Abstract
Background:
To quantify the impact of bone metastasis and skeletal-related events (SREs) on mortality among older patients with lung cancer.
Materials and Methods:
Using the linked Surveillance, Epidemiology and End Results-Medicare database, we identified patients aged 65 years or older diagnosed with lung cancer between July 1, 1999 and December 31, 2005 and followed them to determine deaths through December 31, 2006. We classified patients as having possible bone metastasis and SREs using discharge diagnoses from inpatient claims and diagnoses paired with procedure codes from outpatient claims. We used Cox regression to estimate mortality hazards ratios (HR) among patients with bone metastasis with or without SRE, compared to patients without bone metastasis.
Results:
Among 126,123 patients with lung cancer having a median follow-up of 0.6 years, 24,820 (19.8%) had bone metastasis either at lung cancer diagnosis (9,523, 7.6%) or during follow-up (15,297, 12.1%). SREs occurred in 12,665 (51%) patients with bone metastasis. The HR for death was 2.4 (95% CI = 2.4-2.5) both for patients with bone metastasis but no SRE and for patients with bone metastasis plus SRE, compared to patients without bone metastasis.
Conclusions:
Having a bone metastasis, as indicated by Medicare claims, was associated with mortality among patients with lung cancer. We found no difference in mortality between patients with bone metastasis complicated by SRE and patients with bone metastasis but without SRE.
KEY WORDS: Claims, distant stage, fractures, mortality, SEER
INTRODUCTION
Lung cancer is the third most common cancer diagnosed and the most common cause of cancer-related death among both men and women in the United States (US).[1] The American Cancer Society[2] estimates that in 2012, 226,160 patients (men, 116,470; women, 109,690) will be diagnosed with lung cancer and 160,340 (men, 87,750; women, 72,590) will die from the disease.[3]
Lung cancer is often diagnosed at an advanced stage of disease, as early-stage disease is often asymptomatic. The stage distribution of lung cancer cases diagnosed in 1999-2006 included in the Surveillance, Epidemiology and End Results (SEER) program were: distant metastasis at diagnosis, 56%; regional metastasis, 22%; localized, 15%; and unstaged, 8%.[1] Males and females had similar stage distributions. Stage of disease was a strong risk factor for survival, with advanced disease having much lower five-year survival compared to early disease. From 1999-2006, the SEER program reported that the five-year relative survival was 3.5% for distant stage at diagnosis, 24% for regional stage and 52.9% for localized stage.
Bone is one of the most frequent sites of metastasis in patients with lung cancer.[4] Bone metastases from lung cancer are primarily osteolytic.[5] These osteolytic lesions undermine the structural integrity of bone placing lung cancer patients at an increased risk for skeletal-related events (SREs) such as pathological fractures, spinal cord compression and severe pain requiring radiotherapy or surgery for bone lesions.[6–8] These skeletal complications result in impaired mobility and reduced quality of life adding substantially to the overall burden of disease in patients with lung cancer.[9]
Data are limited on the occurrence and outcomes of bone metastasis and SREs among elderly patients who develop lung cancer. A few studies have reported results pertaining to the occurrence of bone metastasis and of SREs among lung cancer patients with bone metastases,[8,10,11] but none have focused on older patients. We therefore undertook this study to quantify the impact of bone metastasis and SREs on mortality among older lung cancer patients by analyzing a combined population-based cancer registry and the Medicare claims database.
MATERIALS AND METHODS
Data source
We analyzed linked SEER-Medicare data for the time period 1999-2006.[12,13] The SEER program, supported by the National Cancer Institute (NCI), collects cancer incidence and survival data from population-based cancer registries from selected geographic areas. During the time period of the present study, the SEER program covered 14 to 26% of the US population from 9 to 13 geographic areas. The SEER data included information on patient demographics, date of cancer diagnosis, tumor stage and other characteristics at diagnosis and summary information on treatments received soon after diagnosis. The Medicare program, administered by the Centers for Medicare and Medicaid Services, covers 97% of US population ages ≥65 years.[14] Medicare data included demographic information on beneficiaries and claims data from hospitals and other institutional and non-institutional providers. The Medicare claims data included dates of service, diagnosis codes (International Classification of Diseases Ninth Revision (ICD-9)) and procedure codes (current procedural terminology (CPT).
Study population
We identified a cohort of patients aged 65 years or older and having a SEER record of a new diagnosis of lung cancer between July 1, 1999 and December 31, 2005 and used SEER and Medicare data to determine possible occurrence of bone metastasis, SREs and deaths occurring through December 31, 2006. We required each subject to have a baseline period of at least six months of full fee-for-service Medicare coverage before his/her lung cancer diagnosis date. The claims data from this baseline period provided information on the presence of comorbidities, and on the presence of possible bone metastasis and SREs at diagnosis (based on ICD-9 codes). We excluded patients who did not have both Medicare Part A and Part B coverage and those who were enrolled in a Medicare Advantage plan during the baseline period because we did not have complete Medicare claims data on the services they may have received. In all analyses, we censored subjects if they died or lost full fee-for-service coverage before the end of 2006 (e.g., if their coverage changed such that we did not have access to all of their medical claims).
Bone metastasis and SREs
Bone metastasis, with or without evidence of SREs, was the time-dependent exposure variable of main interest in our analyses. Throughout this paper, we apply the term “bone metastasis” and “SREs” to patients who have claims-based evidence of these conditions. We used the following Medicare claims data as evidence of bone metastasis: a) at least one inpatient claim with an ICD-9 diagnosis code of 198.5 (“secondary malignant neoplasm of bone and bone marrow”) as the primary or secondary discharge diagnosis; b) at least one outpatient claim with a diagnosis code of 198.5, paired with a code for procedures used to diagnose or treat bone metastasis; or c) at least one outpatient physician evaluation and management claim with a diagnosis code of 198.5 (above algorithm available on request). We classified subjects as having concurrent bone metastasis at the time of diagnosis if they had a claim in the month of the lung cancer diagnosis or in the preceding month. Among subjects without concurrent bone metastasis at diagnosis, we identified new bone metastasis as the earliest occurrence of one of the above claims patterns at any time during follow-up.
For the purpose of this study, we defined SREs as one or more of the following conditions, occurring concurrently with or after the first bone metastasis: Fractures, radiotherapy to bone, surgery to bone and spinal cord compression. We identified SREs using combinations of diagnosis and procedure codes from inpatient and/or outpatient claims (codes available on request). We classified an SRE as concurrent with bone metastasis if it occurred within 30 days before or after the earliest bone metastasis date and as subsequent to bone metastasis if it occurred at any time more than 30 days after the bone metastasis date. We excluded from the analysis patients with claims suggesting bone metastases occurring more than 30 days prior to the lung cancer diagnosis (n = 570). The excluded patients comprising of 2% of overall patients with bone metastases had similar demographic characteristics as the bone metastases patients included in the analysis but were more likely to have unstaged and unspecified histology.
Mortality
The outcome of interest was mortality. We obtained information on date of death using the combined SEER registry and Medicare claims data. We used the concordant date of death in these two sources, if there was agreement between them. If they were discrepant, we used the SEER death date. If the SEER date of death was missing, we used the Medicare date of death.
Covariates
From the SEER data, we obtained information on age, gender, race/ethnicity and stage at cancer diagnosis. From the Medicare claims data, we obtained information on comorbidities. We computed each person's Charlson comorbidity score on the basis of ICD-9 diagnosis codes in his/her inpatient records for the 17 medical conditions [Table 1] comprising the Charlson index in the 12-month period prior to the month of cancer diagnosis. In computing the Charlson index, we used the approach described by Romano et al,[15] and updated by Quan et al.[16]
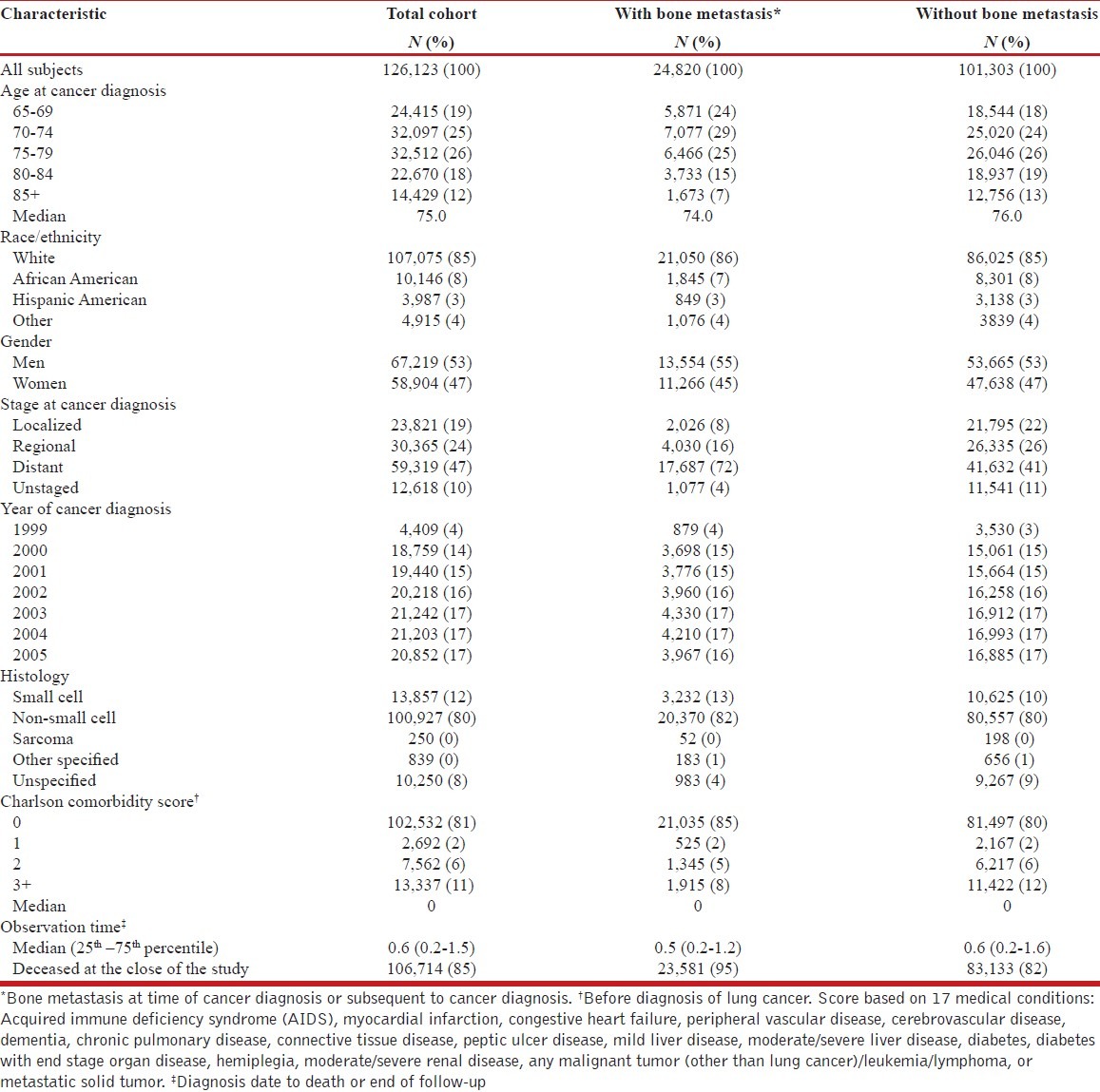
Table 1.
Characteristics of patients with lung cancer, total cohort and according to bone metastasis status: SEERMedicare, July 1, 1999-December 31, 2006
Statistical analysis
Descriptive analyses of the cohort included frequency distributions and median values, where applicable. We computed the proportion of patients with lung cancer having claims-based evidence of bone metastasis at one year from diagnosis for the overall cohort of lung cancer cases and for subgroups specified by tumor stage at diagnosis.[17,18] For these computations, we selected patients diagnosed with lung cancer from July 1, 1999 through December 31, 2005, so that these patients would have a potential observation period of at least one year post lung cancer diagnosis. Other analyses, described below, were unrestricted with regard to length of observation.
We used Cox regression to estimate mortality hazards ratios (HRs) and 95% confidence intervals (CIs) among patients with bone metastasis with or without SREs, compared to patients without bone metastasis, adjusting for covariates.[19] We also estimated mortality HRs for subgroups specified by initial tumor stage, age, gender and race/ethnicity at cancer diagnosis. Confounders were categorical forms of age at cancer diagnosis (65-69, 70-74, 75-79, 80-84, 85+), gender (male, female), race (white, African American, Hispanic American, other), stage at lung cancer diagnosis (distant, localized, regional, unstaged), and Charlson comorbidity score (0, 1, 2, 3+). Analyses were performed using SAS.[20]
This study was approved by the UAB Institutional Review Board and by the NCI SEER-Medicare Program.
RESULTS
The overall cohort of 126,123 patients were predominantly white (85%) with a median age at diagnosis of 75 years and a median follow-up of 0.6 years [Table 1]. About 41% of patients had distant stage of the disease at diagnosis. Eighty-one percent of patients had non-small cell lung cancer. During the study period, 24,820 (19.8%) patients had claims-based evidence of bone metastasis either concurrently with the diagnosis of lung cancer diagnosis (n = 9,523) or during follow-up (n = 15,297). The median time from cancer diagnosis to bone metastasis was 5.4 months among patients without a bone metastasis at diagnosis.
Bone metastasis at lung cancer diagnosis or during follow-up
Patients with, compared to those without, a bone metastasis were more likely to have distant stage disease (72% vs. 41%) and to have died by the end of the study period (95% vs. 82%) [Table 1]. The two groups (patients with and without bone metastasis) were similar with regard to age, race/ethnicity, gender, year of diagnosis, histology, comorbidity score and length of follow-up.
Figure 1 displays the proportion of patients with evidence of bone metastasis at one year post lung cancer diagnosis, according to stage at diagnosis, among the 126,123 patients (localized, n = 23,821; regional, n = 30,365; distant, n = 59,319; unstaged, n = 12,618) diagnosed with lung cancer from July 1, 1999 through December 31, 2005. At one year post diagnosis of lung cancer, the proportion with evidence of bone metastasis was 21% for all stages combined and was 38% for distant, 12% for regional, 11% for unstaged and 5% for localized stages at primary cancer diagnosis. When restricted to patients with non-small cell lung cancer, the proportion with evidence of bone metastasis at one year post diagnosis was 21% for all stages combined and was 39% for distant, 11% for regional, 13% for unstaged and 5% for localized stages.
Figure 1.
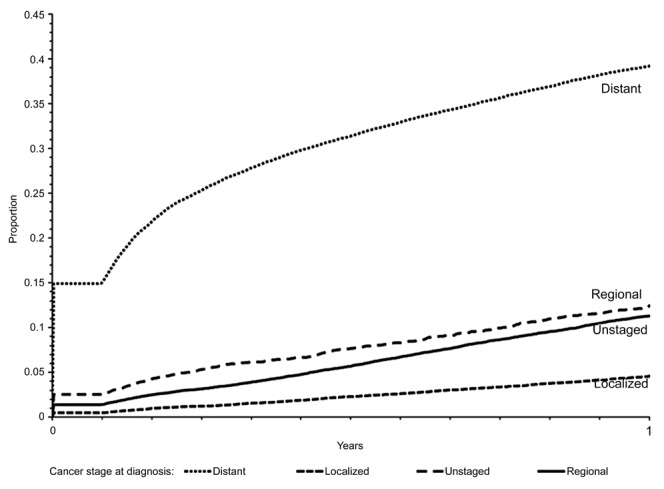
Proportion of 126,123 patients with lung cancer who had evidence of bone metastasis within 1 year by stage
SRE concurrent with or subsequent to bone metastasis
Of the 24,820 patients with a possible bone metastasis, 12,665 (51%) had evidence of a concurrent (n = 11,015, 44%) or subsequent (n = 1,650, 7%) SRE (data not displayed in a table). Among the 12,665 patients with an SRE, most (n = 10,598, 84%) presented with only one skeletal complication at the first diagnosis of an SRE. Of the 10,598 presenting with a single skeletal complication, 8,357 (79%) had radiotherapy to bone, 1,509 (14%) experienced a fracture, 645 (6%) had spinal cord compression and 87 (1%) had surgery to bone.
Of 12,665 patients with SRE, 2,067 (16%) had more than one skeletal complication at the first diagnosis of an SRE. Of these 2,067 patients, 969 (47%) had radiation to bone plus fracture, 617 (30%) had radiation to bone plus spinal cord compression, 183 (9%) had radiation to bone plus fracture plus spinal cord compression, 125 (6%) had fracture plus spinal cord compression; all other combinations accounted for <3% each. Overall, 81% of patients with SRE had radiotherapy to bone either diagnosed as the only SRE or in combination with other SREs.
Among the 2,481 patients who developed a fracture, regardless of whether it was an SRE by itself or in combination with other SREs, 1,625 (65%) had a fracture coded as pathological. The most common site of pathological fracture was the spine (n = 991), followed by hip (n = 290), femur (n = 185), humerus (n = 141), tibia/fibula (n = 14), and distal radius/ulna (n = 4).
Mortality in lung cancer patients with and without bone metastasis and SREs
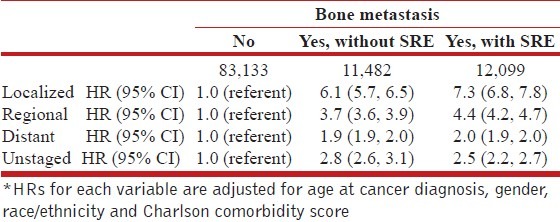
Table 2 displays HRs for death in relation to possible bone metastasis and SREs, adjusted for age at cancer diagnosis, race/ethnicity, gender, stage at cancer diagnosis and Charlson comorbidity score. The HR for risk of death was 2.4 (95% CI = 2.4-2.5), both for patients with bone metastasis but no SRE and for patients with bone metastasis plus SRE, compared to patients without bone metastasis. Stage-specific analyses indicated that HRs associated with bone metastasis complicated with SRE ranged from 2.0 (95% CI = 1.9-2.0) for distant stage disease at cancer diagnosis to 7.3 (95% CI = 6.8-7.8) for localized disease at diagnosis, and bone metastasis without SRE ranged from1.9 (95% CI = 1.9-2.0) for distant stage at diagnosis to 6.1 (95% CI = 5.7-6.5) for localized stage Table 3.
Table 2.
Hazard ratio (HR) for death in relation to bone metastasis and other factors among patients with lung cancer: SEER-Medicare, July 1, 1999-December 31, 2006
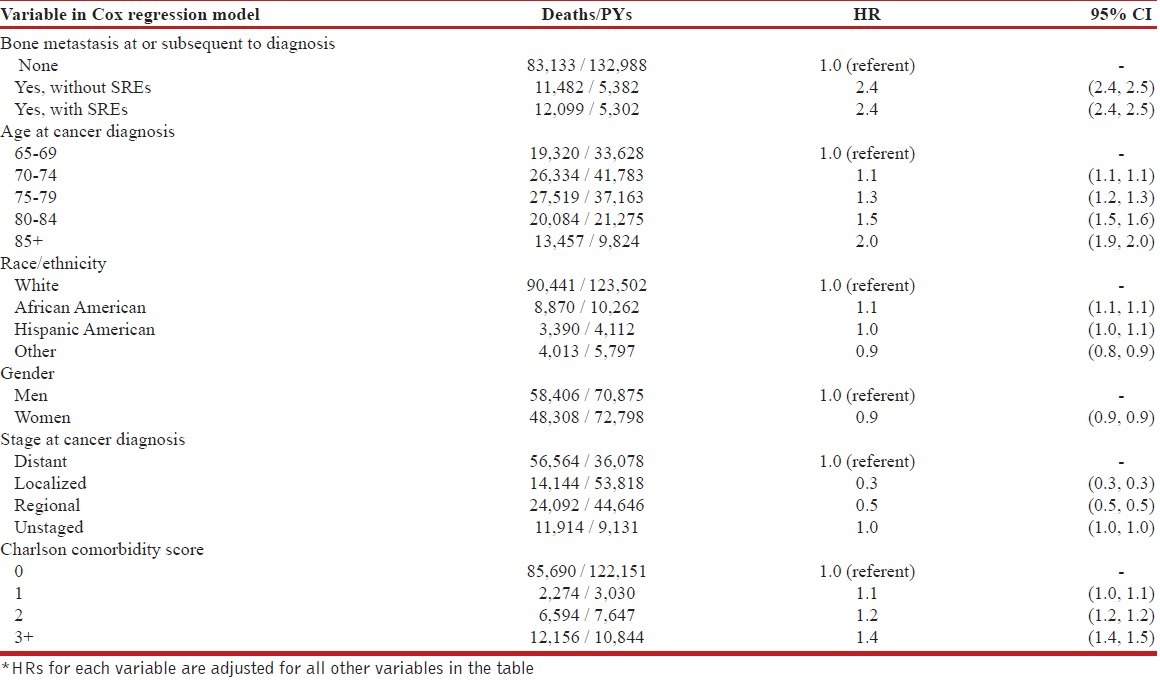
Table 3.
Adjusted hazard ratio (HR)* for death in relation to bone metastasis and skeletal-related factors (SREs) among men with lung cancer, by stage at diagnosis: SEER-Medicare, July 1, 1999-December 31, 2006
HRs for death in relation to bone metastasis in white patients were similar to those for African American patients (white: HR = 2.4, 95% CI = 2.4-2.5); African American, HR = 2.3, 95% CI = 2.2-2.4) (data not displayed in a table). HRs for death among men and women were also similar with regard to bone metastasis (men, HR = 2.4, 95% CI = 2.4-2.5; women, HR = 2.5, 95% CI = 2.4-2.5). We found a positive association between bone metastasis and with bone metastasis complicated with SRE and mortality in all categories of age groups (65-69, 70-74, 75-79, 80-84, 85+).
DISCUSSION
We found that about 19.8% of patients 65 years or older with newly diagnosed lung cancer included in the SEER program from July 1999 to December 2005 presented with or subsequently had evidence of a possible bone metastasis as indicated by Medicare claims. SREs were common, occurring in 51% of these patients. Among patients with a potential follow-up time of one year post lung cancer diagnosis, the proportion with bone metastasis was 21% overall and 38% for distant stage of lung cancer. Having a possible bone metastasis was associated with mortality among patients with lung cancer; the association was similar for bone metastasis complicated with an SRE and for bone metastasis without an SRE.
The skeleton is a frequent site of metastasis in patients with non-small-cell lung cancer which is the most common type of lung cancer.[21] About 85% of lung cancer cases included in the SEER program have a non-small cell histology.[1] In our data, 80% of patients had non-small cell lung cancer. Bone metastasis may be asymptomatic at onset and thus, are likely to be under-diagnosed resulting in inaccurate staging of lung cancer at diagnosis.[22] Studies report that the prevalence of bone metastasis among patients who report bone pain is almost similar to patients who did not report bone pain (27-32% vs. 16-27%).[23,24] Prior to 1991, several studies found skeletal metastases in non-small cell lung cancer to range from 8% to 34%.[25–31] With the advent of newer imaging techniques such as the positron emission tomography (PET) in the 1990s, bone metastasis has been noted to range from 24% to 30% for non-small cell lung cancer.[32,33] The preceding results are consistent with our finding that 21% of Medicare non-small cell lung cancer patients had possible bone metastasis within a year of lung cancer diagnosis.
Lung cancer has a high potential to metastasize. During 1999-2006, the SEER program reports that 56% of lung cancer patients were classified as having distant stage at the time of disease diagnosis.[1] In the present study, 41% of patients had distant disease at diagnosis. Bone metastases are common in patients with distant stage of lung cancer.[8] In the present study, we found that 38% of patients with distant stage lung cancer had possible bone metastasis within a year of their cancer diagnosis. This result is consistent with findings from other studies which have reported that 30-40% of lung cancer patients with distant metastatic disease had a bone metastasis at diagnosis or subsequently developed a bone metastasis.[6,8,34] In addition, autopsies of patients dying of lung cancer indicated that up to 30-40% had evidence of metastatic bone disease.[35]
In the present study, we found that 51% of patients with possible bone metastasis had one or more SREs. This result agrees with findings from a retrospective observational study using data from a health insurance claims database in the US which reported that SREs occurred in 55% of 534 lung cancer patients with bone metastasis.[10] Clinical trials of lung cancer have found that SREs occurred in 48% to 50% of patients with bone metastasis.[8,11] In our study, radiotherapy (81% of patients with SRE) to bone was the most common SRE followed by fracture, spinal cord compression and surgery to bone. Other investigators[10,11] noted similar findings. We observed that among patients with fractures, 65% (or 13% of patients with SRE) had a pathological fracture with the spine as the most common site of occurrence followed by pelvis and extremities. Other studies report that 7-10% of non-small lung cancer patients with SREs developed pathological fractures.[32,33,36]
Our results indicated that having a bone metastasis was associated with mortality among lung cancer patients. The lack of an additional effect of SRE on mortality among patients with bone metastasis may be due to the short survival time in lung cancer patients compared to other tumor types. Our companion studies of breast and prostate cancer, cancers with relatively longer survival time, found stronger associations for bone metastasis complication by SRE than for bone metastasis without SRE.[37,38] Our finding of a stronger association of bone metastasis and for bone metastasis complicated with SRE for patients with localized stage lung cancer than for those with distant metastatic lung cancer at diagnosis may reflect the low mortality among patients with early stage disease and no bone metastasis.
The strengths of the study include the use of the large population-based SEER cancer registry data with pathologically confirmed cancer and the focus on lung cancer in the elderly. The combined cancer registry and Medicare data provide a unique opportunity to evaluate the clinical progress of lung cancer. Other advantages of the SEER data include the continuity of data over time and the quality control measures instituted to ensure completeness of case ascertainment in the participating cancer registries.
The main limitations of our study stem from the characteristics of the SEER-Medicare data. Our study was limited to patients aged 65 years or older diagnosed with lung cancer between 1999 and 2005, who had Medicare Part A and B coverage and who resided in one of the SEER geographic areas. Medicare data do not include claims of Health Maintenance Organization enrollees, care provided in other settings like the Veterans Administration, reimbursement of covered services not captured by the Medicare data such as out-of-pocket expenditures or coverage provided by Medigap, or long term care at home or nursing homes.[39] Therefore, our results will not be generalizable to patients less than 65 years of age and may not represent patients aged 65 years and older who resided in geographic areas not included in the SEER program who had only had Medicare Part A coverage or who were enrolled in Medicare Advantage plan. Second, we have not validated the procedures used to identify bone metastasis in this study. We identified possible bone metastasis from inpatient claims or outpatient claims paired with selected procedure codes, an approach designed to reduce false positives. Despite this approach, we may still have included false positive cases. It is also possible that we may have missed true cases in instances where claims were not obtained from the relevant facilities or where claims were obtained but not coded as bone metastasis. Third, it is possible that the mortality differences we noted for bone metastasis or bone metastasis plus SRE compared to those without these outcomes may be due to other confounding factors such as treatment factors or concurrent comorbid conditions that were not included in our analysis. Finally, the data do not include information on functional status of patients, a predictor of outcomes.
CONCLUSION
In conclusion, the present study found that having a bone metastasis as indicated by Medicare claims, was associated with mortality among patients with lung cancer. We found no difference in mortality between patients with bone metastasis complicated by SRE and patients with bone metastasis but without SRE.
ACKNOWLEDGMENTS
This research is supported by a contract between UAB and Amgen, Inc. Only the authors from UAB have access to the Medicare data used. The analysis, presentation and interpretation of the results are solely the responsibility of the authors.
Footnotes
Source of Support: This research is supported by a contract between UAB and Amgen, Inc. Only the authors from UAB have access to the Medicare data used. The analysis, presentation and interpretation of the results are solely the responsibility of the authors
Conflict of Interest: None declared
REFERENCES
- 1.Kohler BA, Ward E, McCarthy BJ, Schymura MJ, Ries LA, Eheman C, et al. Annual report to the nation on the status of cancer, 1975-2007, featuring tumors of the brain and other nervous system. J Natl Cancer Inst. 2011;103:714–36. doi: 10.1093/jnci/djr077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Cancer Society Facts and Figures 2012. American Cancer Society, Atlanta, Georgia [Internet] [Last cited on 2012 Apr 3]. Available from: http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-031941.pdf .
- 3.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 4.Coleman RE. Risks and benefits of bisphosphonates. Br J Cancer. 2008;98:1736–40. doi: 10.1038/sj.bjc.6604382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roodman GD. Biology of osteoclast activation in cancer. J Clin Oncol. 2001;19:3562–71. doi: 10.1200/JCO.2001.19.15.3562. [DOI] [PubMed] [Google Scholar]
- 6.Coleman RE. Skeletal complications of malignancy. Cancer. 1997;80(8 Suppl):1588–94. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1588::aid-cncr9>3.3.co;2-z. [DOI] [PubMed] [Google Scholar]
- 7.Coleman RE. Management of bone metastases. Oncologist. 2000;5:463–70. doi: 10.1634/theoncologist.5-6-463. [DOI] [PubMed] [Google Scholar]
- 8.Coleman RE. Bisphosphonates: Clinical experience. Oncologist. 2004;9(Suppl 4):14–27. doi: 10.1634/theoncologist.9-90004-14. [DOI] [PubMed] [Google Scholar]
- 9.Berenson JR, Rajdev L, Broder M. Managing bone complications of solid tumors. Cancer Biol Ther. 2006;5:1086–9. doi: 10.4161/cbt.5.9.3308. [DOI] [PubMed] [Google Scholar]
- 10.Delea T, Langer C, McKiernan J, Liss M, Edelsberg J, Brandman J, et al. The cost of treatment of skeletal-related events in patients with bone metastases from lung cancer. Oncology. 2004;67:390–6. doi: 10.1159/000082923. [DOI] [PubMed] [Google Scholar]
- 11.Rosen LS, Gordon D, Tchekmedyian NS, Yanagihara R, Hirsh V, Krzakowski M, et al. Long-term efficacy and safety of zoledronic acid in the treatment of skeletal metastases in patients with nonsmall cell lung carcinoma and other solid tumors: A randomized, Phase III, double-blind, placebo-controlled trial. Cancer. 2004;100:2613–21. doi: 10.1002/cncr.20308. [DOI] [PubMed] [Google Scholar]
- 12.Potosky AL, Riley GF, Lubitz JD, Mentnech RM, Kessler LG. Potential for cancer related health services research using a linked Medicare-tumor registry database. Med Care. 1993;31:732–48. [PubMed] [Google Scholar]
- 13.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: Content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(8 Suppl):IV-3–18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 14.Gornick ME, Warren JL, Eggers PW, Lubitz JD, De Lew N, Davis MH, et al. Thirty years of medicare: Impact on the covered population. Health Care Financ Rev. 1996;18:179–237. [PMC free article] [PubMed] [Google Scholar]
- 15.Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: Differing perspectives. J Clin Epidemiol. 1993;46:1075–9. doi: 10.1016/0895-4356(93)90103-8. [DOI] [PubMed] [Google Scholar]
- 16.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–9. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 17.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: New representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 18.Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. New York: John Wiley & Sons, Inc; 1980. [Google Scholar]
- 19.Cox DR. Regression models and life-tables (with discussion) J Royal Statistical Society. 1972;34:187–220. [Google Scholar]
- 20.SSAS.com [Internet]. SAS Institute Inc. 100 SAS Campus Drive, Cary, NC, 27513-2414, USA. [updated 2012 May 25; cited 2012 Dec 6]. Available from URL: http://www.sas.com .
- 21.Kosteva J, Langer C. The changing landscape of the medical management of skeletal metastases in nonsmall cell lung cancer. Curr Opin Oncol. 2008;20:155–61. doi: 10.1097/CCO.0b013e3282f54cf2. [DOI] [PubMed] [Google Scholar]
- 22.Hirsh V. Skeletal disease contributes substantially to morbidity and mortality in patients with lung cancer. Clin Lung Cancer. 2009;10:223–9. doi: 10.3816/CLC.2009.n.030. [DOI] [PubMed] [Google Scholar]
- 23.Iordanidou L, Trivizaki E, Saranti S, Georgakopoulos A, Bolanos N, Baltagiannis N, et al. Is there a role of whole body bone scan in early stages of non small cell lung cancer patients. J BUON. 2006;11:491–7. [PubMed] [Google Scholar]
- 24.Schirrmeister H, Arslandemir C, Glatting G, Mayer-Steinacker R, Bommer M, Dreinhöfer K, et al. Omission of bone scanning according to staging guidelines leads to futile therapy in non-small cell lung cancer. Eur J Nucl Med Mol Imaging. 2004;31:964–8. doi: 10.1007/s00259-004-1492-2. [DOI] [PubMed] [Google Scholar]
- 25.Michel F, Solèr M, Imhof E, Perruchoud AP. Initial staging of non-small cell lung cancer: Value of routine radioisotope bone scanning. Thorax. 1991;46:469–73. doi: 10.1136/thx.46.7.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tornyos K, Garcia O, Karr B, LeBeaud R. A correlation study of bone scanning with clinical and laboratory findings in the staging of nonsmall-cell lung cancer. Clin Nucl Med. 1991;16:107–9. doi: 10.1097/00003072-199102000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Salvatierra A, Baamonde C, Llamas JM, Cruz F, Lopez-Pujol J. Extrathoracic staging of bronchogenic carcinoma. Chest. 1990;97:1052–8. doi: 10.1378/chest.97.5.1052. [DOI] [PubMed] [Google Scholar]
- 28.Osada H, Nakajima Y, Taira Y, Yokote K, Noguchi T. The role of mediastinal and multi-organ CT scans in staging presumable surgical candidates with non-small-cell lung cancer. Jpn J Surg. 1987;17:362–8. doi: 10.1007/BF02470635. [DOI] [PubMed] [Google Scholar]
- 29.Turner P, Haggith JW. Preoperative radionuclide scanning in bronchogenic carcinoma. Br J Dis Chest. 1981;75:291–4. doi: 10.1016/0007-0971(81)90008-5. [DOI] [PubMed] [Google Scholar]
- 30.Hooper RG, Beechler CR, Johnson MC. Radioisotope scanning in the initial staging of bronchogenic carcinoma. Am Rev Respir Dis. 1978;118:279–86. doi: 10.1164/arrd.1978.118.2.279. [DOI] [PubMed] [Google Scholar]
- 31.Ramsdell JW, Peters RM, Taylor AT, Jr, Alazraki NP, Tisi GM. Multiorgan scans for staging lung cancer. Correlation with clinical evaluation. J Thorac Cardiovasc Surg. 1977;73:653–9. [PubMed] [Google Scholar]
- 32.Kosteva JL. Incidence and distribution of skeletal metastases in NSCLC in the era of PET. Lung Cancer. 2004;46:S45. [Google Scholar]
- 33.Tsuya A, Kurata T, Tamura K, Fukuoka M. Skeletal metastases in non-small cell lung cancer: A retrospective study. Lung Cancer. 2007;57:229–32. doi: 10.1016/j.lungcan.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 34.Coleman RE. Metastatic bone disease: Clinical features, pathophysiology and treatment strategies. Cancer Treat Rev. 2001;27:165–76. doi: 10.1053/ctrv.2000.0210. [DOI] [PubMed] [Google Scholar]
- 35.Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12:6243s–9s. doi: 10.1158/1078-0432.CCR-06-0931. [DOI] [PubMed] [Google Scholar]
- 36.Sun JM, Ahn JS, Lee S, Kim JA, Lee J, Park YH, et al. Predictors of skeletal-related events in non-small cell lung cancer patients with bone metastases. Lung Cancer. 2011;71:89–93. doi: 10.1016/j.lungcan.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 37.Sathiakumar N, Delzell E, Morrisey MA, Falkson C, Yong M, Chia V, et al. Mortality following bone metastasis and skeletal-related events among men with prostate cancer: A population-based analysis of US Medicare beneficiaries, 1999-2006. Prostate Cancer Prostatic Dis. 2011;14:177–83. doi: 10.1038/pcan.2011.7. [DOI] [PubMed] [Google Scholar]
- 38.Sathiakumar N, Delzell E, Morrisey MA, Falkson C, Yong M, Chia V, et al. Mortality following bone metastasis and skeletal-related events among women with breast cancer: A population-based analysis of U.S. Medicare beneficiaries, 1999-2006. Breast Cancer Res Treat. 2012;131:231–8. doi: 10.1007/s10549-011-1721-x. [DOI] [PubMed] [Google Scholar]
- 39.SEER-Medicare: Data Limitations. National Cancer Institute [Internet] [Last cited on 2012 Apr 3]. Available from: http://healthservices.cancer.gov/seermedicare/considerations/limitations.html .


