Abstract
Background
Medication reconciliation is an essential, but resource-intensive process without a “gold standard” to measure medication adherence. Medication reconciliation applications that focus on facilitating clinicians’ decision-making are needed. Since no single available source of medication information is adequate, combining data sources may improve usefulness and outcomes.
Objectives
We aimed to design a medication reconciliation application that could incorporate multiple data sources and convey information about patients’ adherence to prescribed medications. We discuss design decisions integral to developing medication reconciliation applications for the electronic health record. The discussion is relevant for health IT developers, clinical providers, administrators, policy makers, and patients. Three hypotheses drove our design of this application: 1) Medication information comes from a variety of sources, each having benefits and limitations; 2) improvements in patient safety can result from reducing the cognitive burden and time required to identify medication changes; 3) a well-designed user interface can facilitate clinicians’ understanding and clinical decision making.
Methods
Relying on evidence about interface design and medication reconciliation, an application for the electronic health record at an academic medical center in the U.S. was designed. Multiple decisions that considered the availability, value, and display of the medication data are explored: Information from different sources; interval changes in medications; the sorting of information; and the user interface.
Results
The prototype medication reconciliation application design reflects the visual organization, categorization, modality of interactions, and presentation of medication information from three data sources: patient, electronic health record, and pharmacy.
Conclusions
A new medication reconciliation user interface displays information from multiple sources, indicates discrepancies among sources, displays information about adherence, and sorts the medication list in a useful display for clinical decision making. Gathering, verifying, and updating medication data are resource-intensive processes. The outcomes of integrating, interpreting, and presenting medication information from multiple sources remain to be studied.
Keywords: Medication reconciliation, decision making, computerized medical records systems, medication errors/prevention & control, user-computer interface
1. Introduction
Medication errors and preventable adverse drug events between medications documented at home, during office visits, in pharmacies, and during hospitalizations may occur because of medication list discrepancies [2, 9, 11, 12, 15, 34, 43]. Elimination of discrepancies to improve patient safety is the goal of medication reconciliation. Medication reconciliation, as described by The Joint Commission, has five core steps: Gathering and verifying current medication information; prescribing medications; resolving discrepancies; making clinical decisions; and communicating a finalized list to the patient or caregiver [50]. Completion of these steps should lead to creation of a single, most accurate, list of medications, but completing these steps is complex. To assist health care providers in completing these steps, and to improve the accuracy of the reconciled medication list, the design of a system for medication reconciliation needs to address both functional requirements and usability [21, 33].
In their 2010 guidelines about meaningful use, the U.S. Centers for Medicare & Medicaid Services stated that medication reconciliation is “the process of identifying the most accurate list of all medications that the patient is taking, including name, dosage, frequency, and route, by comparing the medical record to an external list of medications obtained from a patient, hospital, or other provider” (8]. Successful medication reconciliation, however, has been hindered by the need to collect and integrate information from multiple, paper-based or electronic, sources [9, 22]. The passage of the U.S. Health Information Technology for Economic and Clinical Health (HITECH) [1] Act could spur more widespread electronic health record (EHR) system adoption [7, 14, 17]. Many early adopters of EHR systems have successfully completed medication reconciliation electronically [10, 19, 39-41, 49]. The focus of attention must now shift to higher-order taxonomic goals: Instead of simply gathering and classifying lists of information, providers should perform the tasks of analysis, synthesis, and evaluation [3, 6].
To move toward these higher-order goals, improved approaches are first needed to aggregate and organize medication lists from multiple sources [20]. In the past, isolation of data sources limited medication data aggregation (e.g. a pharmacy claims database being unable exchange data with an EHR). Now, through the integration of data from multiple sources, such as health information exchanges and regional pharmacy claims databases, aggregating medication data is possible and necessary for successful medication reconciliation. Effective models of data integration are needed. To date, successful integration of medication list information from multiple sources has been reported by only two health care systems: The Veterans Health Administration [24] and Partners HealthCare [30, 36, 45]. Although studies have shown the benefit of presenting the clinician with medication data from multiple sources, we are unaware of any systems that facilitate reconciliation from multiple sources by providing clinicians with organized, computation-based interpretations of data. We describe a new approach that integrates the patient’s report, medication orders, pharmacy dispensing, and insurance claims data in an easily interpreted medication list display. This study offers a novel discussion of design decisions central to the development of electronic medication reconciliation applications.
2. Background
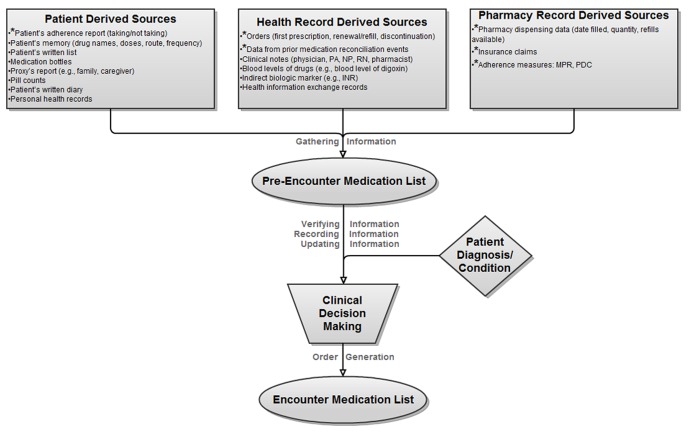
Poon et al. described the inpatient admission and discharge medication reconciliation process, which consists of six steps: Gathering medication information from available sources; verifying the medication information; recording verified medication information; updating the list with new information; making clinical decisions to continue, adjust, or discontinue each medication; and generating appropriate orders [30]. ► Figure 1, adapted from Poon et al. [30], depicts the flow of this process, modified to categorize sources of medication information and cover both the inpatient and outpatient settings. The first four steps gathering, verifying, recording, and updating information can be done with relatively minimal clinical training; in contrast, making clinical decisions and generating prescriptions requires more extensive training. The steps of gathering, verifying, and recording information from health information systems may be assisted by computer-based automation.
Fig. 1.
Flowchart of the medication reconciliation process in the inpatient and outpatient settings (Adapted from Poon et al. [27]). The accurate pre-encounter medication list is compiled based on reconciliation of data from multiple sources. This allows a new encounter medication list to be generated. *Data sources incorporated into our prototype; INR = International normalized ratio; PA = Physician Assistant; NP = Nurse Practitioner; RN = Registered Nurse; MPR = Medication Possession Ratio [30, 31]; PDC = Proportion of Days Covered [30, 31]
2.1 Prior designs of medication reconciliation applications
Published discussion of the design of medication reconciliation applications is sparse. Researchers at Partners HealthCare have published studies of designs that target the clinician [30, 35-38, 45]. The Pre-Admission Medication List (PAML) builder was designed to facilitate medication reconciliation through support of inpatient history taking [30, 36, 45]. Medication information source (outpatient ambulatory setting or inpatient discharge list) is displayed in multiple columns. The authors suggested that this might prompt clinicians to seek additional information when they notice discrepancies. They also discussed the task of merging duplicate medications using the generic name [30]. Subsequent evaluation found an absolute risk reduction of 0.72 for the PAML’s ability to reduce potential adverse drug events due to discrepancies [36].
Similarly focused on clinician-centered design, the Post Discharge Medication Reconciliation Tool targeted reconciliation between the PAML and the hospital discharge medication list [37]. Clinicians in the ambulatory setting were presented with two columns corresponding to the lists to reconcile. They could then reconcile lists by choosing between three actions: Add, modify, or verify. Taking action on every medication in the list was not required. This facilitated subspecialists’ desire to be only partially responsible for medication reconciliation.
In an additional application from Partners HealthCare, the Patient Gateway Medication Module, responsibility is shared between clinician and patient [35, 38]. This application consists of two parts: A patient module, as part of a personal health record; and a clinician application, as part of the EHR. Patients are presented with the active medication list from the EHR for verification and explanation of discrepancies. Information collected from the patients can then be compared with the medication list contained within the EHR by their physicians for reconciliation.
In three other examples, the responsibility for entry of electronic medication reconciliation data falls to someone other than the physician. In one, the use of an ambulatory kiosk directed responsibility to the patient [24]. In the other two, responsibility fell to the nurse or pharmacist to enter and update the electronic medication list [19, 31]. Therefore, these studies also fall outside the scope of our approach of facilitating clinician-centered decision making.
2.2 Barriers to medication reconciliation
The clinician’s task of manually reconciling available medication data is difficult; this is true even independent of barriers in medication data acquisition. The first barrier derives from differences in medication information formatting. Patients’ adherence is frequently collected via oral report; clinical notes commonly exist in paper-based or electronic format. Even when these disparate sources can be found in an EHR, differences in formatting (e.g., tables vs. narrative text) increase the clinician’s cognitive burden. Inpatient studies measuring providers’ time to reconcile medications indicate that this is a lengthy process: 9 to 30 minutes at hospital admission [5, 9, 18, 43, 47]; 60 to 90 minutes on transfer; and 45 to 60 minutes at discharge [18]. Reductions in time required have already been demonstrated, even on paper, through automation and systemization [18, 31, 32].
A second barrier, relying on the patient as the sole source of medication adherence information, can also present problems due to “recall bias, overestimation of adherence, and elicitation of socially acceptable responses” [27]. Furthermore, Trindade et al. showed that clinicians’ accurate identification of problems with medication adherence was only 33% [44]. Clinicians are not limited to the patient as a source, however, since they may have access to multiple additional sources: Patient, health record, and pharmacy (► Figure 1). Each source introduces the chance for discrepancies. As an example, Vogelsmeier et al. showed that clinicians make assumptions about medication-list accuracy and patients’ adherence based upon electronic documentation that may itself be inaccurate[48]. Furthermore, since none of these sources consistently reflects true medication adherence, clinicians lack an adherence “gold standard”.
Next, consider the challenges of extracting adherence information from a clinician’s note. A note often contains medication list information but is frequently stored as narrative text, rather than structured data. Thus, gathering adherence information from notes would require either extensive human and financial resources for manual data entry, or an effective natural language processor; neither of which is easy to implement [16, 46, 51]. Even with such solutions, incomplete documentation and inaccuracy would remain as barriers.
The fourth barrier relates to structured orders from a computerized provider order entry system. Many EHR systems’ methods of reviewing orders and clinical notes are cumbersome; they do not provide “intelligent” means of identifying order relationships. For example, a system may not link the current dose of a medication when it was prescribed by one clinician and increased by another provider at a later time. Further complicating the picture, patients often see providers from multiple health care systems that may not be linked by a health information exchange.
A final barrier arises from difficulties in obtaining medication data from multiple pharmacies or insurance claims databases. To overcome this, one would have to aggregate data from sources that have been historically isolated. Recently, this burden has been reduced with increased availability of pharmacy dispensing data; one such source is SureScripts [42]. Yet, even aggregated pharmacy dispensing databases have limitations that preclude their acceptance as a gold standard source for active medications. First, data from these aggregated sources can be expensive. Second, medications such as over-the-counter medications may still be omitted (though this is true less often as medication data processing systems become more advanced). Finally, 16% of new prescriptions are not filled [13]. Pharmacy data, therefore, do not perfectly correspond to active orders.
To address these barriers, we redesigned an approach to medication reconciliation. Improvements derive from the incorporation of multiple medication information sources as well as a design focused on usefulness and usability. Therefore, while important for medication reconciliation overall, no further discussion is given to steps that occur before final prescribing. This includes the various means by which one could gather, verify, or record medication information. Similarly, the necessary tasks of consolidating similar or duplicative lists of medications are left outside the scope of this discussion. Instead, our focus is upon improved aggregation, interpretation, and ultimately presentation of these varied sources of information. While many users of various roles (e.g., nurses, pharmacists, and clinicians) will use this medication reconciliation application, our focus was directed toward the clinician in an outpatient setting. Below are detailed descriptions of design decisions in the user interface that may improve usability for clinicians and promote accurate, efficient medication reconciliation.
3. Methods
3.1 Study Setting
In this study, we targeted clinicians who practice at a tax-supported health institution in a large Midwestern academic medical center. This institution has used a locally developed comprehensive EHR system [25] for over three decades in both the inpatient and outpatient settings. The Indiana Network for Patient Care [26], a regional health information exchange, stores medication data that include records from computerized provider order entry and pharmacy information systems. The network includes clinical data from 90 hospitals, public health departments, local laboratories, imaging centers, and a few large-group practices affiliated with hospital systems. The health information exchange’s data repository contains more than four billion clinical observations, including over 79 million text reports for approximately 12 million patients.
3.2 Design Decisions
While touching all five steps of medication reconciliation depicted in ► Figure 1, the single driving focus of our prototype design was to facilitate clinicians’ understanding and decision-making. This led to design paradigm decisions that considered data availability, value, and display.
Our first design consideration related to medication information coming from diverse sources. Although each source is important, simultaneous display of information from all sources is impractical. Instead, we organized information into three groups: Patient derived sources; health record derived sources; and pharmacy derived sources (► Figure 1). From each category, we included the most readily available sources (items with “*”). Incorporation of additional sources will come in future development.
Our second design consideration involved the ease of identifying medication list changes. Without discrepancies between medication sources, reconciliation is straightforward. We therefore focused on more complex, yet common, scenarios identifying five categories requiring providers’ special attention (►Table 1).
Table 1.
Categories of changes to medications that require special attention
|
Our third design consideration was medication sorting and display. Common convention sorts medications by name, alphabetically. Alphabetization, however, helps only to identify an individual drug rapidly; even for this purpose, users’ expectations may not be met. As most medications have both brand and generic names, they may or may not begin with different letters; simply finding a drug in an alphabetized list may be harder than expected. Therefore, alphabetical ordering was kept merely as a sorting option, rather than a requirement. The alternative design, sorting by adherence, was predicted to facilitate clinicians’ reconciliation and decisions.
Our final design consideration regarded the user interface for medication information display. Our model focused on four principles of heuristic design: Match between system and the real world; consistency and standards; flexibility and efficiency of use; and aesthetic and minimalist design [28]. Common Internet functionality was incorporated: Use of a virtual shopping cart; and a layout reminiscent of an e-mail application. Taking action on medications in the list in several different ways was supported, to provide flexibility. For example, a user could click on an individual medication to change. Alternatively, the user could select multiple medications to change them as a group. Finally, elements for display were chosen for simplicity and aesthetics.
3.3 Prototype Design
Two of the authors (JC and KS) separately developed alternative, novel medication reconciliation prototypes. To gain diverse feedback, the prototypes were shown to clinicians, information-technology professionals, pharmacists, and nurses. Each prototype then underwent iterative revisions to incorporate their feedback. This feedback included suggestions about wording of adherence categories, consideration of various role-specific views of the application based on typical clinical responsibilities, and distinguishing patient-derived from pharmacy-derived information via separate columns while associating the EHR-derived information with the medication name. A final prototype was then created by incorporating the best features of each initial approach.
3.4 Prototype Definitions
3.4.1 Low Adherence
For most medications, we used an adherence threshold of less than 80% to label a medication as “Low Adherence” [23, 29]. This would be helpful for certain classes of medications such as contraceptives and anti-retroviral medications that require a much higher adherence threshold to be effective [29]. Our selected threshold is not absolute; medication threshold customization is planned as a future development.
3.4.2 Adherence Percentage Calculation
To calculate an adherence percentage, we chose to use the proportion of days covered instead of the medication possession ratio, the advantage being incorporation of “credit” for refills dispensed before the current supply is expected to be depleted [4, 23].
4. Results
4.1 Visual Organization of Medications
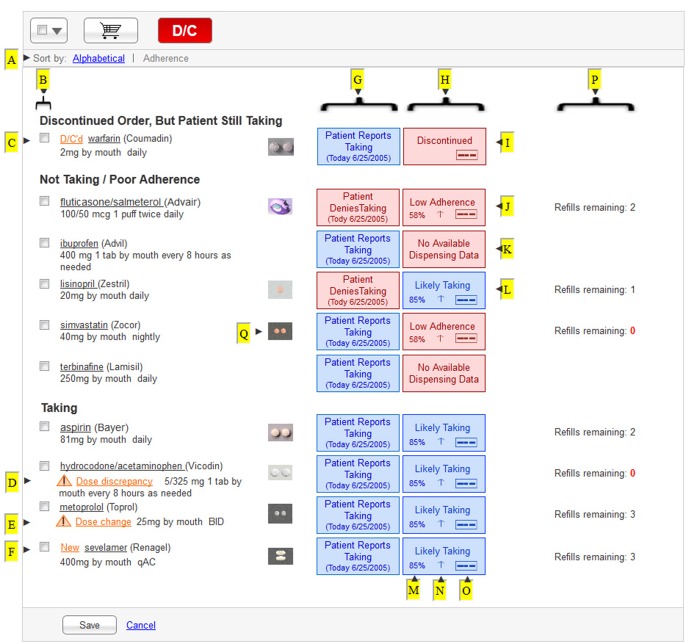
Our prototype is shown in ► Figure 2. We designed the prototype to allow the user to toggle between two alternative views (►Figure 2-A). The first would sort the medication list alphabetically by generic drug name. The second would sort the medication list by three adherence criteria: “Discontinued Order, But Patient Still Taking”; ”Not Taking or Low Adherence”; and “Taking”. Within each adherence group, the medications are sorted alphabetically by generic drug name.
Fig. 2.
Medication List Manager prototype. This display shows medications sorted by computed adherence. Letters with arrows (►) were added to correspond to descriptions provided in ►Table 2 as well as the text.
4.2 Medication Adherence Category Displays
The medication list manager organizes medications into three sections, as follows.
4.4.1 “Discontinued Order, But Patient Still Taking”
The first section in ► Figure 2 is intended to help clinicians identify cases where the patient is still taking a medication despite direction from a clinician to discontinue that medication. For a medication to appear in this section, two conditions must apply: A discontinue order must be the most recent order pertaining to that medication; and the patient reports taking the medication after the order to discontinue the medication was entered. Problems with communication or following instructions could result in this potentially dangerous situation. This might occur upon hospital discharge, for example, if the patient is left without guidance from the discharge medication list specifying which medications should be discontinued.
4.4.2 “Not Taking or Low Adherence”
The second section in ► Figure 2 is intended to present medication data for three types of situations: Patient reports not taking the medication; pharmacy dispensing or insurance claims data indicate low adherence regardless of patient report; or medication dispensing data is unavailable which may occur if the patient never fills a given prescription. While the third scenario might result from incomplete data rather than low adherence, we decided to take a practical and minimalist approach for our adherence categories; this will likely maximize the sensitivity of finding an adherence issue to be verified by the clinician. Therefore, rather than creating separate categories for each possible scenario, we grouped several scenarios into this category. In summary, this category was intended to encompass all medications that were unlikely to be having their intended clinical effect due to low adherence.
4.4.3 “Taking”
The third section in ► Figure 2 lists all ongoing, unchanged medications as well as new medications and changed dosing, as long as three criteria are met: A prescription authorizing this medication is active; adherence exceeds a pre-defined threshold above which the medication could be expected to have its intended clinical effect; and the patient reports adherence to the medication.
4.3 Modality of Interaction with Medication Information
Our prototype uses the concept of a virtual shopping cart to perform actions such as discontinuing a medication or making changes to the dosing instructions. Items chosen by selecting the box in ► Figure 2-B can then be placed into the cart by clicking on the shopping cart button for later review. This same modality gives the user the ability to discontinue multiple selected medications simultaneously. To add medications to the list, users would click on a button not depicted in ► Figure 2.
4.4 Visualization of Health Record Derived Medication Information
To provide alerts to the user about issues directly related to the medication or dosing instructions, we developed four unique flags: D/C’d (discontinued; ► Figure 2-C); Dose Discrepancy (► Figure 2-D); Dose Change (► Figure 2-E); and New (► Figure 2-F). Compared to the user’s previous encounter with the patient, these flags would appear if another provider discontinued a medication (D/C’d), changed the dosing instructions for a medication (Dose Change), or initiated a medication (New). The Dose Discrepancy flag would appear if the patient reported taking a dose that differed from the most recent order for that medication.
4.5 Visualization of Patient Derived Medication Information
In the column denoted by ► Figure 2-G, text boxes display the patient’s report of adherence as “Patient Reports Taking” or “Patient Reports Not Taking”. Blue and red color schemes give additional distinction respectively. To provide the user with information about the age of the data, the date upon which this patient’s adherence report was collected is also displayed.
4.6 Visualization of Pharmacy Record Derived Medication Information
The column denoted by ► Figure 2-H was designed to provide the user with pharmacy-based or insurance claims-based data about medications. The options here are “Discontinued” (► Figure 2-I), “Low Adherence” (► Figure 2-J), “No Available Dispensing Data” (► Figure 2-K), and “Likely Taking” (► Figure 2-L). The wording was chosen to convey the best overall estimate of adherence.
One option, “Low Adherence”, indicates failing to meet the threshold sufficient to consider a patient adherent with a given medication as detailed above. More detailed information is displayed through the calculated percentage for adherence (► Figure 2-M), indicators of an increase or decrease in adherence (► Figure 2-N), and an icon that upon hover-over displays an estimated adherence graph for that medication (► Figure 2-O & ► Figure 3). Calculation of adherence, based on pharmacy derived sources, will be done by the EHR system.
Fig. 3.
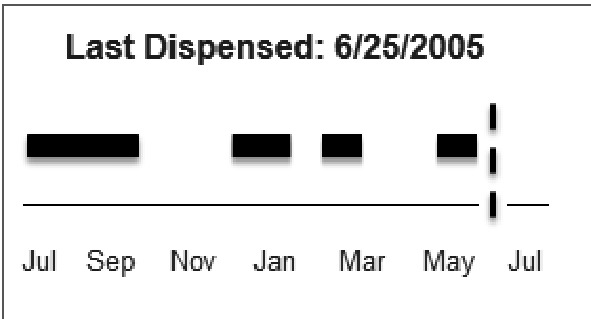
Medication adherence graph. Depiction of a patient’s adherence based on pharmacy dispensing data demonstrating gaps in the available supply. This graph appears when the clinician hovers over the icon depicted in ► Figure 2-0.
Another option, “Likely Taking”, is exclusive of the other three, yet the wording was chosen to convey that the EHR system cannot completely identify adherence. This would appear when the pharmacy-derived or insurance claims-derived data indicated adherence of at least 80%. As with “low adherence”, additional details would be available on demand.
Three commonly encountered scenarios influenced the wording for “No Available Dispensing Data”. First, medication data from pharmacy dispensing records and insurance claims will occasionally be missing or inaccurate. Second, some patients pay for their own medications (“out of pocket”), thus limiting our ability to ascertain dispensing data completely. Finally, medications that are available without a prescription (i.e., over the counter) are inconsistently captured by electronic data systems. Without access to pharmacy data or insurance claims data, there is no additional detailed data to display on hover-over.
The final pharmacy/insurance claims data descriptor, “Discontinued”, would be displayed if the most recent order for that medication had directed the patient to discontinue that medication. We do not provide the additional information of adherence or indicator of change, but we do provide the icon for the user to review a graph of recent adherence (► Figure 2-O and ► Figure 3).
4.7 Visualization of Other Available Data
Although it could also be described as pharmacy data, the number of available refills is displayed in its own column denoted by ► Figure 2-P, distinct from the column denoted by ► Figure 2-H. This decision was made to give visual distinction between data related to past adherence and data that would be more valuable to the provider in the steps of clinical decision-making and generation of orders depicted in ► Figure 1.
In addition, to keep the user interface simple, we decided to make detailed medication information available upon demand. Clicking on the name of a medication would bring up a dialog box that enables adjustment to the dosing instructions for that medication and display of the name of the prescribing clinician. Hovering over a pill icon (► Figure 2-Q) would reveal a larger picture of the medication that was actually dispensed to the patient. A click on the “patient report” box, in the column denoted by ► Figure 2-G, would open a dialog box displaying reports about adherence. Finally, clicking on the “adherence” box would open a dialog box with additional pharmacy dispensing information, such as the name of the prescribing clinician, the name of the pharmacy where the prescription was filled, and the dates of dispensing.
5. Discussion
Based on principles of design and others’ experience, we designed a new medication reconciliation user interface that displays information from multiple sources, indicates discrepancies among sources, and sorts the list in a useful display for clinical decision making. This approach incorporates and extends the work of previously published medication reconciliation applications in several ways. Although the PAML displays discrepant information, the clinician must determine the nature of the discrepancies without further assistance [30, 36, 45]. Our enhancements of computing, interpreting, organizing, and displaying information about adherence might improve outcomes further. Through more direct presentation of discrepant medication information, we anticipate improved facilitation of discussions with patients about any medication adherence barriers. Similar to the Post Discharge Medication Reconciliation Tool, our approach is facilitative of subspecialists’ workflow as it does not require action on every medication [37].
We hypothesize that addressing the user interface design considerations that we described above will facilitate clinicians’ understanding and decisions during medication reconciliation. Minimizing adverse drug events likely cannot be accomplished by merely integrating disparate sources of information; medication reconciliation systems should assist clinicians in the interpretation of information displayed. Such systems should assist clinicians in answering five fundamental questions of medication reconciliation: What medication information is available? What are the sources of this information? What has changed since the last encounter? What is our estimate of the patient’s adherence? What should be done now? A system’s facilitative and interpretive qualities will allow clinicians to focus on interacting with patients about adherence and other issues, rather than just documenting a list of medications.
Medication information must be gathered from multiple diverse sources. No source is universally reliable; each has benefits and limitations. Integration of multiple sources of medication information can improve a clinician’s interpretation. For example, a patient may report taking a medication while pharmacy records demonstrate large gaps in dispensing. With such knowledge, a physician might explore the cause of missing data, or a patient’s barriers to improved adherence, rather than deciding to change the dose or start additional medications. Keeping the minimalist heuristic in mind, our model facilitates rapid comparison of information with clear indication of its source; this is accomplished through arrangement of information into unique columns ( Figure 2-G & 2-H). Involvement of patients in the documentation and verification of their medical histories is ultimately desirable for efficiency, accuracy, reinforcement, identification of problems with adherence, and educational purposes. Our current model does not target patients in this way but could be adapted to do so through future modifications.
Because health care is increasingly shared among multiple clinicians, staying abreast of medication changes becomes more difficult for each provider. Through the steps of verifying, recording, and updating medication information, the types of changes to medications that are listed in ►Table 1 can be elucidated. To aid the clinician’s rapid recognition of such changes, four indicators (► Figure 2-C, 2-D, 2-E, & 2-F) were designed. With this design, clinicians can scan the medication list for the presence of any flags. Once identified, each flag provides a clear indication of the type of changes made since the clinician’s last encounter with that patient. We hypothesize that reducing the cognitive burden of identifying medication changes will facilitate the higher order taxonomic goals of analysis, synthesis, and evaluation; as a result patient safety will improve.
The design of a medication reconciliation application should help to identify the medications that require the most attention. When both the patient and the other sources of medication information indicate high adherence, relatively little attention to adherence is needed. This does not, however, remove the need for clinical decision-making about the ongoing appropriateness of each medication. In contrast, discrepancies in adherence among multiple sources of information should warrant more attention. Ordering of presentation is one such way to emphasize priority; we placed the most important information at the top. We hypothesize that categorizing and prioritizing medications will decrease the time required for providers to identify potential medication discrepancies. One limitation to this approach is that patients’ stated reasons for medication non-adherence are not captured and displayed to assist reconciliation further.
The design described herein includes available sources of medication information already present at the study institution (► Figure 1 items with “*”). These same medication information sources may not be readily incorporated at other institutions. Furthermore, institutions without direct control over their EHR design and development may not be able to incorporate the design elements discussed herein, but EHR vendors and designers could use the results to improve their applications. Flexibility for expansion to accommodate additional sources of information was considered during the design process. Therefore, regardless of medication source availability, the design would still have several key features of importance to many institutions (► Table 3).
Table 3.
Key design features of the prototype medication reconciliation application
| Multiple sources of information presented simultaneously | |
|
|
| Indication of changes to the medication lists | |
|
|
| Automatic calculation of adherence using Proportion of Days Covered | |
| Sorting of medications based on adherence groupings | |
6. Conclusions
In summary, we designed this medication reconciliation application with the intent to incorporate it into our locally developed EHR system. The design decisions discussed in this study, however, are broadly applicable. While approaches in design may vary among medication reconciliation applications, any system that seeks to optimize clinical decision making will need to address the same issues discussed here. Despite being limited in the scope of medication information data included, this application seeks to integrate more sources than discussed in other published literature. We chose to include the medication information most readily available and easiest to incorporate at our institution (items with “*” in ► Figure 1). The next step is usability testing of the prototype. We have designed specific performance tasks and user experience surveys that will be used to refine the design.
7. Clinical Relevance Statement
Medication reconciliation is an essential but resource-intensive process without a gold standard to measure medication adherence. Minimization of discrepancies to improve patient safety is the goal of medication reconciliation. This study reviews the design of a new medication reconciliation user interface that displays information from multiple sources, indicates discrepancies among sources, displays computed adherence, and sorts the list in a useful display for clinical decision making.
Human Subjects Protections
The study was conducted in accordance with ethical principles for medical research. No human or animal subjects were included in the study. The study was approved by the Institutional Review Board of Indiana University.
Conflict of Interest Statement
All authors declare that they have no relevant conflict of interest.
Table 2.
Descriptive key for lettered labels in Figure 2
| A | Toggle between alphabetic and adherence views |
| B | Checkbox to facilitate action(s) on selected item(s) |
| C | Discontinued medication flag |
| D | Dose discrepancy flag |
| E | Dose change flag |
| F | New medication flag |
| G | Column of patient report of adherence |
| H | Column of pharmacy data on adherence |
| I | Pharmacy indication medication is discontinued |
| J | Pharmacy indication patient has low adherence with medication due to <80% dispense history |
| K | Pharmacy indication there is no available dispensing data |
| L | Pharmacy indication patient is likely taking medication due to ≥80% dispense history |
| M | Proportion of days covered adherence percentage based on dispense history |
| N | Indicator of trend in adherence; increase, decrease, or stable |
| O | Thumbnail graph of medication adherence based on pharmacy dispense history |
| P | Column indicating refills remaining |
| Q | Thumbnail image of medication |
Acknowledgements
This project was supported in the U.S. by Grant Number T15LM007117 from the National Library of Medicine and by grant number R18HS018183 from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Library of Medicine, the National Institutes of Health, the Agency for Healthcare Research and Quality, or the Department of Veterans Affairs. Dr. Weiner is Chief of Health Services Research and Development at the Richard L. Roudebush Veterans Affairs Medical Center in Indianapolis, Indiana.
References
- 1.American Recovery and Reinvestment Act of 2009. United States Government Printing Office. 2009 [Google Scholar]
- 2.Akwagyriam I, Goodyer L, Harding L, Khakoo S, Millington H.Drug history taking and the identification of drug related problems in an accident and emergency department. J Accid Emerg Med 1996; 3: 166-168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson LW, Sosniak LA.Bloom's Taxonomy: National Society for the Study of 1994 [Google Scholar]
- 4.Andrade SE, Kahler KH, Frech F, Chan KA.Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidem Dr S 2006; 8: 565-574 [DOI] [PubMed] [Google Scholar]
- 5.Beckett RD, Crank C W, Wehmeyer A.Effectiveness and Feasibility of Pharmacist-Led Admission Medication Reconciliation for Geriatric Patients. J Pharm Pract 2011; 2: 136-141 [DOI] [PubMed] [Google Scholar]
- 6.Bloom BS, Engelhart M, Furst EJ, Hill WH, Krathwohl DR.Taxonomy of educational objectives: Handbook I: Cognitive domain. New York: David McKay; 1956: 56 [Google Scholar]
- 7.Blumenthal D.Stimulating the adoption of health information technology. New Engl J Med 2009; 15: 1477-1479 [DOI] [PubMed] [Google Scholar]
- 8.Centers for Medicare & Medicaid Services Eligible Hospital and Critical Access Hospital Meaningful Use Menu Set Measures - Measure 6. November 7, 2010. http://www.cms.gov/EHRIncentivePrograms/Downloads/6_Medication_Reconciliation.pdf(March 8, 2012)
- 9.Cornish PL, Knowles SR, Marchesano R, Tam V, Shadowitz S, Juurlink DN, Etchells EE.Unintended medication discrepancies at the time of hospital admission. Arch Intern Med 2005; 4: 424 [DOI] [PubMed] [Google Scholar]
- 10.Crosswhite R, Beckham SH, Gray P, Hawkins PR, Hughes J.Using a multidisciplinary automated discharge summary process to improve information management across the system. Am J Manag Care 1997; 3: 473-479 [PubMed] [Google Scholar]
- 11.Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW.The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med 2003; 3: 161-167 [DOI] [PubMed] [Google Scholar]
- 12.Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW.Adverse drug events occurring following hospital discharge. J Gen Intern Med 2005; 4: 317-323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gadkari AS, McHorney CA.Medication nonfulfillment rates and reasons: narrative systematic review. Curr Med Res Opin 2010; 3: 683-705 [DOI] [PubMed] [Google Scholar]
- 14.Gans D, Kralewski J, Hammons T, Dowd B.Medical groups’ adoption of electronic health records and information systems. Health Affair 2005; 5: 1323-1333 [DOI] [PubMed] [Google Scholar]
- 15.Gleason KM, Groszek JM, Sullivan C, Rooney D, Barnard C, Noskin GA.Reconciliation of discrepancies in medication histories and admission orders of newly hospitalized patients. Am J Health Syst Pharm 2004; 16: 1689-1694 [DOI] [PubMed] [Google Scholar]
- 16.Jagannathan V, Mullett CJ, Arbogast JG, Halbritter KA, Yellapragada D, Regulapati S, Bandaru P.Assessment of commercial NLP engines for medication information extraction from dictated clinical notes. Int J Med Inform 2009; 4: 284-291 [DOI] [PubMed] [Google Scholar]
- 17.Jha AK, Des Roches CM, Campbell EG, Donelan K, Rao SR, Ferris TG, Shields A, Rosenbaum S, Blumenthal D.Use of electronic health records in US hospitals. New Engl J Med 2009; 16: 1628-38 [DOI] [PubMed] [Google Scholar]
- 18.Ketchum K, Grass CA, Padwojski A.Medication reconciliation: verifying medication orders and clarifying discrepancies should be standard practice. Am J Nurs 2005; 11: 78 [DOI] [PubMed] [Google Scholar]
- 19.Kramer JS, Hopkins PJ, Rosendale JC, Garrelts JC, Hale LDS, Nester TM, Cochran P, Eidem LA, Haneke RD.Implementation of an electronic system for medication reconciliation. Am J Health Syst Pharm 2007; 4: 404-422 [DOI] [PubMed] [Google Scholar]
- 20.Kushniruk A, Santos S, Pourakis G, Nebeker J, Boockvar K.Cognitive analysis of a medication reconciliation tool: applying laboratory and naturalistic approaches to system evaluation. St Heal T 2011: 203. [PubMed] [Google Scholar]
- 21.Kushniruk AW, Patel VL.Cognitive and usability engineering methods for the evaluation of clinical information systems. J Biomed Inform 2004; 1: 56 [DOI] [PubMed] [Google Scholar]
- 22.Lau HS, Florax C, Porsius AJ, De Boer A.The completeness of medication histories in hospital medical records of patients admitted to general internal medicine wards. Brit J Clin Pharmaco 2000; 6: 597-603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leslie S, Gwadry-Sridhar F, Thiebaud P, Patel B.Calculating medication compliance, adherence and persistence in administrative pharmacy claims databases. Pharmaceutical Programming 2008; 1: 13-19 [Google Scholar]
- 24.Lesselroth BJ, Felder RS, Adams SM, Cauthers PD, Dorr DA, Wong GJ, Douglas DM.Design and implementation of a medication reconciliation kiosk: the Automated Patient History Intake Device (APHID). J Am Med Inform Assn 2009; 3: 300-304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McDonald CJ, Overhage JM, Tierney WM, Dexter PR, Martin DK, Suico JG, Zafar A, Schadow G, Blevins L, Glazener T.The Regenstrief medical record system: a quarter century experience. Int J Med Inform 1999; 3: 225-253 [DOI] [PubMed] [Google Scholar]
- 26.McDonald CJ, Overhage JM, Barnes M, Schadow G, Blevins L, Dexter PR, Mamlin B.The Indiana network for patient care: a working local health information infrastructure. Health Affairs 2005; 5: 1214-20 [DOI] [PubMed] [Google Scholar]
- 27.Morisky DE, Ang A, Krousel Wood M, Ward HJ.Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens 2008; 5: 348-354 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Nielsen J.Ten Usability Heuristics. http://www.useit.com/papers/heuristic/heuristic_list.html(March 8, 2012)
- 29.Osterberg L, Blaschke T.Adherence to medication. New Engl J Med 2005; 5: 487-497 [DOI] [PubMed] [Google Scholar]
- 30.Poon EG, Blumenfeld B, Hamann C, Turchin A, Graydon-Baker E, McCarthy PC, Poikonen J, Mar P.Design and implementation of an application and associated services to support interdisciplinary medication reconciliation efforts at an integrated healthcare delivery network. J Am Med Inform Assn 2006; 6: 581-592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pronovost P, Hobson D, Earsing K, Lins E, Rinke M, Emery K, Berenholtz S, Lipsett P, Dorman T. A.practical tool to reduce medication errors during patient transfer from an intensive care unit. J Crit Care 2004; 1: 26-35 [Google Scholar]
- 32.Rozich J, Resar R.Medication safety: one organization's approach to the challenge. J Clin Outcomes Manage 2001; 10: 27-34 [Google Scholar]
- 33.Saleem JJ, Russ AL, Sanderson P, Johnson TR, Zhang J, Sittig DF.Current challenges and opportunities for better integration of human factors research with development of clinical information systems. Yearb Med Inform 2009: 48-58 [PubMed] [Google Scholar]
- 34.Schnipper JL, Kirwin JL, Cotugno MC, Wahlstrom SA, Brown BA, Tarvin E, Kachalia A, Horng M, Roy CL, McKean SC.Role of pharmacist counseling in preventing adverse drug events after hospitalization. Arch Intern Med 2006; 5: 565 [DOI] [PubMed] [Google Scholar]
- 35.Schnipper JL, Gandhi TK, Wald JS, Grant RW, Poon EG, Volk LA, Businger A, Siteman E, Buckel L, Middleton B.Design and implementation of a web-based patient portal linked to an electronic health record designed to improve medication safety: the Patient Gateway medications module. Inform Prim Care 2008; 2: 147-155 [DOI] [PubMed] [Google Scholar]
- 36.Schnipper JL, Hamann C, Ndumele CD, Liang CL, Carty MG, Karson AS, Bhan I, Coley CM, Poon E, Turchin A.Effect of an electronic medication reconciliation application and process redesign on potential adverse drug events: a cluster-randomized trial. Arch Intern Med 2009; 8: 771 [DOI] [PubMed] [Google Scholar]
- 37.Schnipper JL, Liang CL, Hamann C, Karson AS, Palchuk MB, McCarthy PC, Sherlock M, Turchin A, Bates D W.Development of a tool within the electronic medical record to facilitate medication reconciliation after hospital discharge. J Am Med Inform Assn 2011; 3: 309-313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schnipper JL, Gandhi TK, Wald JS, Grant RW, Poon EG, Volk LA, Businger A, Williams DH, Siteman E, Buckel L.Effects of an online personal health record on medication accuracy and safety: a cluster-randomized trial. J Am Med Inform Assn 2012; 5: 728-734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simonaitis L, Belsito A, Overhage JM.Enhancing an ePrescribing system by adding medication histories and formularies: the Regenstrief Medication Hub. AMIA Annual Symposium 2008. [PMC free article] [PubMed] [Google Scholar]
- 40.Simonaitis L, Dixon BE, Belsito A, Miller T, Overhage JM.Building a Production-Ready Infrastructure to Enhance Medication Management: Early Lessons from the Nationwide Health Information Network. AMIA Annual Symposium 2009. [PMC free article] [PubMed] [Google Scholar]
- 41.Simonaitis L, Belsito A, Cravens G, Shen C, Overhage JM.Continuity of Care Document (CCD) Enables Delivery of Medication Histories to the Primary Care Clinician. AMIA Annual Symposium 2010. [PMC free article] [PubMed] [Google Scholar]
- 42.SureScripts.com. http://www.surescripts.com/ (July 20, 2012).
- 43.Tam VC, Knowles SR, Cornish PL, Fine N, Marchesano R, Etchells EE.Frequency, type and clinical importance of medication history errors at admission to hospital: a systematic review. Can Med Assoc J 2005; 5: 510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trindade AJ, Ehrlich A, Kornbluth A, Ullman TA.Are your patients taking their medicine? Validation of a new adherence scale in patients with inflammatory bowel disease and comparison with physician perception of adherence. Falk Symp 2011; 2: 599-604 [DOI] [PubMed] [Google Scholar]
- 45.Turchin A, Hamann C, Schnipper JL, Graydon-Baker E, Millar SG, McCarthy PC, Coley CM, Gandhi TK, Broverman CA.Evaluation of an inpatient computerized medication reconciliation system. J Am Med Inform Assn 2008; 4: 449-452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uzuner Ö, Solti I, Cadag E.Extracting medication information from clinical text. J Am Med Inform Assn 2010; 5: 514-518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vira T, Colquhoun M, Etchells E.Reconcilable differences: correcting medication errors at hospital admission and discharge. Qual Saf Health Care 2006; 2: 122-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vogelsmeier A, Pepper GA, Oderda L, Weir C.Medication reconciliation: A qualitative analysis of clinicians' perceptions. Research in Social and Administrative Pharmacy 2012. [DOI] [PubMed] [Google Scholar]
- 49.Weeks G, Stanley L, Vinson MC.Automation of the medication history process: a case report. Hosp Pharm 2005; 12: 1057-1061 [Google Scholar]
- 50.Wolzt M, Samama MM, Kapiotis S, Ogata K, Mendell J, Kunitada S.Effect of edoxaban on markers of coagulation in venous and shed blood compared with fondaparinux. Thrombosis and haemostasis 2011; 6: 1080-90 doi: 10.1160/TH10-11-0705. [DOI] [PubMed] [Google Scholar]
- 51.Xu H, Stenner SP, Doan S, Johnson KB, Waitman LR, Denny JC.MedEx: a medication information extraction system for clinical narratives. J Am Med Inform Assn 2010; 1: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]