Abstract
In two-bottle preference tests with water and solutions of monosodium glutamate (MSG) and inosine-5′-monophosphate (IMP), mice from the C57BL/6ByJ inbred strain consumed more and had higher preferences for these solutions compared with mice from the 129/J strain. The C57BL/6ByJ mice consumed 300 mmol/L MSG in large amounts, which were comparable to intakes of highly preferred solutions of sweeteners. The strain differences in voluntary consumption of 300 mmol/L MSG depended at least in part on postingestive effects because prior experience with MSG influenced the expression of the strain difference in MSG acceptance. The strain difference in MSG acceptance was in the opposite direction to the strain difference in NaCl acceptance and was not affected by previous consumption of saccharin. Although the C57BL/6ByJ mice had higher avidity for both MSG and sweeteners than did the 129/J mice, there was no correlation between preferences for these solutions in the second hybrid generation (F2) derived from these two strains. Thus, the strain differences in MSG acceptance are not related to the strain differences in salty or sweet taste responsiveness and most likely represent specific umami taste responsiveness. High acceptance of MSG solutions by the C57BL/6ByJ mice was inherited as a recessive trait in the F2 generation. Further genetic linkage analyses using the F2 hybrids are being conducted to map chromosomal locations of genes determining the strain difference in MSG acceptance.
Keywords: monosodium glutamate, inosine-5′-monophosphate, C57BL/6 and 129 mouse strains, taste, consumption
When the sodium salt of glutamic acid, monosodium glutamate (MSG)5 is added to certain foods in relatively small quantities, the palatability of those foods is increased (Yamaguchi 1998). There is substantial evidence that the sensory basis for this effect is that MSG stimulates the sense of taste (Dove 1948, Kawamura and Kare 1987). Indeed, recent studies have provided convincing evidence that the taste quality elicited by MSG and related substances such as inositol monophosphate (IMP), herein labeled “umami,” is unique. That is, it is not some combination of sweet, sour, salty and bitter, the presumed other primary taste qualities [but see (Halpern 1997)].
The mechanism(s) underlying detection and recognition of umami substances and the basis for their powerful ability to enhance palatability and increase intake remain controversial or unknown. Physiologic and behavioral studies using animal models and genetic dissection of umami taste responses can potentially unravel these mechanisms. MSG and other umami substances are detected and preferred by several species of animals; most of the work, albeit still a very small amount, has concentrated on rats and mice (Ninomiya and Funakoshi 1989a and 1989b, Yamamoto et al. 1991 and 1988).
We recently reported differences in MSG acceptance between two inbred mouse strains, C57BL/6ByJ and 129/J (Beauchamp et al. 1998). Here, we further characterize ingestive responses to MSG and IMP in these two strains and provide a preliminary report of genetic analyses based on C57BL/6ByJ × 129/J hybrids.
MATERIALS AND METHODS
Subjects
Mice of the C57BL6/ByJ (B6) and 129/J (129) strains were obtained from The Jackson Laboratory (Bar Harbor, ME). The mice were housed in individual cages in a temperature-controlled room at 23°C on a 12-h light:dark cycle; mice had free access to deionized water and Teklad Rodent Diet 8604, which contains 0.31% sodium, 0.99% potassium and 1.46% calcium.
For genetic analyses, the B6 and 129 mice were outcrossed to produce the first filial generation of hybrids (F1); these were intercrossed to produce the second hybrid generation (F2). The F1 was generated by reciprocal crosses using both strains and genders: 1B F1 (♀ 129 × ♂ B6) and B1 F1 (♀ B6 × ♂ 129). Three types of the F2 hybrids (n = 455) were obtained: 1B × 1B F2 ( ♀ 1B F1 × ♂ 1B F1; 92 females and 103 males), B1 × Bl F2 (♀ B1 F1 × ♂ Bl F1; 106 females and 91 males) and Bl × 1B F2 (♀ B1 F1 × ♂ 1B F1; 29 females and 34 males). Pups were weaned at 21–30 d of age and reared in same-sexed groups of 4–6.
Measurement of fluid intake
Fluid intake was measured using two-bottle preference tests of individually caged mice. Construction of drinking tubes and other experimental details have been described previously (Bachmanov et al. 1996). The drinking tubes were positioned to the right of the feeder with their tips 15 mm apart, and each extended 25 mm into the cage. Each tube had a stainless steel tip with a 3.175-mm diameter hole from which the mice could lick fluids.
Solutions of monosodium salt of l-glutamic acid (MSG), disodium salt of inosine-5′-monophosphate (IMP), sucrose, d-phenylalanine and sodium saccharin (Sigma Chemical, St. Louis, MO) were prepared in deionized water. The mice were presented with one tube containing a solution and the other tube containing deionized water. The positions of the tubes were switched every 24 h to control for side preferences. Daily measurements were made in the middle of the light period by reading fluid volume to the nearest 0.2 mL. Body weights (BW) of individual mice were measured before and after each test series.
Data analyses
Because fluid intakes can depend on BW (Bachmanov et al. 1998a and 1998b), we analyzed fluid intakes expressed per mouse (raw intakes) and per BW. In experiments presented here, the B6 mice consumed more MSG and IMP solutions than did the 129 mice, and they also were heavier than the 129 mice. Therefore, in all cases when intake per BW was higher in the B6 mice, the strain difference was even larger for raw intakes. On the contrary, in some cases when raw intakes were significantly higher in the B6 mice than in the 129 mice, intakes expressed per BW did not differ significantly. Because intake per BW is a more conservative index for characterizing strain differences, we report only intakes per BW and preferences.
In all experiments with free access to MSG solutions, the B6 mice gained more weight than did the 129 mice. Among individual mice, there was a positive correlation between MSG intakes and BW gain. Thus, mice that consumed more MSG gained more weight. Therefore, in tests with MSG, we corrected MSG intakes using body weights measured before access to MSG. Because during access to IMP or sweeteners changes in BW were similar in the two strains, we used average BW before and after the tests to calculate intake of these solutions per BW.
Indices of fluid acceptance were calculated using average daily (24-h) fluid intakes for each mouse for each solution concentration. We determined the following: 1) raw intakes per mouse, 2) intakes per 30 g of BW (approximate weight of an adult mouse), and 3) preference scores [the ratio of the averaged solution intake to averaged total fluid (solution + water) intake, as a percentage].
The data were analyzed using Pearson correlation coefficients, t tests, ANOVA and planned comparisons as appropriate. The significance of preference/avoidance of a solution over water in the two-bottle tests was determined by comparing the solution and water intakes using paired t tests. Dominance in the F2 generation was detected when the F2 value differed significantly from a midparental value (this was achieved by collapsing B6 and 129 values, assigning the coefficient 1 for each of them, and assigning the coefficient −2 to the F2 value in planned comparison tests). All statistical tests used a two-tailed criterion for significance of P < 0.05.
RESULTS
MSG acceptance by B6 and 129 mice
Experiment 1
0.1–1000 mmol/L MSG solutions were tested in ascending order in 5.5 mo old male B6 (n = 12) and 129 (n = 12) mice. Each MSG concentration was tested with water for 2 d. Before this experiment, the mice had been tested in two-bottle tests with series of concentrations of glycyrrhizic acid, SC-45647 (a guanidineacetic acid sweetener), sucralose and Polycose.
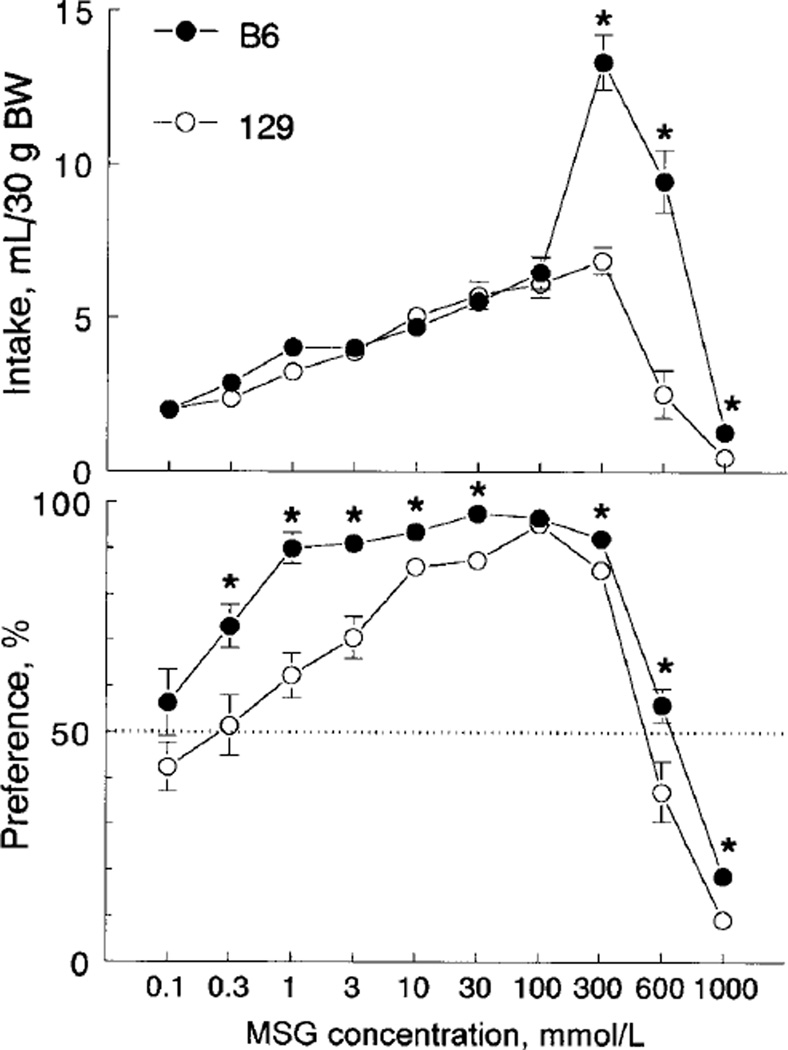
MSG intakes and preferences were significantly affected by MSG concentration [F(9,198) = 69.9 and 93.3, respectively, P < 0.0001, two-way ANOVA] and were higher overall in the B6 strain compared with the 129 strain [F(1,22) = 25.6 and 64.4, P < 0.0001]. The strain differences depended on MSG concentration [strain × concentration interaction F(9,198) = 19.0 for intakes and 2.2 for preferences, P < 0.05]. The two strains drank similar amounts of 0.1–100 mmol/L MSG, but the B6 mice drank more 300–1000 mmol/L MSG than did the 129 mice (Fig. 1, top). Consumption of 300 mmol/L MSG by the B6 mice was remarkably high, i.e., for some B6 mice, daily consumption of MSG exceeded one half of their body weight.
FIGURE 1.
Average daily monosodium glutamate (MSG) intake (upper panel) and preference (lower panel) by male B6 and 129 mice in two-bottle 48-h preference tests (Experiment 1). Vertical bars represent sem. *Significant difference between B6 and 129 mice (P < 0.05, planned comparisons).
The B6 mice preferred 0.3–600 mmol/L MSG solutions (consumed significantly more MSG solution than water, P < 0.05, paired t tests), exhibited high (≥90%) preference over the range of 1–300 mmol/L MSG concentrations and avoided 1000 mmol/L MSG (Fig. 1, lower panel). The 129 mice preferred 1–300 mmol/L MSG, reached peak of preference (95%) at 100 mmol/L concentration, were indifferent to 600 mmol/L MSG and avoided 1000 mmol/L MSG. The B6 mice exhibited significantly higher MSG preference scores compared with the 129 mice at 0.3–30 and 300–1000 mmol/L concentrations. Thus, the B6 mice preferred a wider range of MSG concentrations and had higher MSG preference scores than did the 129 mice.
This experiment demonstrated concentration-specific strain differences in MSG acceptance. At lower MSG concentrations (0.3–30 mmol/L), the B6 mice had higher preference scores than did the 129 mice, but they did not differ significantly in intakes. At higher MSG concentrations (especially 300 and 600 mmol/L), the B6 mice consumed much more MSG than did the 129 mice. To confirm this pattern of strain differences, we conducted several additional experiments testing 1 and 300 mmol/L MSG (Experiments 2, 3 and 4).
Experiment 2
In this experiment, 4.5 mo old male B6 (n = 10) and 129 (n = 10) mice were given two-bottle tests with 1 mmol/L MSG and water for 4 d; for the next 4 d they received 300 mmol/L MSG and water. Before this experiment, the mice had been tested in two-bottle tests with solutions of saccharin, glycine, d-phenylalanine and sucrose.
Consistent with the results of Experiment 1, the two strains had similar intakes of 1 mmol/L MSG, but the B6 mice had higher preferences for this solution (Table 1). When given 300 mmol/L MSG, the B6 mice had higher MSG preferences and intakes compared with the 129 mice.
TABLE 1.
Average daily monosodium glutamate (MSG) solution intakes and preferences by male B6 and 129 mice (Experiment 2)1
| MSG concentration |
Index | B6 | 129 |
|---|---|---|---|
| 1 mmol/L | Intake, mL/30 g BW | 3.1 ± 0.2 | 3.2 ± 0.2 |
| Preference, % | 82.7 ± 4.3 | 64.9 ± 3.2** | |
| 300 mmol/L | Intake, mL/30 g BW | 10.1 ± 1.0 | 3.4 ± 0.6*** |
| Preference, % | 84.5 ± 3.1 | 52.7 ± 5.9*** |
Values are means ± sem;
P < 0.01,
P < 0.001, significant difference between B6 and 129 mice, t tests.
Experiment 3
The goal of this experiment was to extend the observation of strain differences in MSG acceptance to females. Male and female B6 and 129 mice (4.25–6 mo old, n = 9–10/group) were given 4-d tests of 1 mmol/L and 300 mmol/L MSG and water. Between testing the two MSG concentrations, the mice were given water in both drinking tubes for 1 d. Before this experiment, the mice had been tested in two-bottle tests with solutions of glycine, d-phenylalanine, saccharin and sucrose.
Fluid intakes and preferences were analyzed using three-way ANOVA to assess the effects of strain, gender and period of test (1–2 d vs. 3–4 d of the 96-h test). There were no significant changes in 1 mmol/L MSG acceptance during the experiment; therefore all results are presented as 4-d averages (Table 2). Consistent with the previous observations, the two strains did not differ significantly in 1 mmol/L MSG intakes [Table 2; effect of strain F(1,35) = 3.5, P > 0.05], and the B6 mice had higher preferences for 1 mmol/L MSG compared with the 129 mice [Table 2; effect of strain F(1,35) = 29.2, P < 0.0001].
TABLE 2.
Average daily monosodium glutamate (MSG) solution intakes and preferences of B6 and 129 mice (Experiment 3)1
| Males |
Females |
||||
|---|---|---|---|---|---|
| MSG concentration |
Index | B6 | 129 | B6 | 129 |
| 1 mmol/L | Intake, mL/30 g BW | 4.3 ± 0.3 | 3.2 ± 0.4 | 6.6 ± 0.2 | 6.0 ± 0.7 |
| Preference, % | 86.2 ± 2.7 | 63.6 ± 5.7*** | 91.9 ± 1.0 | 70.9 ± 5.0*** | |
| 300 mmol/L | Intake, mL/30 g BW | 12.3 ± 1.2 | 4.2 ± 0.6*** | 20.9 ± 1.1 | 7.9 ± 1.3*** |
| Preference, % | 85.5 ± 3.5 | 68.3 ± 5.8* | 91.0 ± 1.8 | 74.7 ± 6.1* | |
Values are means ± sem;
P < 0.05,
P < 0.01,
P < 0.001, significant difference between B6 and 129 mice for the same gender, planned comparison tests based on three-way ANOVA.
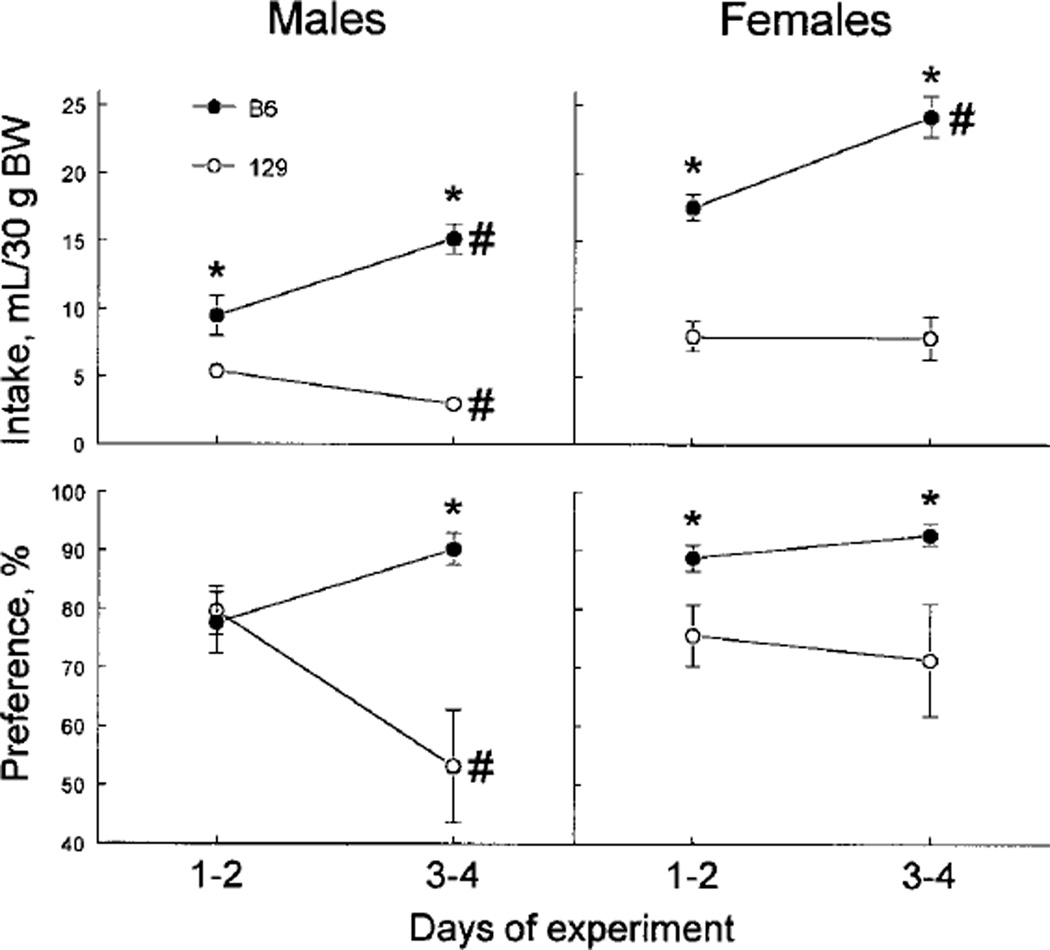
The 300 mmol/L MSG intakes and preferences were higher overall in the B6 mice than in the 129 mice [Table 2; effect of strain F(1,35) = 93.2 and 11.8, respectively, P < 0.01]. However they changed during the 4-d test in a strain-specific manner [strain × period interaction F(1,35) = 61.0 for intakes and 14.1 for preferences, P < 0.001]. Male and female B6 mice drank more 300 mmol/L MSG in the second half of the test compared with the first half, whereas the MSG intakes decreased in the 129 males and did not change in the 129 females (Fig. 2, upper panel). Preferences for 300 mmol/L MSG decreased significantly in 129 males, and they did not change in the other groups (Fig. 2, lower panel). As a result, the strain differences in 300 mmol/L MSG acceptance were larger during the last 2 d of the experiment compared with the first 2 d.
FIGURE 2.
Changes of average daily 300 mmol/L monosodium glutamate (MSG) intake (upper panel) and preference (lower panel) by B6 and 129 mice during 96-h two-bottle preference tests (Experiment 3). Vertical bars represent sem. *Significant difference between B6 and 129 mice (P < 0.05, planned comparisons). #Significant difference between d 1–2 and d 3–4 of the test (P < 0.05, planned comparisons).
Experiment 4
In the previous experiments, mice were tested with MSG after they had been exposed to solutions of sweeteners. To exclude possible carry-over effects from previous tests with sweeteners, in this experiment, MSG acceptance was tested using naïve male B6 and 129 mice (n = 12/group). The mice were 2 mo old when the experiment started. After 5 d of adaptation to individual cages and two drinking tubes with water, the mice were given an MSG solution and water for 2 d. Half of mice from each strain (n = 6) received 1 mmol/L MSG, and the other half received 300 mmol/L MSG. After 5 d with only water available, the second 2-d test was conducted, with the two groups receiving correspondingly 300 and 1 mmol/L MSG. Therefore, each mouse was tested with two MSG concentrations.
There were no significant differences in 1 mmol/L MSG intakes [4.3 ± 0.5 and 3.2 ± 0.4 mL/(30 g BW · d) for the B6 and 129 strains, respectively] or preferences (64 ± 3 and 59 ± 5%, respectively). The strain differences in acceptance of 300 mmol/L MSG depended on whether this solution was tested before or after 1 mmol/L MSG, i.e., for both intakes and preferences, there was a significant interaction between strain and group (Table 3). The B6 mice that received first 1 mmol/L MSG and then 300 mmol/L MSG had higher indices of 300 mmol/L MSG acceptance than similarly treated 129 mice and than B6 mice that received 300 mmol/L MSG before 1 mmol/L MSG. Thus, these results are consistent with the results of Experiment 3, indicating that exposure to MSG exaggerates strain differences in its acceptance. It is also consistent with the following experiment conducted on the same mice showing a strain difference in 300 mmol/L MSG acceptance in 4-d tests.
TABLE 3.
Average daily 300 mmol/L monosodium glutamate (MSG) solution intakes and preferences by male B6 and 129 mice (Experiment 4)1
| Group 1 |
Group 2 |
ANOVA2: Effect of |
|||||
|---|---|---|---|---|---|---|---|
| Index | B6 | 129 | B6 | 129 | Strain | Group | Strain × Group |
| 300 and 1 mmol/L MSG | 1 and 300 mmol/L MSG | ||||||
| Intake, mL/30 g BW | 3.3 ± 0.7 | 3.6 ± 0.3 | 6.7 ± 1.4b | 2.3 ± 0.3a | 6.0* | 1.6 | 7.9* |
| Preference, % | 39.8 ± 3.8 | 53.7 ± 2.9 | 60.8 ± 9.8b | 42.4 ± 3.3a | 0.2 | 0.7 | 7.5* |
Values are means ± sem;
Significant difference between B6 and 129 strains from the same group, P < 0.05, planned comparisons;
Significant difference between Group 1 and Group 2 for the same strain, P < 0.05, planned comparisons.
F values for degrees of freedom 1 and 19;
P < 0.05.
Experiment 5
The goal of this experiment was to assess whether MSG acceptance is affected by previous exposure to sweeteners. After completion of Experiment 4, the same mice were given only water to drink for 5 d and then tested with 300 mmol/L MSG and water for 4 d. On the basis of 300 mmol/L MSG acceptance in this initial test, the B6 and 129 mice were divided into two groups (n = 6), so that in both groups of the same strain, averages and range of variation for MSG preferences and intakes were similar. Then, after 4 d with only water available, one group of mice from each strain was given 20 mmol/L saccharin with water for 4 d, and the other group had only water in both drinking tubes. For the next 2 d, all groups had only water available, and for the following 4 d they had access to 300 mmol/L MSG and water.
During the initial 4-d test with 300 mmol/L MSG, the B6 mice drank more MSG than did the 129 mice [6.4 ±1.3 and 3.0 ± 0.4 mL/(30 g BW · d), respectively, P < 0.05, t test]. Preference scores were 57 ± 8 and 53 ± 5%, respectively, (not significant).
Both B6 and 129 mice tested with saccharin strongly preferred it over water (preference scores were 96 ± 1 and 88 ± 4%, respectively; not significant, t test). Saccharin intakes were higher in the B6 mice than in the 129 mice [10.3 ± 0.5 and 6.1 ± 1.0 mL/(30 g BW · d), respectively, P < 0.01, t test]. Compared with the water-treated 129 mice, the water-treated B6 mice consumed more water [6.1 ± 0.3 and 5.2 ± 0.2 mL/(30 g BW · d), respectively, P < 0.05].
Indices of 300 mmol/L MSG acceptance before and after saccharin or water tests were compared using three-way ANOVA with strain, group (saccharin- or water-treated) and period (before and after saccharin or water tests) as factors. During both MSG tests, saccharin- and water-exposed B6 mice had higher 300 mmol/L MSG intakes than did the 129 mice from corresponding groups [Fig. 3; effect of strain F(1,20) = 6.2, P < 0.05]. All mice slightly but significantly increased MSG intakes in the second test compared with the first test [Fig. 3; effect of period F(1,20) = 6.0, P < 0.05]. However there were no significant differences between saccharin- and water-exposed groups (no significant effects of group or interactions between group and other factors). No significant effects on 300 mmol/L MSG preferences were detected in this experiment.
FIGURE 3.
Average daily 300 mmol/L monosodium glutamate (MSG) intake by male B6 and 129 mice in two-bottle 96-h preference tests before (black columns) and after (shaded columns) exposure to 20 mmol/L saccharin or water (Experiment 5). Vertical bars represent sem; Sac, group tested with 20 mmol/L saccharin and water; Wat, group tested with water in both tubes.
IMP acceptance by B6 and 129 mice
IMP solutions (0.01–100 mmol/L) were tested in ascending order in the same male B6 (n = 12) and 129 (n = 12) mice tested in Experiments 4 and 5. Each IMP concentration was tested with water for 2 d.
IMP intakes and preferences were significantly affected by IMP concentration [F (8,176) = 44.2 and 35.5, respectively, P < 0.0001, two-way ANOVA] and were overall higher in the B6 strain compared with the 129 strain [F(1,22) = 9.5 for intakes and 6.6 for preferences, P < 0.05]. The strain differences depended on IMP concentration [strain × concentration interaction F(8,176) = 2.2 for intakes and 3.9 for preferences, P < 0.05]. Compared with the 129 mice, the B6 mice had higher IMP intakes at 0.03–0.1 and 1–30 mmol/L concentrations (Fig. 4, upper panel). The B6 mice preferred all IMP solutions tested in this experiment (consumed significantly more IMP solution than water, P < 0.05, paired t tests) and exhibited high (≥80%) preference for 3–100 mmol/L IMP (Fig. 4, lower panel). The 129 mice preferred IMP solutions with concentrations ≥0.3 mmol/L (the significant preference for 0.01 mmol/L IMP by this strain is probably due to chance) and exhibited high (≥80%) preference for 30 and 100 mmol/L IMP. The B6 mice had significantly higher IMP preference scores compared with the 129 mice at 0.03–0.1 and 1–10 mmol/L concentrations. Thus, the B6 mice preferred a wider range of IMP concentrations and had higher IMP preference scores than did the 129 mice.
FIGURE 4.
Average daily inosine-5′-monophosphate (IMP) intake (upper panel) and preference (lower panel) by male B6 and 129 mice in two-bottle 48-h preference tests. Vertical bars represent sem. *Signifi-cant difference between B6 and 129 mice (P < 0.05, planned comparisons).
Acceptance of MSG and sweeteners by B6 × 129 F2 hybrids
The B6 and 129 mice (data from Experiment 3) were tested simultaneously with the same solutions as the F2 mice (n = 455). The F2 animals were 4–7 mo old when the tests began. Each taste solution was tested with water for 4 d. The solutions were tested in the following sequence: d-phenylalanine, saccharin, sucrose, 1 mmol/L MSG and 300 mmol/L MSG. One or 2 d with only water available separated tests of each solution. Because there were no (or only small) differences between F2 cross types, all F2 mice were combined in one group.
Mode of inheritance
In male and female B6, 129 and F2 mice, we analyzed daily taste solution intakes and preferences averaged for all 4 d of the test and for the last 2 d of the test (when the strain differences were more pronounced, see Fig. 2, Experiment 3). The results were similar for both genders, for all indices of acceptance (raw intakes, intakes per BW and preferences), and for 4-d and the last 2-d averages. We therefore present here only 4-d average intakes per BW and preferences for males (Fig. 5).
FIGURE 5.
Average daily monosodium glutamate (MSG) and sweetener solutions intake (upper panel) and preference (lower panel) by male B6, 129 and F2 mice. Vertical bars represent sem Horizontal brackets show significant differences between groups (P < 0.05, planned comparisons). *Significant difference between F2 and midparental values (P < 0.05, planned comparisons).
MSG solution intakes and preferences in the F2 mice were lower than in the B6 mice, and did not differ significantly from the 129 mice. The F2 values for 300 mmol/L MSG were also significantly lower than the midparental values. This demonstrates that high MSG acceptance by the B6 mice is inherited as a recessive trait. Acceptance of three sweetener solutions by the F2 mice was significantly different from both parental values and did not differ from corresponding midparental values, except for sucrose preference, which did not differ significantly from the B6 value and exceeded the midparental level. This demonstrates that for sweetener acceptance, mode of inheritance was additive, with partial dominance of B6 alleles.
Analysis of correlations
The F2 mice varied substantially in body weight. Fluid intakes, both raw and expressed per unit of BW, covaried with body weight, which resulted in positive correlations among intakes of all fluids tested. Correlations among preferences for solutions of MSG and sweeteners for all F2 mice (males and females together) are shown in Table 4. Correlations calculated for each gender separately (not shown) were very close to each other and the correlations in the combined F2 group. Preferences for sucrose, saccharin and d-phenylalanine correlated strongly with each other. However, no significant correlations were found between preferences for the two MSG solutions and between MSG and sweetener preferences.
TABLE 4.
Correlations among monosodium glutamate (MSG) and sweetener solution preferences in F2 mice (n = 450–454)
| Solutions | MSG | Sucrose | Saccharin | d-Phenylalanine |
|---|---|---|---|---|
| 300 mmol/L | 120 mmol/L | 20 mmol/L | 30 mmol/L | |
| MSG, | ||||
| 1 mmol/L | +0.07 | +0.11 | +0.10 | +0.03 |
| MSG, | ||||
| 300 mmol/L | 0.00 | +0.01 | −0.04 | |
| Sucrose, | ||||
| 120 mmol/L | +0.72* | +0.50* | ||
| Saccharin, | ||||
| 20 mmol/L | +0.56* |
Significant correlations; the significance level was determined using Bonferroni correction for 10 correlation coefficients estimating critical P level as 0.05/10 = 0.005.
DISCUSSION
The data presented here demonstrate that mice from the B6 strain have a greater avidity for MSG and IMP solutions than do mice from the 129 strain. The strain difference in ingestive responses to MSG was confirmed in several experiments, and it appeared robust across different genders, various ages and previous experiences with taste solutions. This high MSG avidity is inherited as a recessive trait.
MSG and IMP taste similarly to humans. It has also been suggested that IMP itself is tasteless, but it enhances the taste of glutamate and thus can make the subthreshold concentrations of glutamic acid contained in saliva have a taste (Yamaguchi 1991 and 1998). In any case, our data are consistent with a common mechanism underlying taste responses to MSG and IMP in both humans and mice.
We have also confirmed in several experiments a difference between responses to low (e.g., 1 mmol/L) and high (especially 300 mmol/L) MSG concentrations. The B6 mice had higher 1 mmol/L MSG preferences than did the 129 mice, but the two strains did not differ significantly in 1 mmol/L MSG intakes expressed per BW. In contrast, 300 mmol/L MSG intakes were much higher in the B6 than in the 129 mice. Preferences for 1 and 300 mmol/L MSG did not correlate in the F2 hybrid mice, suggesting that acceptance of these solutions depends on different mechanisms determined by different genes. It is possible that low and high MSG concentrations have different taste qualities or flavors. However, the difference between these two solutions may also depend on the postingestive effects of 300 mmol/L MSG, as discussed below.
The amount of 300 mmol/L MSG ingested by the B6 mice is remarkable and rivals the amounts of very highly preferred sweet substances ingested by this same strain (see Fig. 5). What motivates this high consumption is unknown. There may be several mechanisms, one of which may be responsible for this high MSG intake, or they may work in concert. The 300 mmol/L MSG solution elicits strong responses in mouse MSG-best and NaCl-best fibers of the glossopharyngeal and chorda tympani nerves (Ninomiya et al. 2000) and thus must have an easily detectable taste. Therefore, high 300 mmol/L MSG consumption by the B6 mice relative to the 129 mice may be due to the difference in taste perception of MSG between these mouse strains. However, it is evident that postingestive mechanisms also play a role. First, the B6 mice given access to MSG gained more weight than did the 129 mice. This could be due to accumulation of water (300 mmol/L MSG is an osmotically hypertonic solution and contains a substantial amount of sodium) or to changes in metabolism (Jungas et al. 1992), but in any case, the difference in body weight gain suggests that the physiologic consequences of MSG consumption for the B6 and 129 mice are different. Second, the strain differences in 300 mmol/L MSG acceptance increased when mice were exposed to this solution for a longer time (Experiments 3 and 4). In particular, the B6 mice tended to increase, and the 129 mice tended to decrease their MSG acceptance during a 4-d test (Fig. 2). This suggests that the postingestive effects of MSG may be positively reinforcing to the B6 mice and aversive to the 129 mice. It is unclear what kind of postingestive effects modify MSG acceptance, but it is possible that the postingestive effects of MSG may interact with its taste. For example, if osmotically hypertonic 300 mmol/L MSG is palatable to the B6 animals, drinking this solution would make the mice thirsty and would further stimulate consumption of this solution, creating a vicious circle. Regardless of the underlying mechanism, these studies demonstrate the importance of controlling for previous experience in long-term taste acceptance tests. In this regard, another possible carry-over effect, prior testing with sweet compounds, did not appear to influence the results (Experiment 5).
Our data indicate that responsiveness to sweet or salty taste, which have both been suggested as being involved in MSG and/or IMP perception, cannot explain the strain differences in acceptance of the umami-tasting compounds. There are several lines of evidence that the strain differences in MSG acceptance do not depend on sweet taste. First, previous exposure to saccharin did not affect acceptance of subsequently tested MSG (Experiment 5). Second, the modes of inheritance were different for MSG (recessive B6 alleles) and sweeteners (additive or partial dominance of B6 alleles). Third, among the F2 hybrid mice, preferences for several sweeteners correlated positively with each other, but not with preferences for MSG (Table 4). Thus, high acceptance of MSG and sweeteners fortuitously coincides in the B6 mice (and must have been fixed during inbreeding), and these traits are determined by different and genetically unlinked genes. The independence of MSG and sweetener acceptance also provides additional evidence that for mice, the taste quality of MSG is not “sweet,” as has been suggested from some previous rodent studies (Kawamura and Kare 1987, Yamamoto et al. 1991).
The strain differences in MSG and IMP acceptance were opposite to those for NaCl, i.e., the B6 mice avoided and the 129 mice preferred NaCl (Bachmanov et al. 1996 and 1998b, Beauchamp and Fisher 1993, Gannon and Contreras 1993, Lush 1991). Thus, the strain differences in MSG and IMP acceptance depend on factors other than the salty taste of Na+ in MSG and IMP. The most parsimonious hypothesis concerning taste quality for MSG and IMP in the mouse is that it is unique. This is consistent with the results of studies using a number of other species including humans (Ninomiya and Funakoshi 1989a and 1989b, Yamaguchi 1998).
Obtaining F2 hybrids between mouse strains with high and low MSG acceptance and characterizing their MSG consumption is an important step in identifying genes that determine the strain differences. We have demonstrated that high MSG acceptance by the B6 strain is inherited as a recessive trait (correspondingly, low MSG acceptance by the 129 strain is inherited as a dominant trait), and that this variation in MSG acceptance depends on different genes than does the variation in sweetener acceptance. Using the F2 mice, we are currently conducting a genome screen to identify chromosomal regions containing genes that determine the strain difference in MSG acceptance. On the basis of chromosomal locations, it should then be possible to identify the genes themselves (Collins 1992). Thus, we expect that this study will eventually result in understanding the molecular genetic basis of umami taste responsiveness.
To summarize, we have described mouse strain differences in acceptance of the umami-tasting compounds, MSG and IMP, and characterized inheritance of this trait. To our knowledge, this is the first evidence for a specific genetic effect on the response to MSG, and thus should provide a useful tool to investigate the genetic basis for orosensory control of MSG sensitivity and preference.
Footnotes
Presented at the International Symposium on Glutamate, October 12–14,. 1998 at the Clinical Center for Rare Diseases Aldo e Cele Daccó, Mario Negri Institute for Pharmacological Research, Bergamo, Italy. The symposium was sponsored jointly by the Baylor College of Medicine, the Center for Nutrition at the University of Pittsburgh School of Medicine, the Monell Chemical Senses Center, the International Union of Food Science and Technology, and the Center for Human Nutrition; financial support was provided by the International Glutamate Technical Committee. The proceedings of the symposium are published as a supplement to The Journal of Nutrition. Editors for the symposium publication were John D. Fernstrom, the University of Pittsburgh School of Medicine, and Silvio Garattini, the Mario Negri Institute for Pharmacological Research.
Presented in part at the XII International Symposium on Olfaction and Taste, San Diego, CA[Beauchamp et al. 1998].
Supported by National Institutes of Health grant DC 00882.
Abbreviations used: 129, 129/J mouse strain; B6, C57BL/6ByJ mouse strain; BW, body weight; IMP, disodium salt of inosine-5′-monophosphate; MSG, monosodium salt of l-glutamic acid.
LITERATURE CITED
- Bachmanov AA, Schlager G, Tordoff MG, Beauchamp GK. Consumption of electrolytes and quinine by mouse strains with different blood pressures. Physiol. Behav. 1998a;64:323–330. doi: 10.1016/s0031-9384(98)00069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmanov AA, Tordoff MG, Beauchamp GK. Ethanol consumption and taste preferences in C57BL/6ByJ and 129/J mice. Alcohol. Clin. Exp. Res. 1996;20:201–206. doi: 10.1111/j.1530-0277.1996.tb01630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmanov AA, Tordoff MG, Beauchamp GK. Voluntary sodium chloride consumption by mice: differences among five inbred strains. Behav. Genet. 1998b;28:117–124. doi: 10.1023/a:1021471924143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp GK, Bachmanov AA, Stein L. Development and genetics of glutamate taste preference. In: Murphy C, editor. Olfaction and Taste XII, An International Symposium. vol. 855. New York, NY: New York Academy of Sciences; 1998. pp. 412–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp GK, Fisher AS. Strain differences in consumption of saline solutions by mice. Physiol. Behav. 1993;54:179–184. doi: 10.1016/0031-9384(93)90063-l. [DOI] [PubMed] [Google Scholar]
- Collins FS. Positional cloning: let’s not call it reverse anymore. Nat. Genet. 1992;1:3–6. doi: 10.1038/ng0492-3. [DOI] [PubMed] [Google Scholar]
- Dove WF. Flavor and Acceptability of Monosodium Glutamate: Proceedings of the Symposium, Quartermaster Food and Container Institute for the Armed Forces, and Associates. Chicago, IL: Food and Container Institute; 1948. [Google Scholar]
- Gannon K, Contreras RJ. Sodium intake linked to amiloride-sensitive gustatory transduction in C57BL/6J and 129/J mice. Physiol. Behav. 1993;57:231–239. doi: 10.1016/0031-9384(94)00279-e. [DOI] [PubMed] [Google Scholar]
- Halpern BP. Psychophysics of taste. In: Beauchamp GK, Bartoshuk L, editors. Tasting and Smelling. San Diego, CA: Academic Press; 1997. pp. 77–123. [Google Scholar]
- Jungas RL, Halperin ML, Brosnan JT. Quantitative analysis of amino acid oxidation and related gluconeogenesis in humans. Physiol. Rev. 1992;72:419–448. doi: 10.1152/physrev.1992.72.2.419. [DOI] [PubMed] [Google Scholar]
- Kawamura Y, Kare MR. Umami: A Basic Taste. New York, NY: Marcel Dekker; 1987. [Google Scholar]
- Lush IE. The genetics of bitterness, sweetness, and saltiness in strains of mice. In: Wysocki CJ, Kare MR, editors. Genetics of Perception and Communication. New York, NY: Marcel Dekker; 1991. pp. 227–241. [Google Scholar]
- Ninomiya Y, Funakoshi M. Behavioural discrimination between glutamate and the four basic taste substances in mice. Comp. Biochem. Physiol. 1989a;92:365–370. doi: 10.1016/0300-9629(89)90577-x. [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, Funakoshi M. Peripheral neural basis for behavioural discrimination between glutamate and the four basic taste substances in mice. Comp. Biochem. Physiol. 1989b;92:371–376. doi: 10.1016/0300-9629(89)90578-1. [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, Nakashima K, Fukuda A, Nishino H, Sugimura T, Hino A, Danilova V, Hellekant G. Responses to umami substances in taste bud cells innervated by the chorda tympani and glossopharyngeal nerves. J. Nutr. 2000;130:950S–953S. doi: 10.1093/jn/130.4.950S. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S. Basic properties of umami and effects on humans. Physiol. Behav. 1991;49:833–841. doi: 10.1016/0031-9384(91)90192-q. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S. Basic properties of umami and its effects on food flavor. Food Rev. Int. 1998;14:139–176. [Google Scholar]
- Yamamoto T, Matsuo R, Fujimoto Y, Fukanaga I, Miyasaka A, Imoto T. Electrophysiological and behavioral studies on the taste of umami substances in the rat. Physiol. Behav. 1991;49:919–925. doi: 10.1016/0031-9384(91)90204-2. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Matsuo R, Kiyomitsu Y, Kitamura R. Taste effects of umami substances in hamsters as studied by electrophysiological and conditioned taste aversion techniques. Brain Res. 1988;451:147–162. doi: 10.1016/0006-8993(88)90759-7. [DOI] [PubMed] [Google Scholar]