Abstract
Objectives
Health Risk Appraisals (HRAs) have been implemented in a variety of settings, however few studies have examined the impact of computerized HRAs systematically in primary care. The study aimed at the development and pilot testing of a novel, comprehensive HRA tool in primary care practices.
Methods
We designed, implemented and pilot tested a novel, web-based HRA tool in four pair-matched intervention and control primary care practices (N = 200). Outcomes were measured before and 12 months after the intervention using the HRA, patient surveys, and qualitative feedback. Intervention patients received detailed feedback from the HRA and they were encouraged to discuss the HRA report at their next wellness visit in order to develop a personalized wellness plan.
Results
Estimated life expectancy and its derivatives, including Real Age and Wellness Score were significantly impacted by the HRA implementation (P<0.001). The overall rate of 10 preventive maneuvers improved by 4.2% in the intervention group vs. control (P = 0.001). The HRA improved the patient-centeredness of care, measured by the CAHPS PCC-10 survey (P = 0.05). HRA use was strongly associated with better self-rated overall health (OR = 4.94; 95% CI, 3.85–6.36) and improved up-to-dateness for preventive services (OR = 1.22; 95% CI, 1.12–1.32). A generalized linear model suggested that increase in Wellness Score was associated with improvements in patient-centeredness of care, up-to-dateness for preventive services and being in the intervention group (all P<0.03). Patients were satisfied with their HRA-experience, found the HRA report relevant and motivating and thought that it increased their health awareness. Clinicians emphasized that the HRA tool helped them and their patients converge on high-impact, evidence-based preventive measures.
Conclusions
Despite study limitations, results suggest that a comprehensive, web-based, and goal-directed HRA tool can improve the receipt of preventive services, patient-centeredness of care, behavioral health outcomes, and various wellness indicators in primary care settings.
Keywords: Health risk appraisal, health information technology, goal-directed care, prioritization, wellness
1. Background
Our healthcare system is focused predominantly on biomedical determinants of health, diagnosis of individual health problems, and targeted interventions that may correct abnormalities. The prevailing paradigm is rooted in a reductionist scientific approach that aims at generating evidence from highly controlled (“clean”) experiments and the availability of instruments that can quantify bio-medical targets and record their change over time (e.g., HbA1c level) [13]. Although effective for solving specific clinical problems, this thinking has resulted in a disease-driven and problem-oriented care approach. Unfortunately, whole-person care, patient-centeredness, consideration of patient goals and preferences, care coordination across conditions and settings, and continuity of care relationships have often been lost in the process [17].
Although the importance and substantial impact of behavioral risk factors (e.g., level of physical activity, dietary habits, smoking, etc) on health and health care are well known [24], clinicians and their patients may not always appreciate their long-term consequences. Clinicians and patients may feel challenged to modify unhealthy behaviors or personal circumstances, due to the lack of resources, time or sufficient skills. They may also have limited access to means and metrics that can help them gauge the level of behavioral risks and project their impact on long-term health outcomes.
Health information technologies have been developed recently to capture a more granular set of parameters that influence clinical outcomes, including behavioral health parameters [15, 20]. However, simply documenting more psychosocial and behavioral measures within existing data management systems (e.g., in the electronic health record - EHR) may not be sufficient.
Systematic use of comprehensive personal health information has been challenging in primary care due to the lack of financial support for information management, efficient data collection methods (e.g., patient-driven health risk assessment), and sophisticated point-of-care data analysis approaches (e.g., clinical decision support) [8, 14]. Patient-driven e-health technologies have shown some potential to align the goal of patient empowerment with the need for more rounded and actionable clinical data [20, 22]. The effectiveness of health risk appraisal (HRA) instruments was reviewed in a U.S. Department of Human Services Health Care Financing Administration report in 2003 [21]. This review concluded that the HRA questionnaire without follow-up interventions is a suboptimal health management strategy and that HRA instruments combined with linked health promotion programs are more likely to produce favorable improvements. A more recent environment scan commissioned by the Agency for Healthcare Research and Quality (AHRQ) and reported by Econometrica, Inc on the utilization and impact of HRAs [7] echoes the findings of the 2003 report. Despite regular use in worksite and wellness settings, HRAs have not been widely and systematically adopted and studied in primary care, most likely due to the lack of standardization, difficulty of generalization of risk scoring and feedback, limited integration into the care delivery process, and suboptimal resources for patient support and follow-up, especially in the area of behavioral interventions [4, 9, 12].
2. Objectives
In this paper, we suggest an alternative, comprehensive HRA -based approach that operates in a patient goal-directed, outcomes-oriented framework. Goal-directed medicine is a conceptual model of care in which shared patient and clinician goals and objectives are determined prior to deciding on diagnostic and treatment strategies [16, 17]. When shared goals are set as the starting point of care decisions, other steps will follow in a logical manner that may effectively support a patient-centered care model. These include the need to individualize and contextualize guidelines; the prioritization of evidence-based interventions by considering their long-term impact on health; and the integration of preventive measures that aim at life extension and improving current quality of life in a more coherent approach (►Figure 1). This study aimed specifically at the exploratory measurement of the impact of the HRA on estimated life expectancy and its patient-friendly derivatives (Real Age and Wellness Score), uptake of preventive services, and patient-centeredness of care, controlling for patient demographics, comorbidities, care delivery patterns, and behavioral risk factors.
Fig. 1.

Figure 1 Conceptual Model of the Health Risk Appraisal (HRA) Driven, Goal-Directed Care Approach (*) A comprehensive, web-based HRA is completed by the patient before each annual wellness visit as a “homework assignment” (from home, work, or via a wireless tablet in the practice) and a personalized -prioritized health report is generated to aid strategic care planning based on patient goals, needs, preferences, and constraints. (**) In this model, existing chronic health conditions and disease processes are considered risk factors for future adverse outcomes (e.g., myocardial infarction or stroke) that can result in decreased life expectancy and diminished quality of life. This holistic, patient goal-directed care approach encompasses the majority of primary care and integrates biomedical, behavioral, and psychosocial determinants of health into a unified health improvement strategy.
3. Methods
3.1 Development of the Integrated HRA Tool
In the context of a five-year, AHRQ-sponsored K-Award, our team has designed, implemented and pilot tested a novel, web-based HRA tool. The tool was embedded within a previously developed, comprehensive personal health record (PHR), the “My Wellness Portal” (https://mpsrs.us/WPortal) [20]. The development of the HRA tool followed a rigorous user-centered design process including extensive literature search, study of a dozen existing tools, interviews with seven national HRA developers and vendors, and input from key informants. This was followed by multiple development and testing cycles led by a patient advisory panel and in-depth semi-structured interviews (cognitive testing and “think-out-loud” sessions) with a convenience sample of HRA users representing a diverse patient panel. Finally, the HRA was beta-tested with 30 patients in two community primary care practices to refine the tool.
3.2 Architecture of the HRA Risk Engine
We incorporated existing risk prediction methods into an innovative approach that has not been implemented in primary care practices before. The HRA risk engine takes its baseline information from National Center for Health Statistics (NCHS) population life tables for the 15 leading causes of death and all others as a 16th cause, for all ages (0-110 years), both genders, and several races/ethnicities (Caucasian, African American, Hispanic/Latino, Native American, and Asian/Pacific Islander). Age, gender, and race-specific probability of death (qd) values are used in a standard life-table calculation procedure to provide a baseline estimated life expectancy (ELE). The baseline estimate is the life expectancy of an “average” person in the particular age, gender, and ethnicity group (national peer group).
To calculate an individualized ELE, baseline probabilities of death (qd values) are converted into cumulative hazard (λ) values assuming an exponential survival distribution for each year (∑ = - ln (1–Pdeath[t]). Lambda values are then distributed among the 15 leading causes of death (and all other causes as a sixteenth cause) according to the naturally occurring distribution ratios of deaths as indicated by NCHS life tables. As part of this project, a list of over 200 significant modifiable and unmodifiable risk factors encompassing 13 health domains that are connected to the main causes of death has been carefully identified by an expert physician panel in our academic center via evidence-review and consensus. The HRA tool asks specifically about these risk factors (►Table 1). Consequently, using relative risk (RR) values available from meta-analyses and large, representative epidemiological studies, the sixteen lambda values are adjusted according to individual risk factors and personal preferences gleaned from the HRA based on the Cox Proportional Hazards Model. [6] Finally, adjusted lambda values are summed for each year and corresponding probability of death values for each year of life are calculated. These personalized probabilities of death are then used in a second life-table calculation to arrive at a life expectancy estimate that is highly tailored to each person.
Table 1.
Health and wellness domains incorporated into the comprehensive Health Risk Appraisal (HRA) tool. The web-based risk engine dynamically constructs a personalized questionnaire at run-time with branching logic based on personal characteristics, past- and current entries, and available evidence on risk factors. The user can define the granularity and, in some cases, the specificity of data entry.
| HRA Domains | HRA Sub-Domains | Number of Items |
|---|---|---|
| Demographics | Age; Gender; Race/Ethnicity; Residence; Education; Marital Status; Employment; Income Level; Insurance | 10 |
| Vitals/Labs | Weight; Height; (Body-Mass Index); Waist Circumference; Lipid Panel; Blood Pressure; Glycosilated Hemoglobin (HbA1c) | 11 |
| Behavioral Health | Dietary Habits; Physical Activity; Tobacco Use; Alcohol Use; Drug Use | 22 |
| Environmental Health | Stress/Anxiety; Hazardous Materials; Outdoors Risks; Crime Rate; Violence/Abuse; Unemployment | 14 |
| Self Health History | 35 Leading Chronic Conditions and Their Varieties | 47 |
| Family Health History | 16 Leading Hereditary Risk Factors (1st and 2nd Degrees) | 16 |
| Healthcare Utilization | Outpatient Visits; Hospitalizations/Nursing Home Admissions; Emergency Department Visits; Major Surgeries | 4 |
| Allergies & Misc. Risks or Risk Modifiers | Immune Compromise; Vitamin Deficiency; Egg Allergy; G-globulin; Varicella; Guillain-Barre; Organ Removal | 10 |
| Personal Safety | Sun Protection; Car-Related Risks; Risky Sports; Fire/Smoke Alarm; Pool Safety; Motor Cycle; Firearms | 15 |
| Sexual Health | Menses; Sexual Activity; Pregnancy; Menopause; Birth Control; Hormon Replacement Therapy; Fertility; Sexually Trans. Disease | 16 |
| Mental Health | Depression/Anxiety; Mental/Cognitive Activity; Suicide; Trauma; (PHQ-9 test triggered by a positive PHQ-2 test) | 16 |
| Preventive Services | Preventive Services History Related To 15 Preventable Or Modifiable Health Conditions | 23 |
| Health-Related Quality of Life & Personal Goals | Self-Rated Overall Health; Self-Rated Quality of Life; Refusal Of or Concern With Care; Meaningful Life Activities | 11 |
An individualized list of preventive care recommendations is created for a particular person by a separate recommendation algorithm based on age, gender, and personal preferences, utilizing set theory and United States Preventive Services Task Force (USPSTF) guidelines [3, 19]. This list of recommendations is then prioritized based on the estimated impact on length of life. To predict the estimated impact of individual preventive interventions on life expectancy, a difference is calculated between the LE associated with the patient's current risk profile and the LE of a risk profile modified by the preventive maneuver. Repeating this procedure for each recommendation results in a list of estimated impacts on life expectancy for each personalized intervention. Items are then ranked according to their efficacy, but in the future, could also be ranked according to their cost, acceptability, or “effort effectiveness”, a measure that could be defined as an analogue of cost-effectiveness. In addition to LE estimates that are provided for clinicians, the HRA tool also delivers “patient-friendly” derivatives, including a Real Age and a Wellness Score [18] that are transformed from LE estimates. In our HRA tool, Real Age was a risk-matched virtual age estimate (risk of death from 16 causes) and the Wellness Score was an age-standardized outcome calculated from life years gained or lost compared to peers. The score ranged between 0 and 135, including the range around 100 that indicated “average” health. These parameters were represented both numerically and on a color-coded visual-analog scale (VAS) in the web-based HRA report which was presented to the patient for discussion with his/her primary care clinician.
3.3 Validation of the HRA
Predictions and discriminative performance of the HRA tool have been validated against observations from longitudinal studies and other risk prediction methods in three separate modeling experiments indicating a performance that was not inferior to the performance of 118 existing tools for predicting mortality (AUC-ROC = 0.70) [23].
3.4 Study Protocol
In order to conduct a pilot study, a convenience sample of four Oklahoma Physicians Resource/Research Network (OKPRN) primary care practices were carefully pair matched and assigned to control and intervention groups in a quasi-experimental design (see Figure 2), yielding 200 patients (50 per practice). We considered practice size (small, mid-size, or large), location (urban, suburban or rural) and type (private, academic, or public), in addition to patient mix and number of patients seen per week. We selected a pair of rural, private, small family practices and another pair of suburban, mid-size, clinician group practices. Primarily middle-aged and older adults, with multiple health conditions were invited to participate. The research assistant (RA) scheduled a dedicated, 45-60 minute visit with patients in these practices to obtain written informed consent, complete a baseline HRA and other study surveys. This was necessary to ensure that the impact of the HRA can be adequately measured. Upon need, the RA made a study packet available that contained instructions on participating remotely via the Internet. All patients (including control and intervention groups) completed the same web-based HRA and a corresponding survey. Patient outcomes were measured before and 12 months after the implementation through the HRA questionnaire, patient surveys, and qualitative feedback received via open-ended questions. Control patients completed the HRA questionnaire and a self-administered survey, but did not receive an HRA report. Intervention patients received detailed feedback from the HRA at the time of completing the questionnaire, including estimates of overall health, description of health strengths and challenges, and a prioritized list of tailored recommendations for preventive care with individual impact estimates. They were also encouraged to discuss the HRA report at their next annual wellness visit and follow their personalized wellness plan.
3.5 Patient-Centeredness of Care
We measured patient-centeredness of care as we described it in a previous study [20], using an adapted version of the Consumer Assessment of Health Care Providers and Systems (CAHPS) survey, separately from the HRA [10]. We calculated a composite score from selected CAHPS survey questions, summing scores 1 to 8, 10, and 11 [20]. The 10 questions describe patient-clinician interactions as they relate to preventive care. Pre- and post-intervention differences in patient-centeredness scores were calculated for each patient and the differences were compared between control and intervention groups.
3.6 Provision of Preventive Services
Self-reported HRA feedback was reviewed to determine the number and type of selected preventive services received and patient behavioral health patterns before and during the 12-month study period. We used preventive services reports to populate the HRA risk engine that generated personalized recommendations based on USPSTF [3], Advisory Committee on Immunization Practices (ACIP) [1], and American Academy of Family Physicians (AAFP) [2] guidelines.
3.7 Quantitative Analyses
We analyzed quantitative data with the help of SAS 9.1.3 software (SAS Institute, Cary, NC). We conducted difference-in-differences analyses [5] to determine the impact of the HRA on the patient-centeredness of care. These analyses compared the magnitude of the change in patient-centeredness scores between groups and time points. Logistic regression and generalized linear models were constructed using the backward stepwise approach to examine the relationships between various outcome variables and covariates gleaned from the HRA instrument. Covariates included age, gender, number of comorbidities and office visits, up-to-dateness on preventive services, self-rated health and quality of life, body-mass index, smoking status, and level of physical activity.
3.8 Qualitative Analyses
Open-ended patient feedback was collected via free text entries at the end of the patient survey. Responses were analyzed using standard content analytic techniques. Briefly, two reviewers studied a collection of patient responses and generated propositions for emerging themes. These then were compared between reviewers and argued to consensus to arrive at the essence of participant opinion for each question relating to the utility, usability, perceived impact and future potential of the HRA tool.
4. Results
Strategic pair matching of four OKPRN practices (two control and two intervention) yielded a reasonably homogeneous patient population (N = 200) across study groups. ► Table 2 describes the patient characteristics in both arms of the study. Apart from a moderately elevated frequency of office visits in the intervention group, patient characteristics in the two study arms did not differ significantly. About 77% of intervention patients reported that they considered the HRA report in the course of preventive care planning.
Table 2.
Patient characteristics at baseline by study arm (pair-matched control and intervention practices; N = 4)
| Patient Characteristics | Control (N = 98) |
Intervention (N = 102) |
Significance (P) |
|---|---|---|---|
| Mean age (years) | 59.9±10 a | 60.4±11 a | 0.36 (t Stat) |
| Ratio of female patients | 65% | 72% | 0.07 (Chi2) |
| Ratio of non-Caucasian minorities | 8% | 5% | 0.07 (Chi2) |
| Ratio of married patients | 73% | 67% | 0.20 (Chi2) |
| Ratio of patients with at least high school education | 94% | 95% | 0.52 (Chi2) |
| Household income less than $40K per year | 9% | 12% | 0.22 (Chi2) |
| Average number of chronic conditions per patient | 2.7 | 3.2 | 0.06 (Chi2) |
| Ratio of active smokers | 11% | 15% | 0.20 (Chi2) |
| Mean Body-Mass-Index (kg/m2) | 30.1±7 a | 30.8±6 a | 0.23 (t Stat) |
| Self-rated overall health (0 to 4 scale) | 2.76±0.8 a | 2.61±0.8 a | 0.12 (t Stat) |
| Self-rated satisfaction with life (1–10 scale) | 7.57±2.1 a | 7.48±1.9 a | 0.32 (t Stat) |
| Average number of office visits per patient per year | 3.4 | 4.95 | <0.001 (Chi2) |
(a) Given as Mean ± S.D.
Bivariate analyses suggested that estimated life expectancy (ELE), measured by the HRA and its derivatives, including Real Age and Wellness Score were significantly impacted by the HRA intervention. The mean increase in ELE across the intervention population was 8 months higher than expected simply by aging over a year (13 vs. 5 months; P<0.001) and also significantly higher compared to the ELE measured in the control group (13 vs. 7 months; P<0.001). Correspondingly, while the mean increase in Real Age was 7 months in the control group (P = 0.03), it did not change significantly in the intervention group during the study period (P = 0.20), suggesting a decreasing estimated risk of death from multiple causes. Wellness Score, a third health indicator showed a modest, but significant mean increase from 67.6 to 69.9 in the intervention group (P = 0.03) compared to control where there was no significant change (74.34 and 74.65 respectively; P = 0.29).
Patient-reported recommended preventive maneuvers included receiving smoking counseling, mammography, Papanicolaou test, prostate-specific antigen test, colon cancer screening, aspirin chemoprophylaxis in cardiovascular disease, HbA1c measurement for diabetics, healthy diet, physical activity, and seatbelt use. The overall rate of preventive services improved from 59.1% to 63.3% in the intervention group (P = 0.001) compared to control (55.1% and 54.0% respectively; P = 0.40).
A difference-in-differences analysis that accounted for time trends suggested that the HRA modestly improved the patient-centeredness of care, measured by the CAHPS PCC-10 ambulatory survey on a 10-point scale. There was no significant difference between the two groups’ scores at baseline (7.54 and 7.63 respectively; P = 0.49), while the pre-post score difference between the intervention and control groups was 0.81 points (+0.28 and -0.53 respectively; P = 0.05).
Logistic regression models indicated that HRA use was strongly associated with better self-rated overall health (OR = 4.94; 95% CI, 3.85-6.36) and improved up-to-dateness for preventive services (OR = 1.22; 95% CI, 1.12-1.32). Modeling the relationship of treatment to patient-centeredness of care revealed that increase in the CAHPS PCC-10 score was more likely in the intervention group (OR = 1.21; 95% CI, 1.12-1.30).
We applied an exploratory generalized linear model to examine the relationship of Wellness Score to covariates in the intervention group. Increase in patient Wellness Score showed significant association with improvements in patient-centeredness of care, up-to-dateness for preventive services and being in the intervention group, while it suggested an inverse relationship to the number of patient visits, comorbidities, BMI, and smoking (all P<0.03).
Open-ended feedback indicated that patients were satisfied with their HRA-experience and found the HRA report relevant and motivating. Patients expressed that the HRA helped them “focus on maintaining healthy choices” and provided a “roadmap to getting well”. They emphasized that the HRA report improved their awareness of medical issues they had as well as future issues that may arise (e.g., “[the HRA] makes me look at what I am doing to my body“). Both patients and clinicians underscored that the HRA helped them focus and converge on high-impact, evidence-based preventive measures (e.g., “[the HRA] reminds me to go to the doctor more often and stick to what he tells me to do” and “it shows me what areas to focus on improving or minimizing to make my life healthier”). When asked about ways to improve the HRA tool, patients responded that more granular data entry options and open-ended feedback capability would help them perceive that their personal record was accurate. They also suggested that reminding them to prepare for the completion of the HRA by collecting their records (e.g., blood pressure readings, labs, etc) in advance, could facilitate the assessment process.
5. Discussion
The findings of the HRA pilot study suggest that a patient goal-directed and integrated health risk assessment approach can improve intermediate health outcomes and patient perceptions of the quality of care in primary care settings. Our study may give renewed impetus for further research that can determine the impact of health risk appraisal technology on the quality of care and long-term clinical outcomes, such as morbidity and mortality. New lessons learned about HRAs in the past decade need to be translated into practice-based implementations that can correspond to the challenges of patient-centered and outcomes-oriented care.
As part of the HRA development process, we conducted a systematic review of the literature and evaluation of existing tools available in the United States (unpublished results). We found that the majority of current HRAs do not provide metrics and feedback that are conducive to strategic and longitudinal planning for personalized, goal-directed care. This finding compelled us to develop a novel HRA tool that may facilitate a paradigm-shift from problem-focused medicine to viewing health and health care through the lens of patients which may be best captured by establishing care upon patient goals and priorities.
Our study suggests that in order to achieve holistic, person-centered care, a more suitable set of health indicators must be developed and tested that can gauge overall health, and more importantly, can help track changes in health as individual personal goals are accomplished (e.g., quitting smoking, eating 5 servings of vegetables and fruits a day, buckling the seatbelt, decreasing the level of stress or anxiety, or increasing the number of hours in sleep). These indicators should be patient-friendly and their change must be tightly linked to behavioral and psychosocial determinants of health based on the best available evidence. The challenge of the current clinical approach to longitudinal health is that no metrics are used systematically to project or measure the impact of behavioral health improvements, resulting in a decreased enthusiasm for recommending or following through lifestyle changes. We found that over 90% of our computerized care recommendations that ranked in the top three based on their estimated health impact, included behavioral interventions. Given the enormous burden of behavioral health problems, a more accurate, technology-assisted projection and continuous measurement of the impact of behavioral interventions is an imperative.
The mean Wellness Score in both groups was below the national population “average” (90-100). We believe that this may be due to some self-selection bias (less healthy persons may tend to be seen and/or sign up for an HRA in the context of primary care) and that Oklahoma ranks in the bottom quintile in most health indicators nationally.
Both patients and clinicians emphasized that a variety of attributes, risk factors, and preferences should be collected before the patient visit via electronic means as part of the patient’s “homework assignment” and automated clinical decision support should be applied using evidence-based guidelines, in order to facilitate the patient-clinician discussion. They agreed that an updated HRA report should be reviewed annually at each wellness visit (e.g., the recently instituted Medicare Annual Wellness Visit) that can function as a starting point for developing a personalized and prioritized wellness plan. Taking time to systematically prepare for comprehensive office visits is rarely recommended to patients. Based on lessons learned from our study, we propose that empowering patients with comprehensive HRA technology may significantly facilitate person-centered care and help substantiate the central principle of the Chronic Care Model: “informed, activated patients should meet a prepared, proactive practice team” [25]. In this pilot study, we did not find a significant change in the patient activation measure (PAM-13) [11] in either group, although trends were observed. More research is needed to elucidate the relationship between patient activation and patient-centeredness of care or uptake of preventive services in the context of HRA use.
Most patients found the HRA interesting, useful, and a stimulating tool. The majority of comments was positive and articulated the value of the patient-centered, goal-directed design, including activating and informing patients in new ways, triggering insights into one’s health, the impact of lifestyle choices, and personalized health improvement. The HRA approach seemed to resonate with patients and clinicians and it facilitated congruency between patient and clinician goals around evidence-based guidelines. This may be the most notable and desirable immediate result of the goal-directed HRA approach, which could be translated into the improvement of long-term health and wellness. Interestingly, several patients requested HRA features that would allow them to document their health status and risk factors in a more granular fashion, even via free-text entries (e.g., personal “progress notes”). We concluded that the patient’s perception of accuracy and completeness of personal records may have an intrinsic value pertaining to the overall impact of the HRA approach on health outcomes.
This study strengthens our previous finding [20] that primary care practices need substantial assistance to implement e-patient technologies (e.g., HRAs) and care delivery (“clinical pathways”) linked to these technologies to ensure a consistent and systematic response to clinical needs that arise from a wellness visit. Establishment of an efficient “prevention on rails” office system often requires external help (e.g., practice facilitation), incorporation of new resources (e.g., supportive health IT), workflow redesign, and the development of new capacity or skills in order to improve the coordination of care that requires the participation of the entire community. A patient-centered, technology-driven HRA approach may have the potential to catalyze practice transformation and help change the current, suboptimal paradigm of health and health care delivery.
5.1 Limitations
Our pilot study had insufficient power to draw unequivocal conclusions pertaining to the impact of the HRA intervention on patient outcomes. A more robust, randomized trial is needed to solidify these findings. The exploratory approach also did not allow us to corroborate patient-reported outcomes by medical record abstractions. Using the same instruments for pre-and post-implementation measurements may have introduced a response bias. In addition, although the overall completion rate for study instruments was high (97% for surveys with electronic validation and 92% for the HRA questionnaire), the possibility of skipping responses or choosing not to respond to certain HRA questions (e.g., sexual and psychosocial risk factors) that we have permitted for ethical reasons, may have resulted in lower power for specific scales. Since it was not methodologically possible to impute personal risk factors, we omitted 13 records from the pilot study analyses. In the future, some of these variables could be collected automatically from other clinical data repositories, if available (e.g., the EHR). Since we ensured the completion of the HRA and other surveys at baseline and at the end of the study through RA-assisted, 45-60 minute encounters with patients (“efficacy” trial), the current study could not provide sufficient information about the effectiveness of the implementation. A future clinical trial will yield more information about the practice-based implementation of the HRA and how the tool may impact care delivery in a more realistic setting. Other limitations included access to a sample that tended to be more Caucasian, educated, and female, a known confounder in un-stratified investigations of technology use in primary care.
6. Conclusions
Our study suggests that a comprehensive, web-based HRA tool that operates in a goal-directed framework may improve the receipt of preventive services, patient-centeredness of care, and overall health indicators in primary care settings. The study also suggests that patient-friendly derivatives of highly individualized estimated life expectancy (e.g., Wellness Score) could potentially function as comparative health metrics that may help longitudinal tracking of changes in overall health. These metrics are feasible to obtain at the point of care via a pre-visit HRA. They also allow reasonable extrapolations to long-term health outcomes and thus they may facilitate prioritization, strategic care planning and systematic health improvement.
Clinical Relevance
The Patient Protection and Affordable Care Act of 2010 mandates that Medicare pay for annual wellness visits (AWV) that include required health risk appraisals (HRAs) with no co-payment. However, no published studies have examined the clinical effectiveness of HRAs in primary care, although strategic implementation and systematic follow-up could be achieved in a longitudinal patient-clinician relationship. Therefore, there is considerable potential for a new generation of enhanced HRAs that combine successful technologies with novel implementation approaches.
Human Subject Research Approval
The study has been approved and monitored by the University of Oklahoma Health Sciences Center Institutional Review Board (IRB# 15294).
Conflict of Interest Statement
The authors report no conflicts of interest in the research.
Fig. 2.
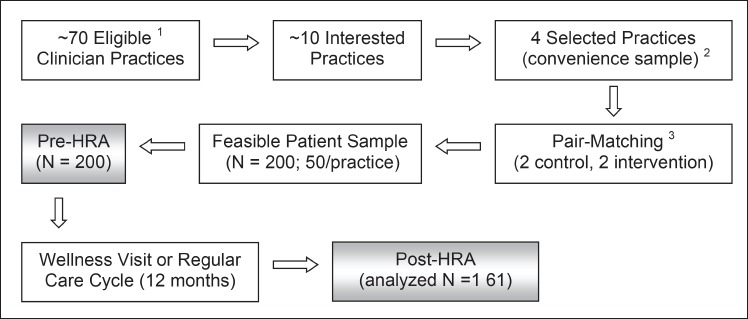
Participant flow diagram of the health risk appraisal pilot study(1) The inclusion criteria for practices were: practice is stable, no concurrent implementation of another major intervention, willingness and capacity to adopt the HRA tool, and clinician sees at least 30 middle-aged and older adults per week. (2) Convenience sample was chosen to maximize the chance of successful implementation and testing based on practice characteristics and available study resources. (3) A quasi-experimental, controlled design was followed, including pair-matching of control and intervention sites based on practice and patient care characteristics.
Acknowledgements
The authors appreciate the enthusiasm and generous contributions of Oklahoma Physicians Resource/Research Network (OKPRN) clinician practices and their patients, who participated in this study. The authors also thank the Agency for Healthcare Research and Quality (AHRQ) that supported the investigation (Grant 1K08HS016470-01A2).
References
- 1.Advisory Committee on Immunization Practices (ACIP) Preventive Services Recommendations. http://www.cdc.gov/vaccines/acip/recs/index.html (December 15).
- 2.American Academy of Family Physicians Clinical Guidelines Clinical Policies and Guidelines. http://www.aafp.org/online/en/home/clinical/clinicalrecs/guidelines.html (December 15).
- 3.United States Preventive Services Task Force (USPSTF) Preventive Services Recommendations. http://www.uspreventiveservicestaskforce.org/ (December 14).
- 4.Beery WL, Greenwald H P. A modified health risk appraisal as a component of a senior health promotion program. HMO Pract 1996; 2: 91-94 [PubMed] [Google Scholar]
- 5.Card D, Krueger AB. Minimum Wages and Employment: A Case Study of the Fast-Food Industry in New Jersey and Pennsylvania. American Economic Review 1994; 4: 774–775 [Google Scholar]
- 6.Cox DR. Regression Models and Life Tables. Journal of the Royal Statistical Society Series B 1972: 187–220 [Google Scholar]
- 7.Cyrus Baghelai MS, Valerie S., Nelkin M.A., Ted R. Miller.Ph.D. (Econometrica, Inc) Health Risk Appraisals in Primary Care: Current Knowledge and Potential Applications To Improve Preventive Services and Chronic Care. Rockville MD: AHRQ Center for Primary Care, Prevention, and Clinical Partnerships; 2010 [Google Scholar]
- 8.Fuji KT, Galt KA, Serocca AB. Personal health record use by patients as perceived by ambulatory care physicians in Nebraska and South Dakota: a cross-sectional study. Perspect Health Inf Manag 2008: 15. [PMC free article] [PubMed] [Google Scholar]
- 9.Halpin HA, McMenamin SB, Schmittdiel J, Gillies RR, Shortell SM, Rundall T, Casalino L. The routine use of health risk appraisals: results from a national study of physician organizations. Am J Health Promot 2005; 1: 34-38 [DOI] [PubMed] [Google Scholar]
- 10.Hays RD, Shaul JA, Williams VS, Lubalin JS, Harris-Kojetin LD, Sweeny SF, Cleary PD. Psychometric properties of the CAHPS 1.0 survey measures. Consumer Assessment of Health Plans Study. Med Care 1999; 3Suppl: MS22-31 [DOI] [PubMed] [Google Scholar]
- 11.Hibbard JH, Stockard J, Mahoney ER, Tusler M. Development of the Patient Activation Measure (PAM): conceptualizing and measuring activation in patients and consumers. Health Serv Res 2004; 4Pt 1: 1005-26 doi: 10.1111/j.1475-6773.2004.00269.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hudson LR, Pope JE. The role of health-risk appraisals in disease management. Manag Care Interface 2006; 2: 43-5 [PubMed] [Google Scholar]
- 13.Kessler R, Glasgow RE. A proposal to speed translation of healthcare research into practice: dramatic change is needed. Am J Prev Med 2011; 6: 637-44 doi: S0749-3797(11)00162-0 [pii] 10.1016/j.amepre.2011.02.023 [DOI] [PubMed] [Google Scholar]
- 14.Kittler AF, Carlson GL, Harris C, Lippincott M, Pizziferri L, Volk LA, Jagannath Y, Wald JS, Bates D W. Primary care physician attitudes towards using a secure web-based portal designed to facilitate electronic communication with patients. Inform Prim Care 2004; 3: 129-138 [DOI] [PubMed] [Google Scholar]
- 15.Krist AH, Peele E, Woolf SH, Rothemich SF, Loomis JF, Longo DR, Kuzel AJ. Designing a patient-centered personal health record to promote preventive care. BMC Med Inform Decis Mak 2011: 73 doi: 1472-6947-11-73 [pii] 10.1186/1472-6947-11-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mold JW, Blake GH, Becker LA. Goal-oriented medical care. Fam Med 1991; 1: 46-51 [PubMed] [Google Scholar]
- 17.Mold JW, Hamm R, Scheid D. Evidence-based medicine meets goal-directed health care. Fam Med 2003; 5: 360-364 [PubMed] [Google Scholar]
- 18.Morgan RF, Wilson J. Growing Younger. How to Measure and Change Your Body’s Age: Morgan Foundation September, 2000 [Google Scholar]
- 19.Nagykaldi Z, Mold J W. The role of health information technology in the translation of research into practice: an Oklahoma Physicians Resource/Research Network (OKPRN) study. J Am Board Fam Med 2007; 2: 188-95 doi: 10.3122/jabfm.2007.02.060084 [DOI] [PubMed] [Google Scholar]
- 20.Nagykaldi Z, Aspy CB, Chou A, Mold J W. Impact of a Wellness Portal on the delivery of patient-centered preventive care. J Am Board Fam Med 2012; 2: 158-67 doi: 10.3122/jabfm.2012.02.110130 [DOI] [PubMed] [Google Scholar]
- 21.Rubenstein LSP, Tucker J, Maglione M, Morton S, Roth E, Chao B, Rhodes S, Wu S, Newberry S. Health Risk Appraisals and Medicare. Baltimore: US Department of Health and Human Services, Health Care Financing Administration; 2003 [Google Scholar]
- 22.Silvestre AL, Sue VM, Allen JY. If you build it, will they come? The Kaiser Permanente model of online health care. Health Aff (Millwood) 2009; 2: 334-44 doi: 28/2/334 [pii] 10.1377/hlthaff.28.2.334 [DOI] [PubMed] [Google Scholar]
- 23.Siontis GC, Tzoulaki I, Ioannidis J P. Predicting death: an empirical evaluation of predictive tools for mortality. Arch Intern Med 2011; 19: 1721-6 doi: archinternmed.2011.334 [pii] 10.1001/archinternmed.2011.334 [DOI] [PubMed] [Google Scholar]
- 24.Sobel DS. Rethinking medicine: improving health outcomes with cost-effective psychosocial interventions. Psychosom Med 1995; 3: 234-244 [DOI] [PubMed] [Google Scholar]
- 25.Wagner EH, Austin BT, Davis C, Hindmarsh M, Schaefer J, Bonomi A. Improving chronic illness care: translating evidence into action. Health Aff (Millwood) 2001; 6: 64-78 [DOI] [PubMed] [Google Scholar]


