Abstract
Context:
Non-resolving pneumonia is often an area of concern for pulmonologists. Fiber optic bronchoscopy (FOB) may have a special role in etiologic evaluation of non-resolving pneumonias. There is paucity of recent studies in this field.
Aims:
This study aimed to assess the patients of non-resolving or slowly resolving pneumonia with special emphasis on efficacy of FOB and computed tomography (CT)-guided fine needle aspiration cytology (FNAC) in diagnosis.
Settings and Design:
Prospective, observational study conducted in a tertiary care institute over a period of one year.
Materials and Methods:
After fulfilling the definition of non-resolving pneumonia by clinical and radiological parameters, patients were evaluated by FOB with relevant microbiological, cytological, histopathological investigations and CT scan of thorax. CT-guided FNAC was done in selected cases where FOB was inconclusive.
Results:
Sixty patients were enrolled in the study. Mean age was 51.33 ± 1.71 years with male to female ratio 2:1. Right lung was more commonly involved (65%), and right upper lobe was the commonest site (25%). Pyogenic infection was the commonest etiology (53.3%), bronchogenic carcinoma and tuberculosis accounted for 26.7% and 16.7% cases, respectively. Both, FOB (85.7%) and CT-guided FNAC (91.67%) were very useful for etiological diagnosis of non-resolving pneumonia. Both the procedures were safe, and no major complication was observed.
Conclusions:
Because of the high yield of FOB, it is very useful and safe diagnostic tool for evaluation of non-resolving pneumonia. CT-guided FNAC also gives good yield when cases are properly selected.
KEY WORDS: Fiber optic bronchoscopy, lung cancer, non-resolving pneumonia, tuberculosis
INTRODUCTION
The term non-resolving or slowly resolving pneumonia is not an uncommon clinical entity and has been used interchangeably to refer the persistence of radiographic abnormalities beyond the expected time limit. Inadequate knowledge regarding the expected clinical course and outcome of a community-acquired or nosocomial pneumonia is a common reason for pulmonary consultation; selection of patients and appropriate timing of further evaluation can be challenging.[1] It accounts for 10% - 15% of nosocomial pneumonias and is estimated to be responsible for approximately 15% of inpatient pulmonary consultation and 8% of bronchoscopies.[2,3] Delay in diagnosis and treatment may lead to rise of mortality by 3-5% in both community-acquired pneumonia and nosocomial pneumonia. Incorrect diagnosis, inadequate antibiotic therapy, impaired host defence, atypical organisms, resistant pathogens, non-infectious causes, tuberculosis, endobronchial lesions, etc. are the common causes of non-resolving pneumonia or slowly resolving pneumonia.[4–7] Slow or incomplete resolution of pneumonia, despite treatment, needs a more aggressive evaluation. Fiberoptic bronchoscopy (FOB), computed tomography (CT) scan of the thorax and CT-guided fine needle aspiration cytology (FNAC) may be helpful in the evaluation of non-resolving or slowly resolving pneumonia. Microbiological, cytological and histopathological tests of the specimens can be done for etiological diagnosis of underlying cause. Efficacy of CT-guided FNAC and FOB in the etiological diagnosis of non-resolving or slowly resolving pneumonia has been around 80% and 70-86%, respectively in some studies. In this study, we tried to establish the etiological diagnosis of non-resolving pneumonia or slowly resolving pneumonia and also to evaluate the efficacy of diagnostic procedures, especially FOB and CT-guided FNAC.
MATERIALS AND METHODS
Study design
This study was a prospective cross-sectional study, performed in the department of pulmonary medicine at a tertiary care teaching institution of eastern India, over a period of one year (January 2010 - December 2010).
Study population
Sixty consecutive cases of non-resolving or slowly resolving pneumonia of both genders, attending the department of pulmonary medicine during the study period, were selected by adhering to the inclusion and exclusion criteria.
Inclusion criteria
Non-resolving or slowly resolving pneumonia was defined in this study by the presence of persistence of clinical symptoms and signs (cough, sputum production, with or without fever more than 100°F), failure of resolution of the radiographic features by 50% in 2 weeks or completely in 4 weeks on serial chest X-ray (indicated in at least 2 consecutive chest X-rays) in spite of antibiotic therapy for a minimum period of 10 days, and sputum for acid fast bacilli (AFB) smear negative for 2 consecutive days.
Exclusion criteria
Known patients of lung cancer or sputum-positive pulmonary tuberculosis, patients having very poor general condition, very severe breathlessness, recent history of myocardial infarction, positive test result for human immunodeficiency virus (HIV) infection, and unwilling patients were excluded from our study.
Study protocol
Detailed demographic and clinical parameters including age, sex, smoking history, clinical symptoms with duration (cough, fever more than 100°F, sputum production, hemoptysis, chest pain, breathlessness) and clinical signs (pallor, cyanosis, clubbing, enlarged neck nodes, pulse, blood pressure, features of consolidation like crepitations, bronchial breath sound) were evaluated in all patients. Presences of any comorbidity, especially diabetes mellitus, were documented. Blood for complete hemogram, blood glucose, urea, creatinine, liver function test, chest X-rays (posteroanterior and lateral view), sputum culture for Mycobacterium tuberculosis in mycobacterial growth indicator tube (MGIT 960) and sputum for malignant cells were sent in all patients. All patients had undergone a contrast-enhanced CT scan of thorax for better anatomical delineation. Fiberoptic bronchoscopy (FOB) was planned next in all patients (4 patients did not give consent for FOB). Macroscopic appearance of bronchial tree during FOB (intraluminal growth, presence of secretions/pus, appearance of bronchial mucosa, etc.) was noted.
Bronchoalveolar lavage (BAL) fluid was sent for cell type, AFB smear and culture for Mycobacterium tuberculosis (MGIT-960), gram stain and culture, fungal stain and culture, and malignant cells in all patients. Bronchial brushing and biopsy were also done in all patients and were sent for AFB smear and cytopathology, and histopathology, respectively. Post-bronchoscopic sputum for AFB smear and malignant cells were also sent. Patients having peripheral lesions on chest radiograph, who did not give consent for FOB or where results of FOB were inconclusive, were further evaluated by CT-guided FNAC. Blood for rheumatoid factor, anti-nuclear antibody (hep-2 method), C-ANCA and p-ANCA, serum angiotensine converting enzyme (SACE) were done additionally in selected patients, wherever found relevant. After evaluation of all the relevant reports, determination of etiology of non-resolving or slowly resolving pneumonia was attempted. Finally, efficacy of FOB and CT-guided FNAC as a diagnostic method for etiological analysis of non-resolving or slowly resolving pneumonia was assessed. The study was approved by the institutional ethics committee.
Statistical analysis
Statistical analysis were performed using SPSS version 10.0 (SPSS Inc, Chicago, IL) software for MS-Windows. Descriptive frequencies were expressed by mean + standard error of mean (SEM). P value was calculated using Fisher's exact test, and P value < 0.05 was considered to be significant.
RESULTS
During the period of 1 year, 60 consecutive patients of both sexes, having chest radiograph suggestive of non-resolving pneumonia or slowly resolving pneumonia as per our study criteria, were enrolled in this study. Overall, mean age of the patients was 51.33 ± 1.71 years (mean + SEM), and most of the patients (81%) were above the age of 40 years; no significant difference was observed in age parameter between different etiological groups. Among the 60 patients, 41 (68.3%) were male and 19 (31.7%) female. Mean duration of illness was 6.87 ± 0.24 weeks (mean + SEM). Twenty five patients (41.6%) were smoker, and smoking was distinctly more common in malignant etiology, compared to other groups (P = 0.006) [Table 1]; 8 patients (19%) were alcoholic. Twelve patients (20%) had past history of treatment with anti-tuberculous medication. Diabetes mellitus was the commonest comorbidity and was noted in 20 patients (33.33%) in the study population. Diabetes was significantly more associated with infective etiology, compared to malignancy (P = 0.023) [Table 1]. Klebsiella pneumoniae was the commonest organism isolated in patients with diabetes and was found in 6 out of 20 diabetics (30%), followed by Mycobacterium tuberculosis (25%, n = 5) and Staphylococcus aureus (20%, n = 4).
Table 1.
Clinical parameters
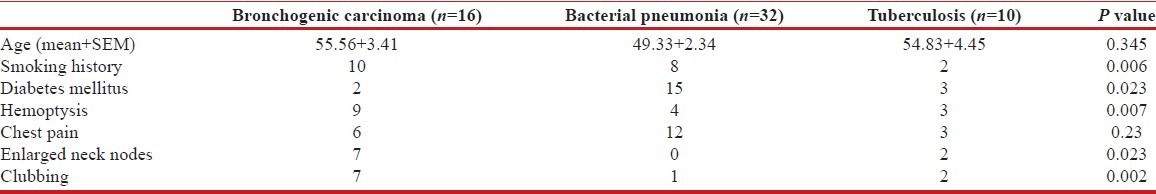
Most common chest symptoms were cough (100%) followed by fever (96%), hemoptysis (53%), chest pain (38%), and breathlessness (33%). Pallor was present in 17 (28.3%) patients in this study group, 16 (26.6%) patients had palpable lymphadenopathy, and 12 (20%) patients had clubbing. On chest X-ray, consolidation was present in 53 patients, consolidation along with cavity was present in 5 patients, and only cavity was present in 2 patients. On the other hand, by CT scan of thorax, cavity along with consolidation was seen in 9 patients and in remaining 51 patients, only consolidation was seen. Presence of cavitary lesion was commonest with tuberculous etiology (n = 6) but was also noted in staphylococcal pneumonia and squamous cell cancer. Cavities in infective etiology were thin-walled and often multiple in contrast to solitary thick-walled cavity in squamous cell cancer. Right lung involvement was seen in 39 (65%) patients, whereas left lung was involved in 21 (35%) patients. Right upper lobe (25%) was the most commonly involved site, followed by right lower lobe (23%), left lower lobe (21%), and left upper lobe (15%). Bilateral and multilobar involvement were seen in 10 (16.67%) cases and were more common with tubercular and staphylococcal etiology (P = 0.0007).
In this study, bacterial pneumonias (n = 32, 53%) were found to be the commonest etiology of non-resolving pneumonia, followed by bronchogenic carcinoma (n = 16, 26.67%) and tuberculosis (n = 10, 16.67%). Wegener's granulomatosis was diagnosed in 1 case (1.67%), and in one case, despite all investigations, no diagnosis could be reached (1.67%) [Table 2]. Among bacterial pneumonia, gram-negative bacilli were the predominant organisms isolated in 30 out of 32 cases of bacterial pneumonia (93.75%); Staphylococcus aureus accounted for 2 cases (6.25%). Klebsiella pneumoniae and Pseudomonas aeruginosa were the two most common gram-negative organisms, isolated in 13 and 11 cases, respectively. Eschericia coli and Acinetobacter spp were found in 3 cases each [Table 2]. Consolidation limited to a single lobe was the commonest radiological finding (93.75%) in cases of bacterial pneumonia.
Table 2.
Etiology of non-resolving pneumonia (n=60)

Bronchogenic carcinoma accounted for 16 cases (26.67%) of non-resolving pneumonia in this study. Squamous cell cancer was the predominant variety (n = 10) followed by adenocarcinoma (n = 5); small cell variety was diagnosed in 1 case. Seven out of 10 patients (70%) of squamous cell carcinoma were diagnosed by bronchoscopic procedures with bronchoscopic biopsy, showing positive histopathology in all the 7 cases and in addition, BAL fluid and bronchial brushings also showed positive result in 3 of these 7 cases; in the remaining 3 cases, diagnosis was established by CT-guided FNAC. On the other hand, all the 5 cases of adenocarcinomas were diagnosed by CT-guided FNAC, of these 2 were found to be of bronchoalveolar variety. Higher yield of bronchoscopy for squamous cell cancer can probably be explained by central location of the tumors as opposed to peripheral location of adenocarcinomas, which are picked up more readily by CT-guided FNAC.
Tuberculosis was responsible for 10 cases (16.67%) in this study. Multilobar and/or bilateral involvement was seen in 8 of those 10 (80%) cases of tuberculosis. Consolidation was the predominant radiological finding (100%) and associated cavitary lesions were present in 6 (60%) cases. Nine out of these 10 cases were diagnosed by fiberoptic bronchochoscopy- 6 patients showed positive mycobacterial culture in BAL fluid. In 2 patients, bronchoscopic biopsy demonstrated epitheloid granuloma with caseous necrosis and langhans’ giant cell, one of these 2 patients was also positive for AFB stain in bronchial brushing; in the remaining one case, all the bronchoscopic procedures were negative, but post-bronchoscopic sputum examination for AFB was positive. One case of tuberculosis was diagnosed by CT-guided FNAC where cytopathology showed epitheloid granuloma with langhans’ giant cell, and Ziehl-Neelsen (Z-N) stain was positive. Sputum for mycobacterial culture was additionally positive in 3 (30%) cases.
In this study, FOB was done in 56 patients (4 patients did not give consent for bronchoscopy). Most common finding in FOB was inflammation with secretions in the bronchial tree (n = 30, 53.6%), only inflamed mucosa was seen in 18 patients (32.1%) and intraluminal growth was observed in 8 cases (14.3%) [Table 3]. BAL fluid showed positive results in 40 out of 56 patients (71.4%) - pyogenic organisms were isolated in 31 patients, mycobacterial culture was positive in 6 cases and squamous cell cancer was diagnosed in 3 cases. Bronchoscopic biopsy was also performed in all the 56 cases. Non-specific inflammation was the commonest finding (n = 46, 82.1%), squamous cell cancer was found in 7 cases (12.5%), small cell cancer was seen in 1 case and tuberculosis was demonstrated in 2 cases. Bronchial brushing showed positive results in 4 cases- 3 cases showed positive cytology for squamous cell cancer, and 1 case was positive for Z-N stain. Overall, FOB showed positive results in 48 cases (85.7%) - Pyogenic infection was diagnosed in 31 cases, tuberculosis and bronchogenic carcinoma were diagnosed in 9 and 8 cases, respectively. However, FOB was inconclusive in 8 cases (14.3%) [Table 4].
Table 3.
Macroscopic findings during bronchoscopy (n=56)

Table 4.
Comparative yield of FOB versus CT-guided FNAC
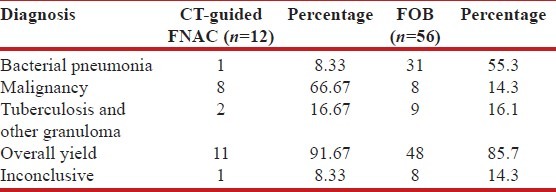
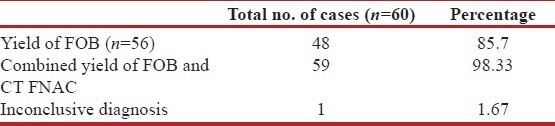
CT-guided FNAC was done in 12 patients (8 patients showed inconclusive result after FOB, and 4 patients did not give consent for FOB) in this study, and it showed a positive result in 11 cases (91.67%); 1 case (8.33%) being inconclusive. Out of the 11 cases where definitive diagnosis could be reached by CT-guided FNAC, bronchogenic carcinoma was diagnosed in 8 cases, granuloma was found in 2 cases, and pyogenic organism was isolated in 1 case from culture of pus aspirated on CT-guided FNA [Table 4]. Of the 2 cases where granuloma was demonstrated on FNAC, one was tuberculosis and other was diagnosed as Wegener's granulomatosis on the basis of clinico-radiological picture and positive c-ANCA in high titer. In this study, finally a specific etiology could be found in 59 out of 60 cases (98.3%), and in one case, no etiology could be established even after thorough investigations [Table 5].
Table 5.
Diagnostic yield of fiberoptic bronchoscopy and CT-guided FNAC
DISCUSSION
Non-resolving or slowly resolving pneumonia is not an infrequent clinical entity to pulmonologists, and at the same time, can be a cause of concern in daily clinical practice. Amberson was the first person to describe the term “unresolved organizing or protracted pneumonia” in 1943.[8] There is lack of uniformity regarding definition for non-resolving pneumonia, but in many studies, the entity of “slow resolution” has been defined as failure of radiographic resolution by 50% in 2 weeks or failure of complete resolution by one month despite adequate antibiotic therapy.[9]
In our study, 80% patients were over the age of 40 years and nearly 50% were over the age of 50 years. El Solh et al. stated that age alone has the most striking influence on resolution of pneumonia, and in their study, rate of resolution on chest X-ray was found to be 35.1% by 3 weeks and 60% by 6 weeks in patients above 70 years of age.[10] Fein has also shown in his study that only 30% of patients above 50 years of age show complete radiologic resolution by 4 weeks.[11] Diabetes mellitus was found to be the commonest comorbidity in this study and was present in 33.3% cases. Avijgan has also reported that diabetes mellitus was a major association with delayed resolution of pneumonia.[12] Klebsiella pneumoniae and Mycobacterium tuberculosis were the two most common etiologies in diabetic patients and were found in 33.3% and 25% cases, respectively. Begamy has also reported increased occurrence of Klebsiella pneumoniae in thoracic infections in diabetic patients.[13] Most common symptoms in this study were cough (100%) followed by fever (96.6%), hemoptysis (53.3%), chest pain (38.5%), and breathlessness (33.3%). Kirtland et al. studied 39 patients of slowly resolving pneumonia and found cough as commonest symptoms (92%) followed by chest pain (38%), breathlessness (38%), fever (36%), and hemoptysis (28%).[14] Boyed observed in his study that right lung appears to be more at risk for slow resolution and has also noted a predilection of chronic infiltrative disease for right upper lobe.[15] In the present study also, right lung involvement was most common (65%), and right upper lobe was most commonly affected (25%). In this study, pyogenic infection was diagnosed as etiological agents in 32 (53.3%) cases. Gram-negative bacteria were found to be the predominant pathogens, accounting for approx. 94% cases of pyogenic infections, and among them klebsiella (40.6%) and pseudomonas (34.4%) were the common pathogens. Fein also shared similar observation of increased occurrence of gram-negative etiology of pneumonia in elderly patients with comorbidities.[11] Tuberculosis was diagnosed in 16.7% cases in the present study. Silver et al. found tuberculosis in 5.7% cases from culture of BAL fluid as a cause of non-resolving pneumonia.[16,17] In our study, bronchogenic carcinoma was found in 16 patients (26.6%), of which squamous cell carcinoma was the commonest variety followed by adenocarcinoma. Ten patients (62.5%) were smokers among the patients with malignancy. Silver et al. found malignancy as a specific cause for non-resolving pneumonia in 11.4% cases in their series of 35 patients.[16]
In the present study, CT-guided FNAC was done in 12 patients, and etiological diagnosis was established in 11 cases (diagnostic yield being 93.7%). Among these patients, malignancy was found in 8 cases (66.7%). In a study conducted by Ferretti et al. in 23 patients of non-resolving pneumonia with negative FOB results, CT-guided core needle biopsy was done. Diagnostic yield of biopsy was 78%, 15 patients were diagnosed as malignancy, and 8 patients were diagnosed to have benign disease. The sensitivity and specificity for malignancy were 87% and 100%, respectively.[17]
Fiberoptic bronchoscopy (FOB) was done in 56 patients, and etiological diagnosis could be established in 48 cases (diagnostic yield 85.7%). BAL fluid was taken in all of these patients, and conclusive diagnosis achieved in 40 patients (71.4%); pyogenic infection was found to be the commonest etiology. In the study by Silver et al., FOB was diagnostic in 86% cases, and infections were the most common etiology obtained at FOB.[16] Balamugesh et al. have also found FOB a very useful tool in evaluating non-resolving pneumonia.[18] In our study, post-bronchoscopic sputum smear examination for AFB was done in all 56 patients, and 2 of them were AFB positive; 1 patient being exclusively positive for AFB by post-bronchoscopic sputum examination only. Therefore, overall diagnostic yield of FOB is very good, and it is a very useful tool for an evaluation of non-resolving or slowly resolving pneumonia. The procedure is safe, and complications are very less if done properly. It is particularly helpful to visualize any intraluminal lesion and to obtain bacteriological samples. Post-bronchoscopic sputum smear examination should not be ignored although it is not a much studied tool till now. Whenever feasible, CT-guided FNAC is also a good procedure, especially for peripherally situated lesions and when FOB is inconclusive. Its diagnostic yield is significantly high, and complications are less if patients are properly selected. In this study, although the yield of CT-guided FNAC (91%) was slightly better compared to yield of FOB (85.7%), it has to be kept in mind that CT-guided FNAC was done only in select cases and FOB should always be the first investigation of choice before CT-guided FNAC in evaluating non-resolving pneumonia No diagnostic procedure is absolutely sensitive for evaluation of non-resolving or slowly resolving pneumonia. Selection of procedure according to the site and clinical findings are important. Multiple procedures may be used in a rational approach to reach the definitive diagnosis [Figure 1].
Figure 1.
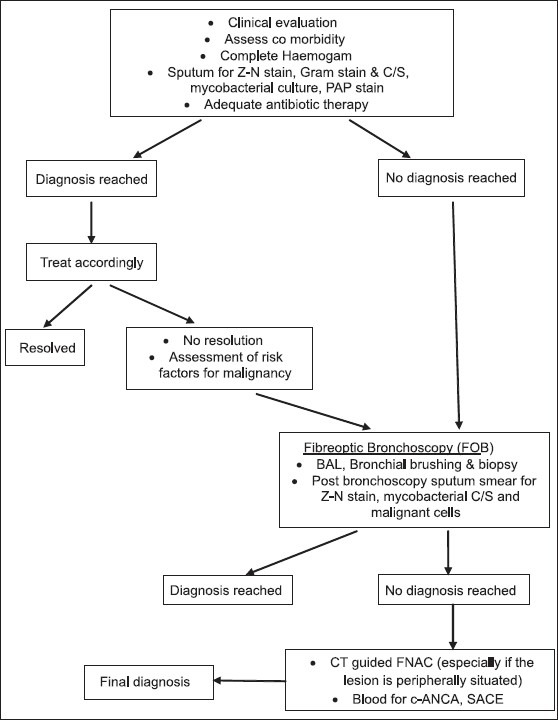
Approach to a patient with non-resolving pneumonia
To summarize, non-resolving pneumonia is often an area of clinical dilemma. Pyogenic infections are the commonest etiology, but microbiological profile is different from that of community-acquired pneumonia; tuberculosis, malignancy, and other non-infectious causes like vasculitis are other important etiologies to be looked for. FOB is an extremely useful investigation; CT-guided FNAC also gives good yield when properly selected.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Weyers CM, Leeper KV. Nonresolving pneumonia. Clin Chest Med. 2005;26:143–58. doi: 10.1016/j.ccm.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 2.Gotway MB, Leung JW, Dawn SK, Hill A. Nonresolving pneumonia in an otherwise healthy patient. Clin Pulm Med. 2004;11:198–200. [Google Scholar]
- 3.Menendez R, Perpina M, Torres A. Evaluation of nonresolving and progressive pneumonia. Semin Respir Infect. 2003;18:103–11. [PubMed] [Google Scholar]
- 4.Arancibia F, Ewig S, Martinez JA, Ruiz M, Bauer T, Marcos MA, et al. Antimicrobial treatment failures in patients with community-acquired pneumonia: Causes and prognostic implications. Am J Respir Crit Care Med. 2000;162:154–60. doi: 10.1164/ajrccm.162.1.9907023. [DOI] [PubMed] [Google Scholar]
- 5.Kuru T, Lynch JP III. Non-resolving or slowly resolving pneumonia. Clin Chest Med. 1999;20:623–51. doi: 10.1016/s0272-5231(05)70241-0. [DOI] [PubMed] [Google Scholar]
- 6.Fayez K, Tamim H, Walid K, Shadi L. Nonresolving pneumonia. Am J Ther. 2011;18:e177–9. doi: 10.1097/MJT.0b013e3181d10a93. [DOI] [PubMed] [Google Scholar]
- 7.White DA, Camus P, Endo M, Escudier B, Calvo E, Akaza H, et al. Noninfectious pneumonitis after everolimus therapy for advanced renal cell carcinoma. Am J Respir Crit Care Med. 2010;182:396–403. doi: 10.1164/rccm.200911-1720OC. [DOI] [PubMed] [Google Scholar]
- 8.Amberson JB. Significance of unresolved organizing or protracted pneumonia. J Mich State Med Soc. 1943;42:599–603. [Google Scholar]
- 9.Rome L, Murali G, Lippmann M. Nonresolving pneumonia and mimics of pneumonia. Med Clin North Am. 2001;85:1511–30. doi: 10.1016/s0025-7125(05)70393-x. [DOI] [PubMed] [Google Scholar]
- 10.El Solh AA, Aquilina AT, Gunen H, Ramadan F. Radiographic resolution of community-acquired bacterial pneumonia in the elderly. J Am Geriatr Soc. 2004;52:224–9. doi: 10.1111/j.1532-5415.2004.52059.x. [DOI] [PubMed] [Google Scholar]
- 11.Fein AM. Pneumonia in the elderly: Overview of diagnostic and therapeutic approaches. Clin Infect Dis. 1999;28:726–9. doi: 10.1086/515218. [DOI] [PubMed] [Google Scholar]
- 12.Avijgan M. Specificity and sensitivity of clinical diagnosis for chronic pneumonia. East Mediterr Health J. 2005;11:1029–37. [PubMed] [Google Scholar]
- 13.Begamy T. Thoracic empyema. Is its microbiology changing? Pul Rev Com. 2000;5:10–2. [Google Scholar]
- 14.Kirtland SH, Winterbauer RH. Slowly resolving chronic and recurrent pneumonia. Clin Chest Med. 1991;12:303–18. [PubMed] [Google Scholar]
- 15.Boyd DH. Failure of resolution. Br J Dis Chest. 1975;69:259–66. doi: 10.1016/0007-0971(75)90094-7. [DOI] [PubMed] [Google Scholar]
- 16.Feinsilver SH, Fein AM, Niederman MS, Schult DE, Faegenburg DH. Utility of firberoptic bronchoscopy in non resolving pneumonia. Chest. 1990;98:1322–6. doi: 10.1378/chest.98.6.1322. [DOI] [PubMed] [Google Scholar]
- 17.Ferretti GR, Jankowski A, Rodiere M, Brichon PY, Brambilla C, Lantuejoul S. CT-guided biopsy of non resolving focal airspace consolidation. J Thorac Imaging. 2008;23:7–12. doi: 10.1097/RTI.0b013e3181453e04. [DOI] [PubMed] [Google Scholar]
- 18.Balamugesh T, Aggarwal AN, Gupta D, Behera D, Jindal SK. Profile of repeat fiberoptic bronchoscopy. Indian J Chest Dis Allied Sci. 2005;47:181–5. [PubMed] [Google Scholar]


