Abstract
CPI-17 (C-kinase-activated protein phosphatase-1 (PP1) inhibitor, 17kDa) is a cytoplasmic protein predominantly expressed in mature smooth muscle (SM) that regulates the myosin-associated PP1 holoenzyme (MLCP). Here, we show CPI-17 expression in proliferating cells, such as pancreatic cancer and hyperplastic SM cells. Immunofluorescence showed that CPI-17 was concentrated in nuclei of human pancreatic cancer (Panc1) cells. Nuclear accumulation of CPI-17 was also detected in the proliferating vascular SM cell culture and cells at neointima of rat vascular injury model. The N-terminal 21-residue tail domain of CPI-17 was necessary for the nuclear localization. Phospho-mimetic Asp-substitution of CPI-17 at Ser12 attenuated the nuclear import. CPI-17 phosphorylated at Ser12 was not localized at nuclei, suggesting a suppressive role of Ser12 phosphorylation in the nuclear import. Activated CPI-17 bound to all three isoforms of PP1 catalytic subunit in Panc1 nuclear extracts. CPI-17 knockdown in Panc1 resulted in dephosphorylation of histone H3 at Thr3, Ser10 and Thr11, whereas it had no effects on the phosphorylation of myosin light chain and merlin, the known targets of MLCP. In parallel, CPI-17 knockdown suppressed Panc1 proliferation. We propose that CPI-17 accumulated in the nucleus through the N-terminal tail targets multiple PP1 signaling pathways regulating cell proliferation.
INTRODUCTION
Abnormal acceleration in epithelial and mesenchymal cell proliferation is a hallmark of tumorigenesis and hyperplasia. Protein phosphatase-1 (PP1) is a dominant Ser/Thr phosphatase in eukaryotic cells, and known to play multiple roles in the regulation of cell proliferation. The catalytic subunits of PP1 (PP1C), consisting of four isoforms (α, δβ, γ1, and testis-specific γ2), are capable of dephosphorylating a range of cellular proteins. Each PP1C isoform is assembled with a specific group of polypeptides, known as targeting subunits or interacting proteins, which regulate specific activity and compartmentalize PP1 at subcellular loci [1,2]. In addition to over 200 PP1 targeting subunits, 10 polypeptides specifically inhibit cellular PP1 holoenzymes in mammalian cells, classified into PP1 inhibitor proteins [1,2,3]. Characterization of PP1 targeting subunits and the endogenous inhibitors that mediate signals regulating cell proliferation is vital to fully understand mechanisms causing hyperplasia,
CPI-17 was discovered as a specific inhibitor for the myosin light chain phosphatase (MLCP), consisting of the PP1C δ (β) isoform associated with MYPT1, the myosin-targeting subunit. CPI-17 is highly expressed (at µM levels) in mature smooth muscles (SM) [4]. In mature SM, G-protein-coupled receptor signals trigger the activation of PKC and ROCK that phosphorylate CPI-17 at Thr38. This phosphorylation enhances the inhibitory potency of CPI-17 over 1,000-fold, resulting in MLCP inhibition, and consequent elevation in myosin light chain phosphorylation, causing SM contraction. The CPI-17-mediated MLCP regulation plays pivotal roles in adjusting responsiveness of SM contraction to stimuli, a process known as Ca2+ sensitization [3,5,6]. Accumulating lines of evidence suggest that changes in CPI-17 levels are associated with impaired excitation-contraction coupling of SM under pathological conditions, such as hypertension, asthma, gastrointestinal diseases, and urinary tract dysfunctions (reviewed in [3,7]).
The CPI-17 protein consists of a central four-helix bundle domain sandwiched with intrinsically unstructured N- and C-terminal tails. The central domain, whose structure is conserved among members of the CPI-17 family, such as PHI-1, KEPI and GBPI, is necessary and sufficient for the phosphorylation-dependent inhibition of MLCP [7]. Purified phospho (P)-CPI-17 inhibits MLCP with IC50 of <10nM and the isolated PP1C with lesser potency (reviewed in [3,7]). The inhibitory phosphorylation site, Thr38, resides in the loop region adjacent to the four-helix bundle. P-Thr38 in the loop directly docks at the bi-metal active site of PP1C, causing competitive inhibition [8]. In the MLCP holoenzyme, MYPT1 contacts both PP1C and CPI-17, stabilizing the enzyme-inhibitor interaction [7,9]. On the other hand, PP1C assembled with other PP1 targeting subunits, such as the glycogen-targeting subunit, rapidly dephosphorylates P-CPI-17 as a substrate and thereby neutralizes the inhibitory action [8]. Thus, PP1 targeting subunits determine whether CPI-17 acts as a specific inhibitor or a substrate of PP1C. What has yet to be fully evaluated is whether P-CPI-17 regulates only MLCP among >200 PP1 holoenzymes in cells.
Upon de-differentiation of SM cells, CPI-17 expression declines to 10% of the level in mature SM [10,11,12]. It is worth noting that the expression level of CPI-17 in de-differentiated SM cells remains over 10-fold greater compared to the IC50 with MLCP. Recently, CPI-17 was suggested to inhibit MLCP in SM cell culture and regulate MEF2C-mediated SM differentiation [13]. Also, it has been claimed that MLCP is a target of CPI-17 in cancer cells [14]. On the other hand, ectopic CPI-17 protein expressed in fibroblasts and in SM cell culture accumulates in nuclei [13,15], leaving open the possibility of CPI-17 regulating PP1 signaling in the nucleus. Here, we determined that CPI-17 is actively imported into nuclei and regulates histone phosphorylation and cell proliferation.
MATERIALS AND METHODS
Immunofluorescence and immunohistochemistry
Immunofluorescence and immunohistochemistry were performed as described previously [11,15]. Briefly, cells seeded on fibronectin-coated coverslip were fixed with 4% paraformaldehyde, and permeabilized with 0.1% Triton X-100, followed by indirect immunostaining. Paraffin-embedded sections of rat aorta at 14 weeks after balloon-catheter injury (gift from Dr. Avril V. Somlyo at University of Virginia) were prepared and processed as described previously [11]. Samples stained without primary antibodies were used as blank (BLK). Fluorescence microscopy and spot densitometry of digital images were done using the spinning-disk confocal microscope (Olympus IX70 plus BD CARV-II) with BD IPLab imaging software [11]. Nuclear / cytoplasmic (N/C) Index was defined as the ratio of staining densities at the nucleus and the cytoplasm of a cell. Mean values ± SEM of N/C index were obtained from 8–10 cells.
Others
Total protein extracts of human pancreatic cancer cell lines (L3.6pl, AsPC-1 and BxPC-3) and the 3xGFP tag expression vector were gifts from Drs J. Thomas Persons and Ian Macara at University of Virginia, respectively. QRTPCR and immunoblotting were done as described previously [12]. Cell proliferation was assayed in triplicate using Panc1 cells treated with negative control or CPI-17 targeting siRNA. Cells were treated with siRNA for 72 h in the presence of 10% FBS, fixed with dry methanol, stained with hematoxylin solution (Sigma), and counted under microscope. Mean values ± SEM of the number of cells were obtained from at least three image fields in each dish. Asterisk in each figure indicates p<0.05 in Student t-test compared to control. Conditions of cell culture, siRNA knockdown, transient transfection [12], subcellular fractionation [16] and pulldown assay [8] are described in Supplemental information. All primary and secondary antibodies used were listed in Supplemental table 1.
RESULTS
Expression and nuclear localization of CPI-17 in cancer and SM cells
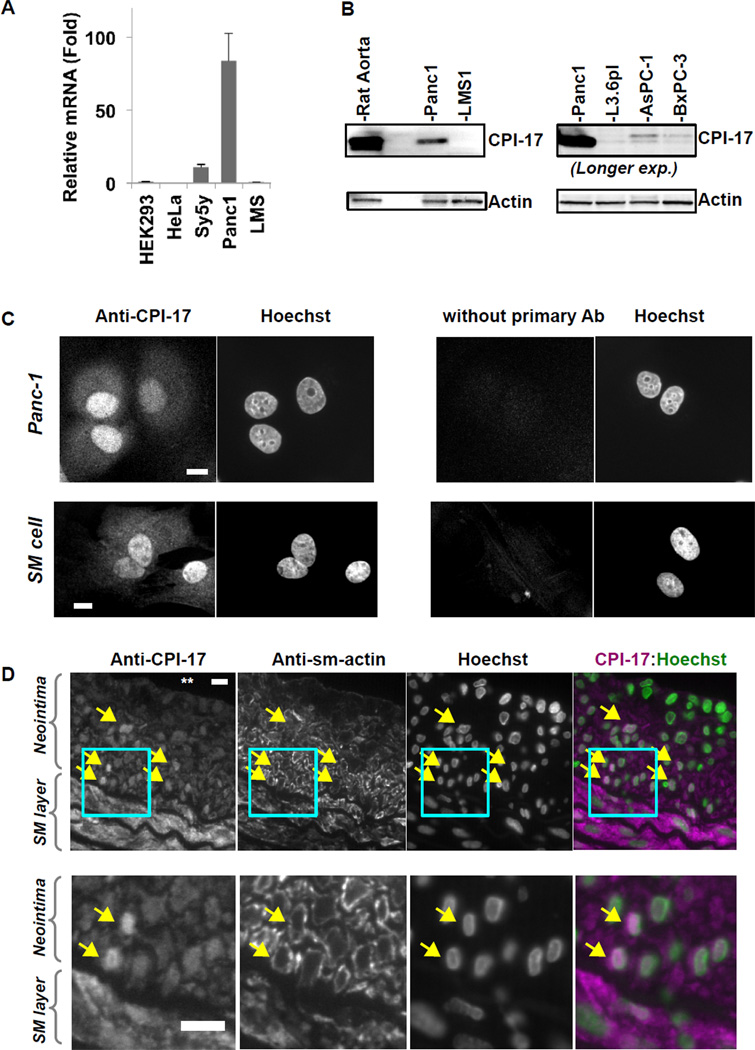
We determined the levels of CPI-17 mRNA and protein using quantitative RTPCR and immunoblotting (Fig. 1A and B). In QRTPCR analysis (Fig. 1A), CPI-17 mRNA was especially abundant in pancreatic cancer cells (Panc1), compared with fibroblasts (HEK293) and other cancer cell lines, such as cervical adenocarcinoma (HeLa), neuroblastoma (SH-SY5Y), and leiomyosarcoma (SK-LMS1). To estimate CPI-17 protein levels, extracts of rat aorta tissue and cancer cells (10µg each) were subjected to immunoblotting with anti-CPI-17 (Fig. 1B). Actin was stained for loading control. CPI-17 protein was detected in Panc1 cells at approximately 10% of the level in aorta tissue, but not in the SM-derived LMS1 cells (Fig. 1B, left). The level in Panc1 cells is equivalent to that of the SM cell culture [10,11]. Among human pancreatic cancer cell lines (Fig. 1B, right), CPI-17 expression was prominent in Panc1 cells and at lower levels in AsPC-1 and BxPC3 pancreatic cancer cell lines. CPI-17 was also expressed in different derivatives of Panc-1 cells (data not shown). Thus, Panc1 can be a model for studying roles of CPI-17 in proliferating cells.
Fig. 1. Expression and localization of CPI-17 in Panc1 cells.
(A) QRTPCR for CPI-17 mRNA. Total RNA (200ng) of the cells indicated was subjected to QRTPCR analysis. Extent of CPI-17 mRNA was normalized against lamin B, and the value of HEK293 was set as 1.0. (B) Immunoblotting of CPI-17 and actin using aorta tissue and cancer cell lysates. Total protein extracts (10µg) were subjected to immunoblotting. The expression of CPI-17 in human pancreatic cancer cells (right panel) was visualized with longer exposure (“Longer exp.”), compared with left panel. HEK293; embryonic fibroblasts, HeLa; cervical adenocarcinoma, SH-SY5Y, neuroblastoma, SK-LMS1; leiomyosarcoma, and Panc1, L3.6pl, AsPC-1, BxPC-3; pancreatic cancers. (C) Immunofluorescence of Panc1 (top) and rat aorta SM cells (bottom) using anti-CPI-17 antibody. Proliferating cells were fixed and stained with or without anti-CPI-17 antibody. Control experiment was carried out without primary antibody (right). Scale bar, 10µm. (D) Representing images of immunohistochemistry of rat aorta cross section with neointimal plaques. At 14-days after balloon-catheter injury, rat aorta was subjected to fixation and sectioning. Sections were co-stained with anti-CPI-17, anti-actin and Hoechst dye. From top to bottom: lumen (indicated with ** in top left), neointima, SM layers. Right panels show merged image of anti-CPI-17 staining with Hoechst staining. Bottom panels show the boxed area indicated in the panels above, respectively. Arrows indicate cells showing prominent nuclear staining with anti-CPI-17. Scale bar, 10µm.
We examined the subcellular localization of CPI-17 by immunofluorescent microscopy of Panc1 and rat aorta SM cell culture (Fig. 1C), and rat aorta wall cross sections (Fig. 1D). Prominent staining for CPI-17 was co-localized with Hoechst staining in Panc1 (Fig. 1C top left), whereas no nuclear staining was detected without the primary antibody as a control for specificity (Fig. 1C, top right).
Release of platelet-derived growth factor and basic fibroblast growth factor converts contractile/quiescent SM cell at vascular media into the proliferative phenotype, which is mimicked by the SM cells explanted from tissues. As shown in Fig. 1C (bottom panels), nuclear localization of CPI-17 was detected in rat aortic SM cell culture, a well-characterized model of the hyperplastic SM. We further examined subcellular localization of CPI-17 using an established in vivo model of hyperplastic SM cells. Physical damage of endothelium of rat aorta triggers proliferation and migration of SM cells, leading to neointima formation, a model of atherosclerosis and restenosis. We have shown that CPI-17 is downregulated in neointima, compared with the cells at SM layers [11]. Fig. 1D show immunohistochemistry of paraffin-embedded artery section at 14 week after injury co-stained with anti-CPI-17, anti-smooth muscle type (sm)-actin and Hoechst. CPI-17 was detected throughout cells at SM layers in tunica media. On the other hand, CPI-17 in the cells at neointima was concentrated in nuclei (Fig. 1D, arrows), which is evident in the merged image of CPI-17 staining with Hoechst staining (right panel). These cells at neointima also expressed sm-actin that was excluded from nuclei (Fig. 1D). These results suggest that CPI-17 nuclear localization occurs when it is expressed in cells with the proliferative phenotype.
Mechanisms underlying CPI-17 nuclear localization
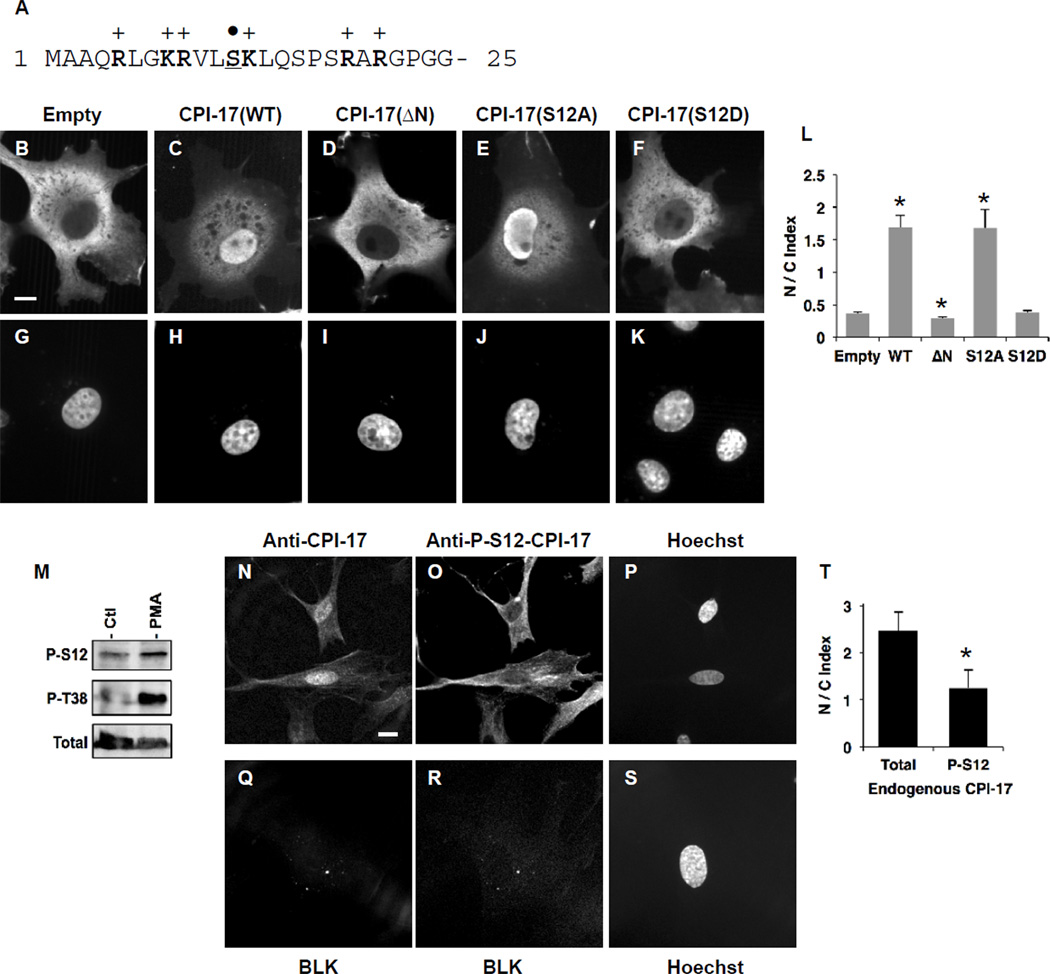
The molecular basis of CPI-17 nuclear localization was investigated using COS1 cells transiently expressing triple-GFP (3xGFP) tagged protein (Fig. 2A–L). To mimic the modest level of the endogenous protein, expression of 3xGFP protein was limited to minimum, and visualized by immunofluorescence using anti-GFP antibody. Intrinsic GFP fluorescence images of live and fixed cells under epifluorescence are shown in Supplemental figure 1. In the confocal images, ectopic 3xGFP protein alone (“empty”) was excluded from nuclei due to the size (Mr=78kDa) (Fig. 2, panel B, G). We quantified staining intensities of the proteins in regions of the nucleus and the cytoplasm by spot densitometry. The ratio of nuclear staining to cytoplasmic staining (“N/C Index”) was below 0.5 (panel L), indicating the exclusion of 3xGFP protein from the nucleus (Fig. 2L, “Empty”). By sharp contrast, 3xGFP protein fused with CPI-17 wild type (WT) was accumulated in nuclei (panel C, H) with N/C Index of over 1.5 (panel L). As shown in Fig. 2A, the N-terminal tail of CPI-17 forms a basic-residue cluster, similar to nuclear import signal [17]. Indeed, the deletion of the N-terminal 21-residue eliminated the nuclear accumulation of CPI-17 (Fig. 2D, I, L: 3xGFP-CPI-17(ΔN)), suggesting a functional nuclear localization signal (NLS) in the unstructured N-terminal tail. Purified kinases, such as PKC, ZIPK, ROCK and ILK, can phosphorylate CPI-17 at Ser12 in the middle of the N-terminal NLS region (Fig. 2A, marked by dot) [7]. Asp-substitution mimicking the phosphorylation at Ser12 (3xGFP-CPI-17(S12D)) diminished the nuclear localization (Fig. 2F, K, L), whereas Ala-substitution at Ser12 had no effect (Fig. 2E, J, L).
Fig. 2. Mechanisms underlying nuclear localization of CPI-17.
(A) Amino acid sequence of CPI-17 at the N-terminal tail. Arg, Lys and Ser are indicated in bold and underline respectively. (B–L) Localization of 3xGFP proteins. Rat aorta SM cells were transfected with the vectors encoding 3xGFP (B, G), 3xGFP-CPI-17(WT) (C, H), ΔN lacking the N-terminal 21 residues (D, I), S12A (E, J), and S12D (F, K), and visualized by immunofluorescence using anti-GFP. Bar indicates 10µm. N/C Index was obtained from 8–10 cells. *, p<0.05 compared to “Empty”. (M) CPI-17 phosphorylation at Ser12 in HEK293 cells. HA-tagged CPI-17 was expressed in HEK293 cells for 24 h. The transfected cells were harvested for 24 h without serum, followed by the stimulation for 10 min with 1 µM PMA, and subjected to immunoblotting using anti-P-S12-CPI-17. Total CPI-17 was detected using anti-HA antibody. (N–T) Localization of phospho-CPI-17 at Ser12 in rat fundus SM cells. TCA-fixed proliferating rat fundus SM cells were subjected to immunoblotting with anti-total CPI-17 (N) plus anti-P-S12-CPI-17 (O) or without primary antibodies (Q, R). Scale bar, 10µm. N/C index of P-S12-CPI-17 and total CPI-17 staining was obtained from 5 independent cells. *, p<0.05, compared to total CPI-17 staining.
To determine roles of Ser12 phosphorylation in the nuclear localization, we developed the antibody recognizing P-Ser12, but not P-Thr38 (Supplemental figure 2). To confirm Ser12 phosphorylation in cells, HEK293 cells expressing HA-tagged CPI-17 was stimulated with phorbol ester (PMA) and subjected to immunoblotting (Fig. 2M). CPI-17 phosphorylated at Ser12 was detected in the cell lysates of the quiescent cells, and cells stimulated with PMA at slightly higher level (Fig.2M). Thr38 phosphorylation was enhanced in response to PMA stimulation, as we reported previously [10]. Rat fundus SM cells expressing higher level of endogenous CPI-17 were fixed with 10% TCA and subjected to co-immunostaining with the anti-P-S12-CPI-17 antibody plus anti-total CPI-17 and Hoechst (Fig. 2N–T). Unlike total CPI-17 staining (panel N), P-Ser12-CPI-17 staining (panel O) did not show any nuclear accumulation with N/C index of around 1.0 (panel T), indicating free diffusion in the cells. On the other hand, cells stained without primary antibodies (panel Q, R, S) did not show any background signal under the condition. These results suggest that the phosphorylation of CPI-17 at the N-terminal tail is sufficient to attenuate the NLS activity.
CPI-17 regulates phosphorylation of nuclear proteins and cell proliferation
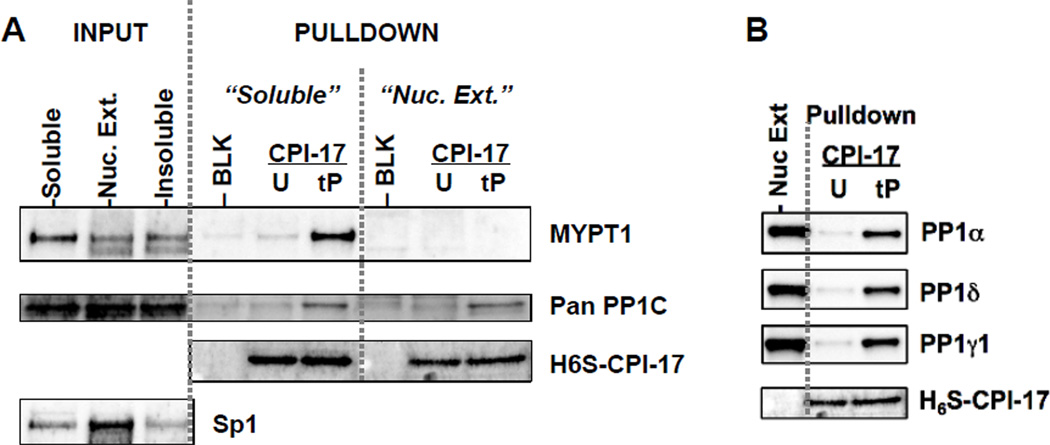
We questioned what was the target of nuclear CPI-17. As shown in Fig. 3A, the sequential extraction of Panc1 cells showed that majority of MYPT1 existed in the soluble fraction, which is consistent with the report by Murata et al [18]. A small amount of MYPT1 was extracted from nuclei along with Sp1 protein a marker of nuclear proteins (Fig. 3A). This is consistent with the data in proteomics that showed MYPT1 in nuclear extracts, in addition to approximately 20 other PP1-binding polypeptides [19,20]. The soluble fraction and nuclear extracts were subjected to a CPI-17 pulldown assay (Fig. 3A, B). Both MYPT1 and PP1C in the soluble fraction bound to tP-CPI-17-beads, but not to unphosphorylated (U)-CPI-17 or blank beads (BLK), demonstrating the phosphorylation-dependent binding of CPI-17 to the MYPT1-PP1C complex (Fig. 3A). MYPT1 in the nuclear extracts failed to bind tP-CPI-17 beads (Fig. 3A), although tP-CPI-17 bound to all isoforms of PP1C, (α, δ, and γ1) in nuclear extracts (Fig. 3B). Because most nuclear PP1 targeting subunits recognize specific PP1C isoforms, CPI-17 likely binds multiple types of nuclear PP1 holoenzymes in nuclei.
Fig. 3. Binding of CPI-17 to nuclear PP1 in Panc1 cells.
(A) Differential extraction and CPI-17 pulldown assay. Proliferating Panc1 cells were subjected to sequential extraction as described in Supplemental information (INPUT). Aliquot of the soluble fraction and the nuclear extracts were mixed with beads conjugated with unphosphorylated (U)-, tP-CPI-17 and no protein (BLK) and the bound proteins were analyzed by immunoblotting with antibodies indicated. Sp1 was used as a marker of nuclear protein. Anti-pan-PP1C antibody recognizes all isoforms. (B) Specificity of CPI-17 binding to PP1 isoforms. PP1 isoforms bound to tP-CPI-17-beads were determined using antibodies recognizing the isoforms. Nuc. Ext.: input sample.
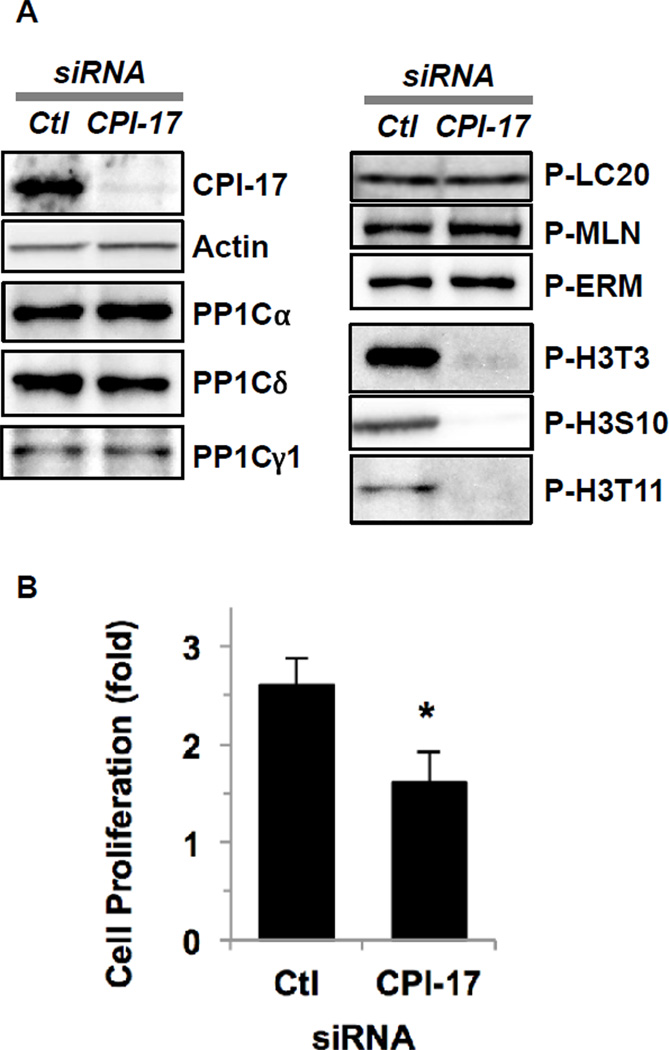
We determined phosphorylation of established substrate proteins for MLCP in Panc1 cells after CPI-17 knockdown (Fig. 4). siRNA knockdown effectively eliminated CPI-17 protein without changing the expression of PP1C isoforms (Fig. 4A, left). Remarkably, the phosphorylation of myosin light chain (LC20), merlin (MLN) and ezrin/radixin/moesin (ERM), the major substrates of MLCP, did not change in response to CPI-17 knockdown (Fig. 4A, right). On the other hand, CPI-17 knockdown resulted in the net de-phosphorylation of histone H3 at Thr3 (P-H3T3), at Ser10 (P-H3S10) and at Thr11 (P-H3T11) (Fig. 4A, right). As shown in Fig. 4B, after a 72-h treatment with control siRNA, the number of Panc1 cells increased 2.5-fold, whereas CPI-17 knockdown resulted in a 40 % reduction in Panc1 proliferation. These results suggest that CPI-17 in the nucleus inhibits a subset of PP1 responsible for dephosphorylation of histone H3, upregulating cell proliferation signaling.
Fig. 4. Effects of CPI-17 knockdown on PP1 signaling in Panc1 cells.
(A) Panc1 cells were transfected for 72 h with siRNA for CPI-17 (right lane) or non-targeting control (left lane, Ctl), and subjected to immunoblotting using antibodies against proteins indicated. P denotes phosphorylated protein. MLN; merlin, ERM; ezrin/radixin/moesin, H3; histone H3. (B) Effects of CPI-17 knockdown on cell proliferation. After 72-h knockdown, cells were stained with hematoxylin, and the number of cells was counted under microscope. The cell number before the knockdown was set as 1.0. n=3, * p<0.05 (Student’s t-test).
DISCUSSION
In this report we present evidence suggesting that CPI-17 regulates nuclear PP1 signaling pathways. Based on the model established in mature SM tissues [3,7,21], CPI-17 was anticipated to inhibit MLCP in cancer cells, amplifying Ras signaling through merlin phosphorylation [14], and in vascular SM cell culture, regulating MEF2C and the differentiation [13]. By sharp contrast, our finding shows that CPI-17 inhibits nuclear PP1 responsible for histone H3 dephosphorylation. The suppression of Panc1 proliferation by CPI-17 knockdown may be attributed to the histone H3 dephosphorylation, because the temporal phosphorylation is required for chromatin remodeling during the cell cycle. MYPT1 possesses an active NLS at the N-terminal domain and is detected in nuclear extracts [20,22]. Clearly, MYPT1 in nuclei is not accessible for CPI-17. We presume that MYPT1 in the nucleus is not in complex with PP1C [23], otherwise it would have bound to tP-CPI-17. Or, unknown posttranslational modifications of MYPT1 interfere CPI-17 binding. Over 20 polypeptides in nuclei are found to bind PP1C, and each of them is a potential target of P-CPI-17 [19,20]. CPI-17 is involved in the phosphorylation of at least histone H3 Thr3, Ser10 and Thr11, offering a clue to find PP1 holoenzymes regulated by nuclear CPI-17. Of particular interest, Repo-Man-PP1Cγ is known to dephosphorylate histone H3 at Thr3, and a candidate of a CPI-17 target [24]. P-histone H3 Ser10 and Thr11 are also dephosphorylated by PP1, but the targeting subunit remains unknown [24]. Further systematic investigation is necessary for elucidating nuclear PP1 signaling regulated by CPI-17 in hyperplastic cancer and SM cells.
The nuclear localization of CPI-17 in proliferating cells, but not in mature SM, was confirmed by fluorescent microscopy of endogenous proteins and ectopic 3xGFP-proteins. The N-terminal 21-residue tail, whose sequence is identical in mouse, pig and human, was mapped as the region required for nuclear localization. This is consistent to our previous finding that FLAG-tagged CPI-17(1–120) and (1–89) proteins are accumulated in nuclei of rat embryo fibroblasts [15]. We presume that the binding of importin complex to the N-terminal tail drives the nuclear import of CPI-17. This active nuclear import of CPI-17 is neutralized upon Ser12 phosphorylation, based on the data using the phospho-mimetic S12D protein and the phospho-specific antibody. Once CPI-17 is phosphorylated at Ser12, it is diffused but not excluded from nuclei, suggesting that nuclear export of CPI-17 is driven by diffusion, but not through active exporting mechanisms, such as CRM1 signaling. 3xGFP-CPI-17(ΔN) and (S12D) was excluded from nuclei, because of the size (>78kDa). It has known that PKC, ZIPK, ROCK and ILK, are capable of the phosphorylation of CPI-17 at Thr38 as well as Ser12 [7]. Our data here suggest that Ser12 phosphorylation occurs in cells. In addition, there are two more Ser residues (Ser16 and Ser18) in the N-terminal tail of CPI-17, which may play an inhibitory role in the nuclear import. It is possible that pathological stresses alter the phosphorylation state at the N-terminal NLS of CPI-17 regulating the subcellular distribution of the signaling. Identification of the kinases phosphorylating at Ser12 and adjacent sites will be a next step toward establishing pathological links between the redistribution of CPI-17 signaling and hyperplastic phenotype.
In addition to CPI-17, other endogenous PP1 inhibitors are known to undergo nuclear shuttling, in response to environmental changes. PP1 inhibitor-2 is redistributed from the nucleus to the cytoplasm, when the cell-cell contact is established [16]. Another soluble PP1 inhibitor, DARPP32, is actively imported into nuclei in response to amphetamine and cocaine treatments due to the phosphorylation at Ser97, resulting in the elevation in histone H3 Ser10 phosphorylation [25]. Nuclear shuttling of PP1 inhibitors may be a mechanism by which mammalian cells adjust phosphorylation levels in the nucleus in response to environmental changes.
Supplementary Material
HIGHLIGHTS.
Non-canonical roles of the myosin phosphatase inhibitor (CPI-17) were studied.
CPI-17 is localized in the nucleus of hyperplastic cancer and smooth muscle cells.
CPI-17 Ser12 phosphorylation may regulate the nuclear import.
CPI-17 regulates histone H3 phosphorylation and cell proliferation.
The nuclear CPI-17-PP1 axis plays a proliferative role in cells.
Acknowledgement
We thank Dr. David L. Brautigan for proofreading and invaluable suggestions. This work was supported by grants from NIH (HL083261, DK088905), Brandywine Valley Hemophilia Foundation, and Pennsylvania C.U.R.E (to M. Eto), Korean Research Foundation (MOEHRD, KRF-2007-357-E00004) and Korean Ministry of Education, Science and Technology (NRF Basic Science Research program 2011-0014919) (to J.I. Kim). Research in this publication includes work carried out by the Kimmel Cancer Center Genomics Facility, supported in part by NCI grant P30 CA56036.
Abbreviations
- PP1
protein phosphatse-1
- CPI-17
PKC-activated PP1 inhibitor (Mr=17kDa)
- MLCP
myosin light chain phosphatase
- MYPT1
myosin phosphatase targeting subunit, GFP, green fluorescence protein
- PKC
protein kinase C
- ROCK
RhoA-associated coiled coil kinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Cohen PT. Protein phosphatase 1--targeted in many directions. J. Cell Sci. 2002;115:241–256. doi: 10.1242/jcs.115.2.241. [DOI] [PubMed] [Google Scholar]
- 2.Bollen M, Peti W, Ragusa MJ, Beullens M. The extended PP1 toolkit: Designed to create specificity. Trends Biochem. Sci. 2011;35:450–458. doi: 10.1016/j.tibs.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eto M, Brautigan DL. Endogenous inhibitor proteins that connect Ser/Thr kinases and phosphatases in cell signaling. IUBMB Life. 2012;64 doi: 10.1002/iub.1067. scope. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woodsome TP, Eto M, Everett A, Brautigan DL, Kitazawa T. Expression of CPI-17 and myosin phosphatase correlates with Ca2+ sensitivity of protein kinase C-induced contraction in rabbit smooth muscle. J. Physiol. 2001;535:553–564. doi: 10.1111/j.1469-7793.2001.t01-1-00553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kitazawa T, Masuo M, Somlyo AP. G protein-mediated inhibition of myosin light-chain phosphatase in vascular smooth muscle. Proc. Natl. Acad. Sci. U.S.A. 1991;88:9307–9910. doi: 10.1073/pnas.88.20.9307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kitazawa T, Kitazawa K. Size-dependent heterogeneity of contractile Ca2+ sensitization in rat arterial smooth muscle. J. Physiol. 2012;590:5401–5423. doi: 10.1113/jphysiol.2012.241315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eto M. Regulation of cellular protein phosphatase-1 (PP1) by phosphorylation of the CPI-17 family, C-kinase-activated PP1 inhibitors. J. Biol. Chem. 2009;284:35273–35277. doi: 10.1074/jbc.R109.059972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eto M, Kitazawa T, Brautigan DL. Phosphoprotein inhibitor CPI-17 specificity depends on allosteric regulation of protein phosphatase-1 by regulatory subunits. Proc. Natl. Acad. SciUS.A. 2004;101:8888–8893. doi: 10.1073/pnas.0307812101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eto M, Kitazawa T, Matsuzawa F, Aikawa S, Kirkbride JA, Isozumi N, Nishimura Y, Brautigan DL, Ohki SY. Phosphorylation-induced conformational switching of CPI-17 produces a potent myosin phosphatase inhibitor. Structure. 2007;15:1591–1602. doi: 10.1016/j.str.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woodsome TP, Polzin A, Kitazawa K, Eto M, Kitazawa T. Agonist- and depolarization-induced signals for myosin light chain phosphorylation and force generation of cultured vascular smooth muscle cells. J. Cell Sci. 2006;119:1769–1780. doi: 10.1242/jcs.02805. [DOI] [PubMed] [Google Scholar]
- 11.Kim JI, Young GD, Jin L, Somlyo AV, Eto M. Expression of CPI-17 in smooth muscle during embryonic development and in neointimal lesion formation. Histochem. Cell Biol. 2009;132:191–198. doi: 10.1007/s00418-009-0604-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim JI, Urban M, Young GD, Eto M. Reciprocal regulation controlling the expression of CPI-17, a specific inhibitor protein for the myosin light chain phosphatase in vascular smooth muscle cells. Am. J. Physiol. Cell Physiol. 2012;303:C58–68. doi: 10.1152/ajpcell.00118.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pagiatakis C, Gordon JW, Ehyai S, McDermott JC. A novel RhoA/ROCK-CPI-17-MEF2C signaling pathway regulates vascular smooth muscle cell gene expression. J. Biol. Chem. 2012;287:8361–8370. doi: 10.1074/jbc.M111.286203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin H, Sperka T, Herrlich P, Morrison H. Tumorigenic transformation by CPI-17 through inhibition of a merlin phosphatase. Nature. 2006;442:576–579. doi: 10.1038/nature04856. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi Y, Senba S, Yazawa M, Brautigan DL, Eto M. Defining the Structural Determinants and a Potential Mechanism for Inhibition of Myosin Phosphatase by the Protein Kinase C-potentiated Inhibitor Protein of 17 kDa. J. Biol. Chem. 2001;276:39858–39863. doi: 10.1074/jbc.M107302200. [DOI] [PubMed] [Google Scholar]
- 16.Leach C, Eto M, Brautigan DL. Domains of type 1 protein phosphatase inhibitor-2 required for nuclear and cytoplasmic localization in response to cell-cell contact. J. Cell Sci. 2002;115:3739–3745. doi: 10.1242/jcs.00052. [DOI] [PubMed] [Google Scholar]
- 17.Marfori M, Mynott A, Ellis JJ, Mehdi AM, Saunders NF, Curmi PM, Forwood JK, Boden M, Kobe B. Molecular basis for specificity of nuclear import and prediction of nuclear localization. Biochim. Biophys. Acta. 2011;1813:1562–1577. doi: 10.1016/j.bbamcr.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 18.Murata K, Hirano K, Villa-Moruzzi E, Hartshorne DJ, Brautigan DL. Differential localization of myosin and myosin phosphatase subunits in smooth muscle cells and migrating fibroblasts. Mol. Biol. Cell. 1997;8:663–673. doi: 10.1091/mbc.8.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trinkle-Mulcahy L, Andersen J, Lam YW, Moorhead G, Mann M, Lamond AI. Repo-Man recruits PP1 gamma to chromatin and is essential for cell viability. J. Cell Biol. 2006;172:679–692. doi: 10.1083/jcb.200508154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moorhead GB, Trinkle-Mulcahy L, Nimick M, De Wever V, Campbell DG, Gourlay R, Lam YW, Lamond AI. Displacement affinity chromatography of protein phosphatase one (PP1) complexes. BMC Biochem. 2008;9:28. doi: 10.1186/1471-2091-9-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dimopoulos GJ, Semba S, Kitazawa K, Eto M, Kitazawa T. Ca2+-dependent rapid Ca2+ sensitization of contraction in arterial smooth muscle. Circ. Res. 2007;100:121–129. doi: 10.1161/01.RES.0000253902.90489.df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu Y, Muranyi A, Erdodi F, Hartshorne DJ. Localization of myosin phosphatase target subunit and its mutants. J. Muscle Res. Cell Motil. 2005;26:123–134. doi: 10.1007/s10974-005-2579-5. [DOI] [PubMed] [Google Scholar]
- 23.Eto M, Kirkbride JA, Brautigan DL. Assembly of MYPT1 with protein phosphatase-1 in fibroblasts redirects localization and reorganizes the actin cytoskeleton. Cell Motil. Cytoskeleton. 2005;62:100–109. doi: 10.1002/cm.20088. [DOI] [PubMed] [Google Scholar]
- 24.Qian J, Lesage B, Beullens M, Van Eynde A, Bollen M. PP1/Repo-man dephosphorylates mitotic histone H3 at T3 and regulates chromosomal aurora B targeting. Curr. Biol. 2011;21:766–773. doi: 10.1016/j.cub.2011.03.047. [DOI] [PubMed] [Google Scholar]
- 25.Stipanovich A, Valjent E, Matamales M, Nishi A, Ahn JH, Maroteaux M, Bertran-Gonzalez J, Brami-Cherrier K, Enslen H, Corbillé AG, Filhol O, Nairn AC, Greengard P, Hervé D, Girault JA. A phosphatase cascade by which rewarding stimuli control nucleosomal response. Nature. 2008;453:879–884. doi: 10.1038/nature06994. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.