck J, Richter M, Straube T, Miltner WH, Weiss T. Affective brain regions are activated during the processing of pain-related words in migraine patients. Pain. 2011;152:1104–1113. doi: 10.1016/j.pain.2011.01.026. [DOI] [PubMed] [Google Scholar]
- Edwards RR, Cahalan C, Mensing G, Smith M, Haythornthwaite JA. Pain, catastrophizing, and depression in the rheumatic diseases. Nat Rev Rheumatol. 2011;7:216–224. doi: 10.1038/nrrheum.2011.2. [DOI] [PubMed] [Google Scholar]
- Egecioglu E, Skibicka KP, Hansson C, Alvarez-Crespo M, Friberg PA, Jerlhag E, Engel JA, Dickson SL. Hedonic and incentive signals for body weight control. Rev Endocr Metab Disord. 2011;12:141–151. doi: 10.1007/s11154-011-9166-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI. The pain of social disconnection: examining the shared neural underpinnings of physical and social pain. Nat Rev Neurosci. 2012;13:421–434. doi: 10.1038/nrn3231. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An FMRI study of social exclusion. Science. 2003;302:290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Elman I, Zubieta JK, Borsook D. The missing p in psychiatric training: why it is important to teach pain to psychiatrists. Arch Gen Psychiatry. 2011;68:12–20. doi: 10.1001/archgenpsychiatry.2010.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsenbruch S, Rosenberger C, Bingel U, Forsting M, Schedlowski M, Gizewski ER. Patients with irritable bowel syndrome have altered emotional modulation of neural responses to visceral stimuli. Gastroenterology. 2010;139:1310–1319. doi: 10.1053/j.gastro.2010.06.054. [DOI] [PubMed] [Google Scholar]
- Fan D, Rossi MA, Yin HH. Mechanisms of action selection and timing in substantia nigra neurons. J Neurosci. 2012;32:5534–5548. doi: 10.1523/JNEUROSCI.5924-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi M, Agosta F, Scola E, Canu E, Magnani G, Marcone A, Valsasina P, Caso F, Copetti M, Comi G, Cappa SF, Falini A. Functional network connectivity in the behavioral variant of frontotemporal dementia. Cortex; a journal devoted to the study of the nervous system and behavior. 2012 doi: 10.1016/j.cortex.2012.09.017. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Shiner T, FitzGerald T, Galea JM, Adams R, Brown H, Dolan RJ, Moran R, Stephan KE, Bestmann S. Dopamine, affordance and active inference. PLoS computational biology. 2012;8:e1002327. doi: 10.1371/journal.pcbi.1002327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavaruzzi T, Carnaghi A, Lotto L, Rumiati R, Meggiato T, Polato F, De Lazzari F. Recalling pain experienced during a colonoscopy: pain expectation and variability. Br J Health Psychol. 2010;15:253–264. doi: 10.1348/135910709X458305. [DOI] [PubMed] [Google Scholar]
- Gear R, Becerra L, Upadhyay J, Bishop J, Walling D, Pendse G, Levine J, Borsook D. Pain Facilitation Brain Regions Activated by Nalbuphine are Revealed by Pharmacological fMRI. PLoS One. 2012 doi: 10.1371/journal.pone.0050169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuter S, Eippert F, Hindi Attar C, Buchel C. Cortical and subcortical responses to high and low effective placebo treatments. Neuroimage. 2013;67:227–236. doi: 10.1016/j.neuroimage.2012.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracely RH, Geisser ME, Giesecke T, Grant MA, Petzke F, Williams DA, Clauw DJ. Pain catastrophizing and neural responses to pain among persons with fibromyalgia. Brain. 2004;127:835–843. doi: 10.1093/brain/awh098. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex. 2009;19:72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guitart-Masip M, Bunzeck N, Stephan KE, Dolan RJ, Duzel E. Contextual novelty changes reward representations in the striatum. J Neurosci. 2010;30:1721–1726. doi: 10.1523/JNEUROSCI.5331-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas C, Guillevin R, Abanou A. In vivo structural and functional imaging of the human rubral and inferior olivary nuclei: A mini-review. Cerebellum. 2010;9:167–173. doi: 10.1007/s12311-009-0145-1. [DOI] [PubMed] [Google Scholar]
- Habas C, Kamdar N, Nguyen D, Prater K, Beckmann CF, Menon V, Greicius MD. Distinct cerebellar contributions to intrinsic connectivity networks. J Neurosci. 2009;29:8586–8594. doi: 10.1523/JNEUROSCI.1868-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall GB, Kamath MV, Collins S, Ganguli S, Spaziani R, Miranda KL, Bayati A, Bienenstock J. Heightened central affective response to visceral sensations of pain and discomfort in IBS. Neurogastroenterol Motil. 2010;22:276–e280. doi: 10.1111/j.1365-2982.2009.01436.x. [DOI] [PubMed] [Google Scholar]
- Ham TE, de Boissezon X, Leff A, Beckmann C, Hughes E, Kinnunen KM, Leech R, Sharp DJ. Distinct Frontal Networks Are Involved in Adapting to Internally and Externally Signaled Errors. Cereb Cortex. 2012 doi: 10.1093/cercor/bhs056. [DOI] [PubMed] [Google Scholar]
- Harris RE, Williams DA, McLean SA, Sen A, Hufford M, Gendreau RM, Gracely RH, Clauw DJ. Characterization and consequences of pain variability in individuals with fibromyalgia. Arthritis Rheum. 2005;52:3670–3674. doi: 10.1002/art.21407. [DOI] [PubMed] [Google Scholar]
- Hashmi JA, Baria AT, Baliki MN, Huang L, Schnitzer TJ, Apkarian AV. Brain networks predicting placebo analgesia in a clinical trial for chronic back pain. Pain. 2012 doi: 10.1016/j.pain.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein G, Singer T. I feel how you feel but not always: the empathic brain and its modulation. Curr Opin Neurobiol. 2008;18:153–158. doi: 10.1016/j.conb.2008.07.012. [DOI] [PubMed] [Google Scholar]
- Heine L, Soddu A, Gomez F, Vanhaudenhuyse A, Tshibanda L, Thonnard M, Charland-Verville V, Kirsch M, Laureys S, Demertzi A. Resting state networks and consciousness: alterations of multiple resting state network connectivity in physiological, pharmacological, and pathological consciousness States. Frontiers in psychology. 2012;3:295. doi: 10.3389/fpsyg.2012.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A, Schlagenhauf F. Dopaminergic dysfunction in schizophrenia: salience attribution revisited. Schizophr Bull. 2010;36:472–485. doi: 10.1093/schbul/sbq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey C, Chelazzi L, Theeuwes J. Reward changes salience in human vision via the anterior cingulate. J Neurosci. 2010;30:11096–11103. doi: 10.1523/JNEUROSCI.1026-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang K, Hallquist MN, Luna B. The Development of Hub Architecture in the Human Functional Brain Network. Cereb Cortex. 2012 doi: 10.1093/cercor/bhs227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongsma ML, Postma SA, Souren P, Arns M, Gordon E, Vissers K, Wilder-Smith O, van Rijn CM, van Goor H. Neurodegenerative properties of chronic pain: cognitive decline in patients with chronic pancreatitis. PLoS One. 2011;6:e23363. doi: 10.1371/journal.pone.0023363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser C, Petkov CI, Logothetis NK. Multisensory interactions in primate auditory cortex: fMRI and electrophysiology. Hear Res. 2009;258:80–88. doi: 10.1016/j.heares.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Kotsis V, Benson S, Bingel U, Forsting M, Schedlowski M, Gizewski ER, Elsenbruch S. Perceived treatment group affects behavioral and neural responses to visceral pain in a deceptive placebo study. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2012;24:935–e462. doi: 10.1111/j.1365-2982.2012.01968.x. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Striatal plasticity and basal ganglia circuit function. Neuron. 2008;60:543–554. doi: 10.1016/j.neuron.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kross E, Berman MG, Mischel W, Smith EE, Wager TD. Social rejection shares somatosensory representations with physical pain. Proc Natl Acad Sci U S A. 2011;108:6270–6275. doi: 10.1073/pnas.1102693108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruit MC, Launer LJ, Overbosch J, van Buchem MA, Ferrari MD. Iron accumulation in deep brain nuclei in migraine: a population-based magnetic resonance imaging study. Cephalalgia. 2009;29:351–359. doi: 10.1111/j.1468-2982.2008.01723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupers R, Lonsdale MN, Aasvang E, Kehlet H. A positron emission tomography study of wind-up pain in chronic postherniotomy pain. Eur J Pain. 2011;15:698, e691–616. doi: 10.1016/j.ejpain.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Kurniawan IT, Seymour B, Talmi D, Yoshida W, Chater N, Dolan RJ. Choosing to make an effort: the role of striatum in signaling physical effort of a chosen action. J Neurophysiol. 2010;104:313–321. doi: 10.1152/jn.00027.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J. Rethinking the emotional brain. Neuron. 2012;73:653–676. doi: 10.1016/j.neuron.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrain V, Iannetti GD, Plaghki L, Mouraux A. The pain matrix reloaded: a salience detection system for the body. Prog Neurobiol. 2011;93:111–124. doi: 10.1016/j.pneurobio.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Lemoine P. [The placebo mystery or neurobiology of the soul]. Bull Acad Natl Med. 2011;195:1465–1476. [PubMed] [Google Scholar]
- Levine JD, Gordon NC. Influence of the method of drug administration on analgesic response. Nature. 1984;312:755–756. doi: 10.1038/312755a0. [DOI] [PubMed] [Google Scholar]
- Linnman C, Moulton EA, Barmettler G, Becerra L, Borsook D. Neuroimaging of the periaqueductal gray: state of the field. Neuroimage. 2012;60:505–522. doi: 10.1016/j.neuroimage.2011.11.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenstein L, Kenton K, Mueller ER, Brubaker L, Heneghan M, Senka J, Fitzgerald MP. Patients with painful bladder syndrome have altered response to thermal stimuli and catastrophic reaction to painful experiences. Neurourol Urodyn. 2009;28:400–404. doi: 10.1002/nau.20676. [DOI] [PubMed] [Google Scholar]
- Luerding R, Weigand T, Bogdahn U, Schmidt-Wilcke T. Working memory performance is correlated with local brain morphology in the medial frontal and anterior cingulate cortex in fibromyalgia patients: structural correlates of pain-cognition interaction. Brain. 2008;131:3222–3231. doi: 10.1093/brain/awn229. [DOI] [PubMed] [Google Scholar]
- Ma N, Liu Y, Li N, Wang CX, Zhang H, Jiang XF, Xu HS, Fu XM, Hu X, Zhang DR. Addiction related alteration in resting-state brain connectivity. Neuroimage. 2010;49:738–744. doi: 10.1016/j.neuroimage.2009.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddock RJ. The retrosplenial cortex and emotion: new insights from functional neuroimaging of the human brain. Trends Neurosci. 1999;22:310–316. doi: 10.1016/s0166-2236(98)01374-5. [DOI] [PubMed] [Google Scholar]
- Maleki N, Becerra L, Nutile L, Pendse G, Brawn J, Bigal M, Burstein R, Borsook D. Migraine attacks the Basal Ganglia. Mol Pain. 2011;7:71. doi: 10.1186/1744-8069-7-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maleki N, Linnman C, Brawn J, Burstein R, Becerra L, Borsook D. Her versus his migraine: multiple sex differences in brain function and structure. Brain. 2012;135:2546–2559. doi: 10.1093/brain/aws175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinen S, Vartiainen N, Hlushchuk Y, Koskinen M, Ramkumar P, Forss N, Kalso E, Hari R. Aberrant temporal and spatial brain activity during rest in patients with chronic pain. Proc Natl Acad Sci U S A. 2010;107:6493–6497. doi: 10.1073/pnas.1001504107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone MA. Irritable bowel syndrome. Prim Care. 2011;38:433–447. viii. doi: 10.1016/j.pop.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Martins MJ, Moura BL, Martins IP, Figueira ML, Prkachin KM. Sensitivity to expressions of pain in schizophrenia patients. Psychiatry Res. 2011;189:180–184. doi: 10.1016/j.psychres.2011.03.007. [DOI] [PubMed] [Google Scholar]
- McBeth J, Morris S, Benjamin S, Silman AJ, Macfarlane GJ. Associations between adverse events in childhood and chronic widespread pain in adulthood: are they explained by differential recall? J Rheumatol. 2001;28:2305–2309. [PubMed] [Google Scholar]
- McClure SM, Daw ND, Montague PR. A computational substrate for incentive salience. Trends in neurosciences. 2003;26:423–428. doi: 10.1016/s0166-2236(03)00177-2. [DOI] [PubMed] [Google Scholar]
- Meerman EE, Verkuil B, Brosschot JF. Decreasing pain tolerance outside of awareness. J Psychosom Res. 2011;70:250–257. doi: 10.1016/j.jpsychores.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Melloni L, van Leeuwen S, Alink A, Muller NG. Interaction between Bottom-up Saliency and Top-down Control: How Saliency Maps Are Created in the Human Brain. Cereb Cortex. 2012 doi: 10.1093/cercor/bhr384. [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith MA, Stein BE. Visual, auditory, and somatosensory convergence on cells in superior colliculus results in multisensory integration. J Neurophysiol. 1986;56:640–662. doi: 10.1152/jn.1986.56.3.640. [DOI] [PubMed] [Google Scholar]
- Moore JW, Stickney KJ. Formation of attentional-as- sociative networks in real time: Role of the hippocampus and im-plications for conditioning. Physiological Psychology. 1980;8:207–217. [Google Scholar]
- Moulton EA, Elman I, Pendse G, Schmahmann J, Becerra L, Borsook D. Aversion-related circuitry in the cerebellum: responses to noxious heat and unpleasant images. J Neurosci. 2011;31:3795–3804. doi: 10.1523/JNEUROSCI.6709-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouraux A, Diukova A, Lee MC, Wise RG, Iannetti GD. A multisensory investigation of the functional significance of the “pain matrix”. Neuroimage. 2011;54:2237–2249. doi: 10.1016/j.neuroimage.2010.09.084. [DOI] [PubMed] [Google Scholar]
- Mouraux A, Iannetti GD. Nociceptive laser-evoked brain potentials do not reflect nociceptive-specific neural activity. J Neurophysiol. 2009;101:3258–3269. doi: 10.1152/jn.91181.2008. [DOI] [PubMed] [Google Scholar]
- Mulckhuyse M, Theeuwes J. Unconscious attentional orienting to exogenous cues: A review of the literature. Acta Psychol (Amst) 2010;134:299–309. doi: 10.1016/j.actpsy.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Mutschler I, Ball T, Wankerl J, Strigo IA. Pain and emotion in the insular cortex: evidence for functional reorganization in major depression. Neurosci Lett. 2012;520:204–209. doi: 10.1016/j.neulet.2012.03.095. [DOI] [PubMed] [Google Scholar]
- Nagy H, Levy-Gigi E, Somlai Z, Takats A, Bereczki D, Keri S. The effect of dopamine agonists on adaptive and aberrant salience in Parkinson's disease. Neuropsychopharmacology. 2012;37:950–958. doi: 10.1038/npp.2011.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SM, Dosenbach NU, Cohen AL, Wheeler ME, Schlaggar BL, Petersen SE. Role of the anterior insula in task-level control and focal attention. Brain Struct Funct. 2010;214:669–680. doi: 10.1007/s00429-010-0260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen MO, Rostrup E, Wulff S, Bak N, Lublin H, Kapur S, Glenthoj B. Alterations of the brain reward system in antipsychotic naive schizophrenia patients. Biol Psychiatry. 2012;71:898–905. doi: 10.1016/j.biopsych.2012.02.007. [DOI] [PubMed] [Google Scholar]
- Nielsen TA, McGregor DL, Zadra A, Ilnicki D, Ouellet L. Pain in dreams. Sleep. 1993;16:490–498. [PubMed] [Google Scholar]
- Petrovic P, Kalso E, Petersson KM, Andersson J, Fransson P, Ingvar M. A prefrontal nonopioid mechanism in placebo analgesia. Pain. 2010;150:59–65. doi: 10.1016/j.pain.2010.03.011. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Kalso E, Petersson KM, Ingvar M. Placebo and opioid analgesia-- imaging a shared neuronal network. Science. 2002;295:1737–1740. doi: 10.1126/science.1067176. [DOI] [PubMed] [Google Scholar]
- Porreca F, Burgess SE, Gardell LR, Vanderah TW, Malan TP, Jr., Ossipov MH, Lappi DA, Lai J. Inhibition of neuropathic pain by selective ablation of brainstem medullary cells expressing the mu-opioid receptor. J Neurosci. 2001;21:5281–5288. doi: 10.1523/JNEUROSCI.21-14-05281.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redelmeier DA, Kahneman D. Patients’ memories of painful medical treatments: real-time and retrospective evaluations of two minimally invasive procedures. Pain. 1996;66:3–8. doi: 10.1016/0304-3959(96)02994-6. [DOI] [PubMed] [Google Scholar]
- Robinson DL. Functional contributions of the primate pulvinar. Prog Brain Res. 1993;95:371–380. doi: 10.1016/s0079-6123(08)60382-9. [DOI] [PubMed] [Google Scholar]
- Robinson DL, Petersen SE. The pulvinar and visual salience. Trends Neurosci. 1992;15:127–132. doi: 10.1016/0166-2236(92)90354-b. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Thorpe SJ, Maddison SP. Responses of striatal neurons in the behaving monkey. 1. Head of the caudate nucleus. Behav Brain Res. 1983;7:179–210. doi: 10.1016/0166-4328(83)90191-2. [DOI] [PubMed] [Google Scholar]
- Sabatini E, Della Penna S, Franciotti R, Ferretti A, Zoccolotti P, Rossini PM, Romani GL, Gainotti G. Brain structures activated by overt and covert emotional visual stimuli. Brain Res Bull. 2009;79:258–264. doi: 10.1016/j.brainresbull.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta JK. Individual differences in reward responding explain placebo-induced expectations and effects. Neuron. 2007;55:325–336. doi: 10.1016/j.neuron.2007.06.028. [DOI] [PubMed] [Google Scholar]
- Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta JK. Placebo and nocebo effects are defined by opposite opioid and dopaminergic responses. Arch Gen Psychiatry. 2008;65:220–231. doi: 10.1001/archgenpsychiatry.2007.34. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminowicz DA, Davis KD. Cortical responses to pain in healthy individuals depends on pain catastrophizing. Pain. 2006;120:297–306. doi: 10.1016/j.pain.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Seminowicz DA, Labus JS, Bueller JA, Tillisch K, Naliboff BD, Bushnell MC, Mayer EA. Regional gray matter density changes in brains of patients with irritable bowel syndrome. Gastroenterology. 2010;139:48–57. e42. doi: 10.1053/j.gastro.2010.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serranova T, Jech R, Dusek P, Sieger T, Ruzicka F, Urgosik D, Ruzicka E. Subthalamic nucleus stimulation affects incentive salience attribution in Parkinson's disease. Mov Disord. 2011;26:2260–2266. doi: 10.1002/mds.23880. [DOI] [PubMed] [Google Scholar]
- Setlow B, Schoenbaum G, Gallagher M. Neural encoding in ventral striatum during olfactory discrimination learning. Neuron. 2003;38:625–636. doi: 10.1016/s0896-6273(03)00264-2. [DOI] [PubMed] [Google Scholar]
- Shimo K, Ueno T, Younger J, Nishihara M, Inoue S, Ikemoto T, Taniguchi S, Ushida T. Visualization of painful experiences believed to trigger the activation of affective and emotional brain regions in subjects with low back pain. PLoS One. 2011;6:e26681. doi: 10.1371/journal.pone.0026681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine JM, Halliday GM, Naismith SL, Lewis SJ. Visual misperceptions and hallucinations in Parkinson's disease: dysfunction of attentional control networks? Mov Disord. 2011;26:2154–2159. doi: 10.1002/mds.23896. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O'Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Singh MK, Giles LL, Nasrallah HA. Pain insensitivity in schizophrenia: trait or state marker? J Psychiatr Pract. 2006;12:90–102. doi: 10.1097/00131746-200603000-00004. [DOI] [PubMed] [Google Scholar]
- Smith BW, Tooley EM, Montague EQ, Robinson AE, Cosper CJ, Mullins PG. Habituation and sensitization to heat and cold pain in women with fibromyalgia and healthy controls. Pain. 2008;140:420–428. doi: 10.1016/j.pain.2008.09.018. [DOI] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF. Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence C, Parise C. Prior-entry: a review. Conscious Cogn. 2010;19:364–379. doi: 10.1016/j.concog.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory systems of the brain: a brief history and current perspective. Neurobiol Learn Mem. 2004;82:171–177. doi: 10.1016/j.nlm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Stanghellini G, Ballerini M, Fusar Poli P, Cutting J. Abnormal bodily experiences may be a marker of early schizophrenia? Curr Pharm Des. 2012;18:392–398. doi: 10.2174/138161212799316181. [DOI] [PubMed] [Google Scholar]
- Starr CJ, Sawaki L, Wittenberg GF, Burdette JH, Oshiro Y, Quevedo AS, Coghill RC. Roles of the insular cortex in the modulation of pain: insights from brain lesions. J Neurosci. 2009;29:2684–2694. doi: 10.1523/JNEUROSCI.5173-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr CJ, Sawaki L, Wittenberg GF, Burdette JH, Oshiro Y, Quevedo AS, McHaffie JG, Coghill RC. The contribution of the putamen to sensory aspects of pain: insights from structural connectivity and brain lesions. Brain. 2011;134:1987–2004. doi: 10.1093/brain/awr117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein BE, Stanford TR. Multisensory integration: current issues from the perspective of the single neuron. Nat Rev Neurosci. 2008;9:255–266. doi: 10.1038/nrn2331. [DOI] [PubMed] [Google Scholar]
- Stein N, Sprenger C, Scholz J, Wiech K, Bingel U. White matter integrity of the descending pain modulatory system is associated with interindividual differences in placebo analgesia. Pain. 2012 doi: 10.1016/j.pain.2012.07.010. [DOI] [PubMed] [Google Scholar]
- Stone AA, Schwartz JE, Broderick JE, Shiffman SS. Variability of momentary pain predicts recall of weekly pain: a consequence of the peak (or salience) memory heuristic. Pers Soc Psychol Bull. 2005;31:1340–1346. doi: 10.1177/0146167205275615. [DOI] [PubMed] [Google Scholar]
- Strigo IA, Simmons AN, Matthews SC, Craig AD, Paulus MP. Association of major depressive disorder with altered functional brain response during anticipation and processing of heat pain. Arch Gen Psychiatry. 2008;65:1275–1284. doi: 10.1001/archpsyc.65.11.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takikawa Y, Kawagoe R, Hikosaka O. Reward-dependent spatial selectivity of anticipatory activity in monkey caudate neurons. J Neurophysiol. 2002;87:508–515. doi: 10.1152/jn.00288.2001. [DOI] [PubMed] [Google Scholar]
- Taylor KS, Seminowicz DA, Davis KD. Two systems of resting state connectivity between the insula and cingulate cortex. Hum Brain Mapp. 2009;30:2731–2745. doi: 10.1002/hbm.20705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason ME, Hamilton JP, Gotlib IH. Stress-induced activation of the HPA axis predicts connectivity between subgenual cingulate and salience network during rest in adolescents. J Child Psychol Psychiatry. 2011;52:1026–1034. doi: 10.1111/j.1469-7610.2011.02422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Kahn RS, Goni J, Sporns O. High-cost, high-capacity backbone for global brain communication. Proc Natl Acad Sci U S A. 2012;109:11372–11377. doi: 10.1073/pnas.1203593109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veilleux S, Melzack R. Pain in psychotic patients. Exp Neurol. 1976;52:535–543. doi: 10.1016/0014-4886(76)90224-7. [DOI] [PubMed] [Google Scholar]
- Weissman-Fogel I, Moayedi M, Tenenbaum HC, Goldberg MB, Freeman BV, Davis KD. Abnormal cortical activity in patients with temporomandibular disorder evoked by cognitive and emotional tasks. Pain. 2011;152:384–396. doi: 10.1016/j.pain.2010.10.046. [DOI] [PubMed] [Google Scholar]
- Wiech K, Lin CS, Brodersen KH, Bingel U, Ploner M, Tracey I. Anterior insula integrates information about salience into perceptual decisions about pain. J Neurosci. 2010;30:16324–16331. doi: 10.1523/JNEUROSCI.2087-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward ND, Rogers B, Heckers S. Functional resting-state networks are differentially affected in schizophrenia. Schizophrenia research. 2011;130:86–93. doi: 10.1016/j.schres.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright J, Morley S. Autobiographical memory and chronic pain. Br J Clin Psychol. 1995;34(Pt 2):255–265. doi: 10.1111/j.2044-8260.1995.tb01460.x. [DOI] [PubMed] [Google Scholar]
- Yen CP, Kuan CY, Sheehan J, Kung SS, Wang CC, Liu CK, Kwan AL. Impact of bilateral anterior cingulotomy on neurocognitive function in patients with intractable pain. J Clin Neurosci. 2009;16:214–219. doi: 10.1016/j.jocn.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Yoshii N, Fukuda S. Effects of unilateral and bilateral invasion of thalamic pulvinar for pain relief. Tohoku J Exp Med. 1979;127:81–84. doi: 10.1620/tjem.127.81. [DOI] [PubMed] [Google Scholar]
- Yuan Z, Qin W, Wang D, Jiang T, Zhang Y, Yu C. The salience network contributes to an individual's fluid reasoning capacity. Behav Brain Res. 2012;229:384–390. doi: 10.1016/j.bbr.2012.01.037. [DOI] [PubMed] [Google Scholar]
- Zajonic Feeling and thinking: Preferences need no inferences. American Psychologist. 1980;35:151–175. [Google Scholar]
- Zhang L, Zhang Y, Zhao ZQ. Anterior cingulate cortex contributes to the descending facilitatory modulation of pain via dorsal reticular nucleus. Eur J Neurosci. 2005;22:1141–1148. doi: 10.1111/j.1460-9568.2005.04302.x. [DOI] [PubMed] [Google Scholar]
- Zink CF, Pagnoni G, Martin ME, Dhamala M, Berns GS. Human striatal response to salient nonrewarding stimuli. J Neurosci. 2003;23:8092–8097. doi: 10.1523/JNEUROSCI.23-22-08092.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]