Abstract
Subpopulations of individuals with alcohol-induced fatty livers and nonalcoholic steatosis develop steatohepatitis. Steatohepatitis is defined histologically: increased numbers of injured and dying hepatocytes distinguish this condition from simple steatosis. The increased hepatocyte death is generally accompanied by hepatic accumulation of inflammatory cells and sometimes increases in myofibroblastic cells, leading to hepatic fibrosis and eventually, cirrhosis. The purpose of this review is to summarize similarities and differences in the pathogenesis of steatohepatitis in alcoholic fatty liver disease (AFLD) and nonalcoholic fatty liver disease (NAFLD).
Keywords: alcoholic, nonalcoholic, pathogenesis, steatohepatitis
Introduction
Steatohepatitis occurs in some, but not all, individuals who develop steatosis due to excessive consumption of alcohol 1,2. It may also occur in some individuals with steatosis due to nonalcoholic fatty liver disease, a condition that is most commonly associated with obesity, insulin resistance and the metabolic syndrome 3–5. Steatohepatitis differs from steatosis mainly in the degree of hepatocyte injury and death, both of which are much worse in steatohepatitis than simple steatosis 6,7. Thus, although hepatocyte accumulation of triglyceride occurs in both steatosis and steatohepatitis, histological features of liver cell injury, such as hepatocyte ballooning and cytoskeletal condensation (Mallory-Denk bodies), and cell death (e.g., apoptotic bodies), occur predominately in steatohepatitis and distinguish the condition from steatosis.
Hepatocyte injury in steatohepatitis is often accompanied by hepatic accumulation of inflammatory cells and myofibroblasts 8–10. The latter sometimes results in deposition of excessive type 1 collagen (i.e., fibrosis). The distribution of fibrosis in steatohepatitis differs somewhat from that of other types of chronic liver injury, with pericellular and sinusoidal fibrosis in acinar zone 3 being more common in steatohepatitis 9,11. However, “typical” peri-portal fibrosis and bridging fibrosis between portal tracts and between portal tracts and central veins, also occur in steatohepatitis 9,12. As in other types of chronic liver disease, bridging fibrosis may eventuate in cirrhosis. Hepatocellular carcinomas have also been demonstrated in rare individuals with steatohepatitis, and occur more commonly in steatohepatitis-related cirrhosis 13.
Evidence that cirrhosis and/or hepatocellular carcinoma are potential outcomes of steatohepatitis, but tend to occur relatively infrequently, if at all, in individuals with simple steatosis, supports the concept that steatohepatitis is a more serious form of liver damage than simple steatosis 14–16. The purpose of this review is to summarize similarities and differences in the pathogenesis of steatohepatitis in alcoholic fatty liver disease (AFLD) and nonalcoholic fatty liver disease (NAFLD).
Pathogenesis of Hepatocyte Injury and Death in AFLD and NAFLD
The extent and severity of hepatocyte injury and death distinguish steatohepatitis from simple steatosis 6,7. Regardless of the specific primary stimulus for steatosis, hepatocyte injury and death result from unsuccessful adaptations to that stimulus 17–19. Ironically, both failure to sufficiently induce “coping” mechanisms and the coping mechanisms themselves can result in hepatocyte lethality. Thus, hepatic steatosis identifies a state of hepatocyte vulnerability 14. Common mechanisms that promote progression from simple steatosis to steatohepatitis are discussed subsequently. It is important to emphasize that these mechanisms are interactive, redundant, and not specific for AFLD or NAFLD. Also, multiple mechanisms may be operative simultaneously within any given individual with either condition.
Lipotoxicity
Altered lipid homeostasis is an initiating force for both alcoholic fatty liver disease (AFLD) and nonalcoholic fatty liver disease (NAFLD) 20,21. While hepatocyte accumulation of triglyceride is the hallmark of hepatic steatosis in both AFLD and NAFLD, it is important to emphasize that triglycerides themselves are not hepatotoxic 22 and, therefore, do not cause steatohepatitis. Rather, storage of fatty acids in triglycerides protects hepatocytes from various potentially noxious consequences of fatty acid accumulation. Before discussing mechanisms for fatty acid toxicity, a brief summary of the factors that influence triglyceride accumulation is justified, since failure to adequately dispose of excess fatty acids by converting them into triglyceride increases the risk for hepatocyte lipotoxicity.
Factors that regulate hepatocyte triglyceride content
Triglycerides are a natural end-product of fatty acid metabolism. Hepatocytes normally increase their rates of triglyceride synthesis when energy consumption exceeds energy utilization. Energy excess is a feature of obesity, because obese subjects typically consume more food energy than they utilize by doing physical activity. Hence, energy excess provides a major stimulus for hepatocyte triglyceride synthesis in NAFLD. It may also contribute to steatosis in AFLD because alcoholic beverages are calorically dense, and this may push energy intake above energy utilization in habitual heavy drinkers.
In both AFLD and NAFLD, triglycerides are ultimately synthesized from fatty acids. There are several potential sources of fatty acids that can be used to generate triglycerides. Dietary fatty acids are an important source of fatty acids in both conditions. In both AFLD and NAFLD, fatty acids derived from lipolysis of adipose tissue triglyceride depots are also delivered to the liver, taken up by hepatocytes, and converted into triglycerides 23,24. Hepatocyte uptake of fatty acids from the diet and from lipid-containing particles that are released from endogenous lipid stores is regulated by several types of proteins, including fatty acid transport proteins (FATPs), fatty acid translocase 25 (also called CD36), and fatty acid binding proteins (FABPs). A detailed discussion of these proteins was recently published elsewhere 21, and is beyond the scope of this review. Briefly, targeted deletion of FATP 26, FAT 27,28 or FABP 29,30 in hepatocytes reduce hepatic lipid accumulation in animal models of diet-induced hepatic steatosis. Although not tested formally, it also seems likely that knock-down of these genes would afford some protection from alcohol-induced steatosis. To date, very little information has been published about whether or not polymorphisms of these genes play an important role in susceptibility to and/or progression of AFLD or NAFLD. However, hepatic expression of FAT/CD36 was reported to be increased and correlated with liver fat content in some patients with NAFLD 31. On the other hand, there has been much discussion about the role of adipocytokines, such as adiponectin, in regulating hepatic fatty acid uptake (and de novo lipogenesis) in both AFLD and NAFLD 32. Reduced adiponectin and/or defective adiponectin function have been demonstrated in both conditions, and are believed to contribute to hepatocyte fatty acid accumulation and increased triglyceride synthesis 33.
De novo lipogenesis (i.e., increased fatty acid biosynthesis) is another factor that contributes to the development of steatosis in both AFLD and NAFLD. This process is regulated by transcription factors that are activated by insulin, particularly sterol regulatory element binding protein (SREBP)-1c 34. Therefore, hyperinsulinemia is an important stimulus for de novo lipogenesis. Hyperinsulinemia is common in NAFLD, but may also occur in AFLD when inflammatory cytokines reduce insulin sensitivity 35,36. SREBP-1 is also activated by endoplasmic reticulum (ER) stress, a condition that occurs in both AFLD and NAFLD 37–39 (see below). Hence, increased de novo lipogenesis may provide a stimulus for increased triglyceride synthesis in both NAFLD and AFLD.
Finally, fatty acids may also accumulate within hepatocytes because their metabolism is impaired. In healthy hepatocytes, fatty acids are oxidized by enzymes in peroxisomes, mitochondria, and the endoplasmic reticulum (microsomes) 21. When fatty acid oxidation is inhibited, but mechanisms for triglyceride synthesis remain intact, the resultant accumulation of fatty acids provides a potent stimulus for triglyceride synthesis.
Regardless of the source of fatty acids that hepatocytes use to produce triglyceride, this triglyceride is normally packaged into lipoproteins in the hepatocyte endoplasmic reticulum, and then exported to adipose depots for storage. Therefore, in both AFLD and NAFLD, triglyceride accumulates within hepatocytes when these export mechanisms become overwhelmed 24. This may occur due to inherited or acquired defects in lipoprotein assembly and secretion 40–42, including ER stress, homocysteinemia, abetaliproteinemia, and choline deficiency. These factors can occur in both AFLD and NAFLD.
Factors that control fatty acid oxidation
Variability in the efficiency of the different mechanisms for fatty acid oxidation, coupled with differences in the ability to cope with residual fatty acids and/or their metabolic by-products, is likely to explain some of the differences in the degree of hepatocyte triglyceride accumulation, and conversely, the severity of hepatocyte injury (i.e., lipotoxicity) that occurs in any given individual over time, as well as among different individuals with AFLD or NAFLD. Lipotoxicity occurs because, unlike triglycerides which are relatively inert, fatty acids physically interact with lipid membranes and other cellular molecules 43,44. Some of these interactions are directly damaging 45,46. Others cause damage by initiating signaling events 47,48. For example, fatty acids are endogenous ligands for certain nuclear hormone receptors, and thereby regulate cellular metabolism and differentiation 49 (see below). They also alter lysosomal permeability in hepatocytes, promoting release of cathepsin B and triggering hepatocyte production of cytokines, such as tumor necrosis factor (TNF) α and interleukin-6 50. In addition, fatty acids are capable of interacting with certain toll like receptors and thus, modulate activation of down-stream kinases and transcription factors that are regulated by these receptors 51.
Fatty acids that are not incorporated into triglyceride are degraded by oxidation. This process may also be hepatotoxic. Fatty acid oxidation is catalyzed by enzymes that are localized within three discrete cellular compartments: mitochondria, peroxisomes and microsomes 52,53 (i.e., smooth endoplasmic reticulum). Transcription of enzymes that catalyze β-oxidation of fatty acids in peroxisomes and mitochondria is regulated by the fatty acid-sensitive nuclear hormone receptor PPAR-α 54. PPAR-α activity is inhibited by chronic consumption of alcohol, but may be more normal in NAFLD 55–57. Changes in PPAR-α activity influence β-oxidation of fatty acids in both conditions. Mitochondrial oxidation of fatty acids generates superoxide (which is generally detoxified efficiently by mitochondrial superoxide dismutase), ATP, ketone bodies and acetyl CoA (which ultimately enters the tricarboxylic acid cycle and is converted to CO2 and H2O). Because mitochondrial damage is common in AFLD 58,59 and also occurs in NAFLD 60,61, the capacity for fatty acid oxidation in this organelle may become limiting, particularly in AFLD. This leads to increased peroxisomal (and microsomal) oxidation of fatty acids. Peroxisomal oxidation of fatty acids generates hydrogen peroxide, a potential source of oxidant stress 53,62. Reactive oxygen species (ROS) are also produced when fatty acids undergo ω-oxidation by cytochrome P450 enzymes within microsomes 63–66. In addition, microsomal ω-oxidation of fatty acids generates dicarboxcylic acids (DCA). DCA uncouple mitochondrial oxidative phosphorylation, reducing the mitochondrial membrane potential 63. This decreases the efficiency of mitochondrial ATP production, and enhances vulnerability to other stresses that promote depolarization of mitochondrial membranes, including TNFα and various other pro-apoptotic signals 67. DCA are also PPAR-α ligands 53, and thus, amplify expression of fatty acid oxidizing enzymes. This re-enforces expression of microsomal fatty acid oxidizing enzymes, such as Cyp2E1, and helps to explain why expression of Cyp2E1 and other microsomal enzymes are increased in both AFLD and NAFLD. Since Cyp2E1 also metabolizes ethanol 68,69, fatty acid-related induction of this enzyme contributes to generation of acetaldehyde, which forms immunogenic adducts 70 with various molecules, and exacerbates ROS production in AFLD.
Based on this discussion, it is evident that the ultimate “impact” of fatty acid oxidation is modulated by the capacity of various endogenous systems to buffer hepatocytes from noxious actions of by-products of fatty acid oxidation. Mitochondria themselves (which progressively degrade fatty acids and dicarboxylic acids to innocuous end-products), and various antioxidant enzymes (which detoxify superoxide anion and hydrogen peroxide that are generated during fatty acid oxidation) are particularly important in this regard 25,71–73. These buffering systems act in concert with other factors that carefully regulate the net content of fatty acids within hepatocytes by controlling their uptake (e.g., FATPs, FAT, FABPs), biosynthesis (e.g., SREBP-1c), non-oxidative metabolism (e.g., DGAT2-mediated conversion into triglyceride), and the availability/activity of fatty acid-sensitive signaling molecules (e.g., PPARs, Toll-like receptors). Lipotoxicity (i.e., hepatocyte injury and death) results when this delicate and complex equilibrium is disturbed.
Oxidative Stress
Increased generation of ROS occurs in both AFLD and NAFLD and this has long been considered to play an important role in progression to steatohepatitis in both conditions. As discussed above, hepatocyte metabolism of ethanol and lipids results in formation of ROS within several intra-cellular compartments, including the mitochondria, peroxisomes, and the endoplasmic reticulum 65,74. When ROS production exceeds the buffering/detoxifying capacity of antioxidant systems, various cellular macromolecules are subject to direct oxidative attack. This may result in DNA mutations, destruction of vital enzymes, peroxidation of lipid membranes, and generation of other toxic molecules such as peroxinitrite and reactive iron species 75,76. At lower levels, ROS function as signaling intermediates, triggering the activation of redox-sensitive transcription factors, such as NF-κB 77,78, that control the transcription of genes that regulate hepatocyte viability, as well as the synthesis of inflammatory mediators, such as TNFα and other proinflammatory cytokines 79–81. These cytokines, in turn, exert both autocrine and paracrine effects: autocrine activation of TNF receptors, for example, may initiate death receptor signaling within hepatocytes themselves 82,83; paracrine activation of TNF receptors on neighboring macrophages, endothelial cells and stellate cells promotes inflammatory and fibrogenic responses 35,84–86.
Despite the compelling rationale that supports the importance of ROS in the pathogenesis of both ASH and NASH, it has been difficult to demonstrate consistent benefit of anti-oxidant therapies in either condition. For example, agents that increase intracellular stores of reduced glutathione (e.g., betaine and S-adenosyl methionine) have been reported to improve ASH and NASH in some animal models, but similar improvements have not been observed reproducibly in patients with either condition 87,88. To date, the benefits of vitamin E therapy have been similarly inconclusive 89,90. In contrast, treatment with pharmacologic inhibitors of NADPH oxidase (the membrane-associated enzyme complex that generates ROS in macrophages and various other cell types, including hepatic stellate cells), as well as generalized knock-down of this enzyme, significantly protected mice from alcohol-induced steatohepatitis 79,81. Conversely, mice that over-expressed a constitutively active mutant form of NADPH oxidase developed significantly more liver injury and fibrosis than wild type controls when treated with carbon tetrachloride 91. Interestingly, in these NADPH oxidase transgenic mice, over-activation of NADPH oxidase was restricted to myofibroblastic cells because the transgene was under the control of α-smooth muscle actin regulatory elements. The latter finding raises the intriguing possibility that ROS production by myofibroblasts, rather than macrophages, is responsible for liver damage during steatohepatitis.
Endoplasmic Reticulum Stress
ER accumulation of proteins that are normally secreted evokes an unfolded protein response 92,93 that restrains the further synthesis of such proteins, while amplifying the production of ER membranes and membrane-associated factors 94,95. It also induces other mechanisms (e.g., autophagy) to cope with the burden of retained proteins. As mentioned above, such responses impact lipid homeostasis. They also have various other “off-target” effects that may be detrimental when superimposed upon hepatocytes that are already struggling to adapt to oxidative- and other forms of metabolic stress 96. ER stress is believed to be an important mechanism of hepatotoxicity in both AFLD and NAFLD.
Cytokines
Production of pro-inflammatory cytokines, particularly TNFα and IL-1, and TNF-inducible cytokines such as interleukin (IL)-6 and IL-8, is increased in both AFLD and NAFLD 97,98. Multiple cell types likely contribute to this process because hepatocytes, cholangiocytes, macrophages, stellate cells, endothelial cells, and adipocytes are all capable of producing cytokines when challenged. In animal models of either AFLD or NAFLD, various strategies that inhibit expression and/or activity of TNFα generally improve steatohepatitis 99–101. This is not surprising because there are multiple mechanisms by which increased TNFα is likely to promote progression from steatosis to steatohepatitis. For example, TNFα inhibits the expression and activity of adiponectin 102 and this exacerbates hepatocyte accumulation of fatty acids, contributing to lipotoxicity (see above). In addition, TNFα increases mitochondrial ROS production and promotes the mitochondrial membrane transition, effects that contribute to oxidant and apoptotic stress 35,103. TNFα also activates down-stream kinases that interfere with insulin-signaling and this promotes hepatic (and systemic) insulin-resistance, hyperinsulinemia, and the consequent perturbations in lipid and glucose metabolism 35,104. Finally, TNFα is a potent inducer of IL-8 and other chemokines and chemokine receptors that promote the hepatic recruitment and accumulation of various types of inflammatory cells 105.
However, despite all of these potentially dangerous effects of TNFα and the apparent benefit that accrues when TNFα signaling is blocked in animal and cell culture models of steatohepatitis, it is critical to acknowledge that specific antagonism of TNFα has not been proven to improve the outcomes in patients with ASH. Indeed, in at least two trials that were performed in patients with severe ASH, TNFα antagonism led to increased morbidity and mortality 106,107. The reasons for the discrepant outcomes in experimental models and patients with ASH are not well-understood, but may relate to differences in the severity of liver injury and/or fibrosis in animals and people with alcohol-related steatohepatitis. Because specific TNFα antagonists are expensive and potentially toxic and patients with NASH seldom, if ever, manifest the same florid features of hepatic decompensation that occur in patients with acute alcoholic hepatitis, specific TNFα antagonists have not been evaluated in humans with NASH.
Interestingly, however, another “anti-cytokine” agent, pentoxifylline, has proven to improve outcomes in both ASH and NASH patients 108,109. Although pentoxifylline inhibits TNFα, it also suppresses production of other cytokines and inhibits phosphodiesterases 110. The latter effect has been linked to its anti-fibrotic actions, including its ability to block stellate cell activation 86,111. Therefore, it is difficult to know which (if any) of these actions underlie the observed benefits of pentoxifylline in patients with steatohepatitis. Corticosteroids are another ASH therapy that is presumed to mediate its benefits by blocking the negative actions of inflammatory cytokines 112. However, because prednisone and prednisolone are known to promote adiposity and exacerbate insulin resistance, and both conditions are risk factors for NASH, these agents have not been evaluated as therapies for NASH. Given evidence that corticosteroids improve mortality in patients with ASH 113, but would likely worsen insulin resistance, which constitutes a major risk factor for NASH, it is curious that certain insulin sensitizing agents improve both ASH and NASH 114,115.
Arguments that these agents are beneficial because they inhibit TNFα may need to be reconsidered in light of emerging evidence that TNFα antagonism is actually harmful in patients with severe ASH. This, in turn, re-directs attention towards other common targets. Thiazolidenediones, for example, increase activity of PPAR-γ. In addition to improving insulin sensitivity, inhibiting inflammatory signaling, and preventing TNFα production, PPAR-γ also suppresses transformation of quiescent stellate cells into activated myofibroblasts 116, and this is likely to be beneficial in both ASH and NASH. Another insulin sensitizing agent, metformin, has also been reported to provide some benefit in both NASH and ASH 117,118. Metformin increases the activity of adenosine monophosphate-activated protein (AMP) kinase 119. This is expected to promote PPAR-α activation, and thus, might promote fatty acid disposal. Increased AMP kinase activity is also expected to improve ATP regeneration. Recent evidence also suggests that increasing AMP kinase activity prevents stellate cell activation 120,121. Thus, like pentoxifylline, two commonly prescribed insulin-sensitizing agents that seem to improve ASH and NASH have TNFα independent effects that may reduce hepatocyte injury, including important actions on liver non-parenchymal cells.
Adipose tissues are also a rich source of cytokines that modulate the biology of various types of liver cells. In addition to TNFα and IL6 (which are thought to be produced by macrophages that accumulate in adipose tissues), adipocytes themselves also produce adipocytokines 102,122. Two of the most extensively studied factors are leptin and adiponectin. Leptin reduces steatosis and lipotoxicity mainly by improving peripheral insulin sensitivity and thereby reducing hepatic exposure to adipose-derived fatty acids 123. It also has significant anti-inflammatory actions 124. However, leptin promotes myofibroblastic activation of hepatic stellate cells and thus, may contribute to fibrogenesis in NASH (and ASH) 125,126. Adiponectin, on the other hand, seems to have generally beneficial effects, inhibiting steatosis, lipotoxicity, and fibrogenesis in both conditions 32,33,127.
Endotoxin and other products of gut bacteria
The healthy liver receives most of its afferent blood supply from the portal venous system and consequently, it is routinely exposed to commensal flora and their products. Intestinal permeability increases significantly in ASH 128,129. It has also been reported to be increased in experimental animals and patients with NASH 130,131. Thus, in both ASH and NASH, hepatic exposure to gut-derived bacterial products increases 100,101,132. Evidence that such factors contribute to the pathogenesis of steatohepatitis was first demonstrated in animal models of ASH 133. Treatment with poorly absorbed oral antibiotics, particularly agents that bound lipopolysaccharide, significantly protected rodents from alcohol-induced liver injury 101. Subsequent studies demonstrated similar protection by deleting cell-surface receptors that promote LPS signaling 134,135. Some benefits were also observed in rodent models of NAFLD/NASH when the mice were treated with probiotics 136. Oral antibiotic therapy also improved liver damage in patients with total parenteral nutrition-related steatohepatitis 137. More recent, elegant studies in germ-free mice proved that the gut flora modulates hepatic lipid homeostasis, and thus, influences lipotoxicity 138. Multiple mechanisms are likely to be involved given that resident intestinal bacteria release various factors that interact with different pathogen-associated molecular pattern (PAMP) recognition receptors on the surface of resident liver cells, including hepatocytes, macrophages, and stellate cells. Ligation of Toll-like receptor 4, for example, activates inflammatory signaling in hepatocytes 139,140. It is also known to play a critical role in activation of hepatic stellate cells 141.
Ethanol and its metabolites
Perhaps the biggest difference in ASH and NASH pertains to the relative exposure to ethanol and its metabolites, which occur at significant levels in the former, but presumably not the latter, condition. On the other hand, it is important to emphasize that ethanol and acetaldehyde can be generated endogenously, albeit in much lower levels than are typically observed in actively drinking alcohol abusers 142,143. Ethanol, for example, is produced by gut bacteria during carbohydrate metabolism. Acetaldehyde is also a by-product of normal intermediary metabolism. The fact that ASH and NASH share many similar histologic features despite the fact that the two conditions clearly differ in the degree to which the liver is exposed to ethanol and acetaldehyde suggests either that these factors are unimportant in the pathogenesis of ASH or that their hepatotoxic effects may be mimicked by (or result from) other molecules. On the other hand, evidence that the natural history of ASH appears to be much more “aggressive” than that of NASH suggests that ethanol and/or acetaldehyde may, indeed, have unique roles in steatohepatitis progression. For example, acetaldehyde interacts with various molecules to form adducts that have immunogenic properties 70. Acetaldehyde has also been shown to directly activate collagen gene expression in hepatic stellate cells 144,145. Ethanol itself disorders lipid membranes. Its (non-oxidative) metabolism also generates fatty acid ethyl esters that may be cytotoxic 146. Therefore, chronic exposure to ethanol and/or its metabolites may further challenge livers that are concomitantly experiencing stresses related to lipotoxicity, oxidative and ER stress, and exposure to cytokines and PAMP recognition receptors, exacerbating hepatocyte injury and related inflammation and fibrogenesis.
Summary
Steatohepatits occurs in subpopulations of individuals with either alcoholic or nonalcoholic fatty liver disease. Steatohepatitis differs from simple steatosis mainly with regard to the severity of hepatocyte injury and extent of hepatocyte death, both being much worse in steatohepatitis than steatosis. A number of common mechanisms contribute to hepatocyte injury in ASH and NASH (Figure 1), including lipotoxicity, oxidant and ER stress, and increased exposure to various cytokines and factors that activate PAMP recognition receptors. Patients with ASH are also chronically exposed to relatively high concentrations of ethanol and its metabolite, acetaldehyde, which superimpose additional toxicities. The latter may help to explain why a greater proportion of patients with ASH than NASH appear to develop cirrhosis and liver-related mortality. Nevertheless, ASH and NASH are generally improved by treatments that reduce hepatocyte fatty acid accumulation and/or that block inflammatory signaling and activation of hepatic stellate cells. This suggests that these shared mechanisms drive the pathogenesis and progression of steatohepatitis in both conditions.
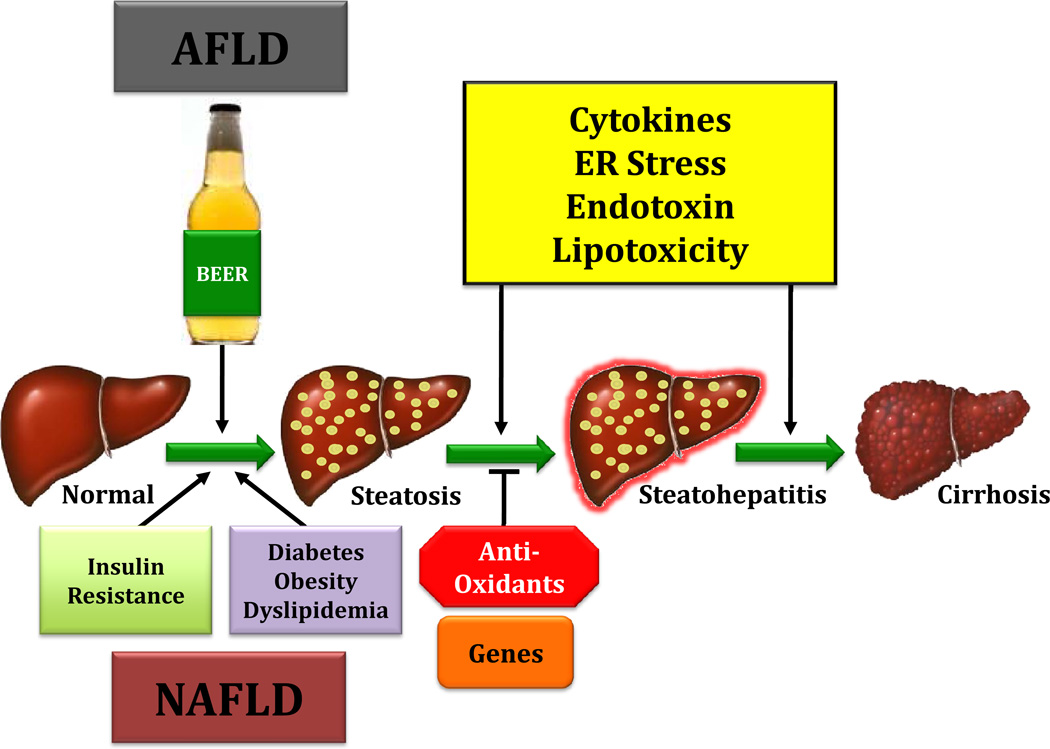
Figure 1.
Under normal conditions, cells respond to increased fatty acid load by up regulating oxidation pathways, increase cellular export of VLDL and suppress fatty acid synthesis. In NAFLD and AFLD, triglyceride accumulation results from excess energy because obese subjects typically consume more food energy than they utilize by doing physical activity and because alcoholic beverages are calorically dense pushing energy intake above energy utilization in habitual heavy drinkers respectively. Hepatic steatosis then occurs when the influx of fatty acids to the liver is coupled with repressed fatty acid oxidation, triglyceride export (VLDL) and dysregulated fatty acid synthesis. When increased fatty acid load exceeds metabolic oxidation pathways, accumulation of potentially toxic by-products and reactive oxygen species (ROS), including hydrogen peroxide results. ROS trigger lipid, protein and DNA peroxidation, and are immunogenic, which lead to the production of proinflammatory cytokines (such as TNFα). When persistent, these cellular stresses overwhelm intrinsic detoxification mechanisms (antioxidants and unfolded protein response), and promote hepatocyte cell death, a hallmark of steatohepatitis. Compounding the problem, obesity, diabetes and chronic ethanol intake are associated with increased gut epithelial permeability and bacterial overgrowth, resulting in endotoxemia (i.e.,lipopolysaccharide, LPS) which activate hepatic stellate cells and Kupffer cells. This triggers the production of additional TNFα and ROS, both of which are pro-apoptotic and promote the inflammatory milieu that drives progressive fibrosis.
Acknowledgements
This work was supported by National Institutes of Health Grants R01 DK053792 (A.M.D.).
Abbreviations
- AFLD
alcoholic fatty liver disease
- AMP
adenosine monophosphate-activated protein
- ASH
alcoholic steatohepatitis
- ATP
adenosine triphosphate
- CYP2E1
cytochrome P450 2E1
- DCA
dicarboxylic acids
- DGAT
diacylglycerol acyltransferase
- ER
endoplasmic reticulum
- FABP
fatty acid binding protein
- FAT
fatty acid translocase
- FATP
fatty acid transport protein
- HCC
hepatocellular carcinoma
- IL
interleukin
- LPS
lipopolysaccharide
- NADPH
nicotinamide adenine dinucleotide phosphate
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- NFkB
nuclear factor kappa B
- PAMP
pathogen-associated molecular pattern
- PPAR
peroxisome proliferator-activated receptor
- ROS
reactive oxygen species
- SREBP
sterol regulatory element binding protein
- TLR
toll-like receptor
- TNF
tumor necrosis factor
References
- 1.Lieber CS. Alcoholic liver disease: new insights in pathogenesis lead to new treatments. J Hepatol. 2000;32(1 Suppl):113–128. doi: 10.1016/s0168-8278(00)80420-1. [DOI] [PubMed] [Google Scholar]
- 2.Molina PE, McClain C, Valla D, et al. Molecular pathology and clinical aspects of alcohol-induced tissue injury. Alcohol Clin Exp Res. 2002;26(1):120–128. [PubMed] [Google Scholar]
- 3.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116(6):1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 4.McCullough AJ. Pathophysiology of nonalcoholic steatohepatitis. J Clin Gastroenterol. 2006;40(Suppl 1):S17–S29. doi: 10.1097/01.mcg.0000168645.86658.22. [DOI] [PubMed] [Google Scholar]
- 5.Neuschwander-Tetri BA. Fatty liver and the metabolic syndrome. Curr Opin Gastroenterol. 2007;23(2):193–198. doi: 10.1097/MOG.0b013e32801421a9. [DOI] [PubMed] [Google Scholar]
- 6.Feldstein AE, Canbay A, Angulo P, et al. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003;125(2):437–443. doi: 10.1016/s0016-5085(03)00907-7. [DOI] [PubMed] [Google Scholar]
- 7.Wieckowska A, Zein NN, Yerian LM, Lopez AR, McCullough AJ, Feldstein AE. In vivo assessment of liver cell apoptosis as a novel biomarker of disease severity in nonalcoholic fatty liver disease. Hepatology. 2006;44(1):27–33. doi: 10.1002/hep.21223. [DOI] [PubMed] [Google Scholar]
- 8.Feldstein AE, Papouchado BG, Angulo P, Sanderson S, Adams L, Gores GJ. Hepatic stellate cells and fibrosis progression in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2005;3(4):384–389. doi: 10.1016/s1542-3565(04)00616-0. [DOI] [PubMed] [Google Scholar]
- 9.Hubscher SG. Histological assessment of non-alcoholic fatty liver disease. Histopathology. 2006;49(5):450–465. doi: 10.1111/j.1365-2559.2006.02416.x. [DOI] [PubMed] [Google Scholar]
- 10.French SW, Nash J, Shitabata P, et al. Pathology of alcoholic liver disease VA Cooperative Study Group 119. Semin Liver Dis. 1993;13(2):154–169. doi: 10.1055/s-2007-1007346. [DOI] [PubMed] [Google Scholar]
- 11.Falck-Ytter Y, Younossi ZM, Marchesini G, McCullough AJ. Clinical features and natural history of nonalcoholic steatosis syndromes. Semin Liver Dis. 2001;21(1):17–26. doi: 10.1055/s-2001-12926. [DOI] [PubMed] [Google Scholar]
- 12.Richardson MM, Jonsson JR, Powell EE, et al. Progressive fibrosis in nonalcoholic steatohepatitis: association with altered regeneration and a ductular reaction. Gastroenterology. 2007;133(1):80–90. doi: 10.1053/j.gastro.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 13.Bugianesi E. Non-alcoholic steatohepatitis and cancer. Clin Liver Dis. 2007;11(1):191–207. doi: 10.1016/j.cld.2007.02.006. x-xi. [DOI] [PubMed] [Google Scholar]
- 14.Day CP, James OF. Steatohepatitis: a tale of two "hits"? Gastroenterology. 1998;114(4):842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 15.Day CP. Natural history of NAFLD: remarkably benign in the absence of cirrhosis. Gastroenterology. 2005;129(1):375–378. doi: 10.1053/j.gastro.2005.05.041. [DOI] [PubMed] [Google Scholar]
- 16.Adams LA, Lymp JF, St Sauver J, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129(1):113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 17.Sanyal AJ. Mechanisms of Disease: pathogenesis of nonalcoholic fatty liver disease. Nat Clin Pract Gastroenterol Hepatol. 2005;2(1):46–53. doi: 10.1038/ncpgasthep0084. [DOI] [PubMed] [Google Scholar]
- 18.Yang S, Koteish A, Lin H, et al. Oval cells compensate for damage and replicative senescence of mature hepatocytes in mice with fatty liver disease. Hepatology. 2004;39(2):403–411. doi: 10.1002/hep.20082. [DOI] [PubMed] [Google Scholar]
- 19.Roskams T, Yang SQ, Koteish A, et al. Oxidative stress and oval cell accumulation in mice and humans with alcoholic and nonalcoholic fatty liver disease. Am J Pathol. 2003;163(4):1301–1311. doi: 10.1016/S0002-9440(10)63489-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sozio M, Crabb DW. Alcohol and lipid metabolism. Am J Physiol Endocrinol Metab. 2008;295(1):E10–E16. doi: 10.1152/ajpendo.00011.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Musso G, Gambino R, Cassader M. Recent insights into hepatic lipid metabolism in non-alcoholic fatty liver disease (NAFLD) Prog Lipid Res. 2008 doi: 10.1016/j.plipres.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Yamaguchi K, Yang L, McCall S, et al. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology. 2007;45(6):1366–1374. doi: 10.1002/hep.21655. [DOI] [PubMed] [Google Scholar]
- 23.Klaus S. Adipose tissue as a regulator of energy balance. Curr Drug Targets. 2004;5(3):241–250. doi: 10.2174/1389450043490523. [DOI] [PubMed] [Google Scholar]
- 24.Jou J, Choi SS, Diehl AM. Mechanisms of disease progression in nonalcoholic fatty liver disease. Semin Liver Dis. 2008;28(4):370–379. doi: 10.1055/s-0028-1091981. [DOI] [PubMed] [Google Scholar]
- 25.Rouach H, Fataccioli V, Gentil M, French SW, Morimoto M, Nordmann R. Effect of chronic ethanol feeding on lipid peroxidation and protein oxidation in relation to liver pathology. Hepatology. 1997;25(2):351–355. doi: 10.1002/hep.510250216. [DOI] [PubMed] [Google Scholar]
- 26.Doege H, Baillie RA, Ortegon AM, et al. Targeted deletion of FATP5 reveals multiple functions in liver metabolism: alterations in hepatic lipid homeostasis. Gastroenterology. 2006;130(4):1245–1258. doi: 10.1053/j.gastro.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 27.Zhou J, Febbraio M, Wada T, et al. Hepatic fatty acid transporter Cd36 is a common target of LXR, PXR, and PPARgamma in promoting steatosis. Gastroenterology. 2008;134(2):556–567. doi: 10.1053/j.gastro.2007.11.037. [DOI] [PubMed] [Google Scholar]
- 28.Febbraio M, Abumrad NA, Hajjar DP, et al. A null mutation in murine CD36 reveals an important role in fatty acid and lipoprotein metabolism. J Biol Chem. 1999;274(27):19055–19062. doi: 10.1074/jbc.274.27.19055. [DOI] [PubMed] [Google Scholar]
- 29.Newberry EP, Xie Y, Kennedy S, et al. Decreased hepatic triglyceride accumulation and altered fatty acid uptake in mice with deletion of the liver fatty acid-binding protein gene. J Biol Chem. 2003;278(51):51664–51672. doi: 10.1074/jbc.M309377200. [DOI] [PubMed] [Google Scholar]
- 30.Newberry EP, Xie Y, Kennedy SM, Luo J, Davidson NO. Protection against Western diet-induced obesity and hepatic steatosis in liver fatty acid-binding protein knockout mice. Hepatology. 2006;44(5):1191–1205. doi: 10.1002/hep.21369. [DOI] [PubMed] [Google Scholar]
- 31.Greco D, Kotronen A, Westerbacka J, et al. Gene expression in human NAFLD. Am J Physiol Gastrointest Liver Physiol. 2008;294(5):G1281–G1287. doi: 10.1152/ajpgi.00074.2008. [DOI] [PubMed] [Google Scholar]
- 32.Arner P. The adipocyte in insulin resistance: key molecules and the impact of the thiazolidinediones. Trends Endocrinol Metab. 2003;14(3):137–145. doi: 10.1016/s1043-2760(03)00024-9. [DOI] [PubMed] [Google Scholar]
- 33.Xu A, Wang Y, Keshaw H, Xu LY, Lam KS, Cooper GJ. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest. 2003;112(1):91–100. doi: 10.1172/JCI17797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen G, Liang G, Ou J, Goldstein JL, Brown MS. Central role for liver X receptor in insulin-mediated activation of Srebp-1c transcription and stimulation of fatty acid synthesis in liver. Proc Natl Acad Sci U S A. 2004;101(31):11245–11250. doi: 10.1073/pnas.0404297101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peraldi P, Spiegelman B. TNF-alpha and insulin resistance: summary and future prospects. Mol Cell Biochem. 1998;182(1–2):169–175. [PubMed] [Google Scholar]
- 36.Hotamisligil GS. Mechanisms of TNF-alpha-induced insulin resistance. Exp Clin Endocrinol Diabetes. 1999;107(2):119–125. doi: 10.1055/s-0029-1212086. [DOI] [PubMed] [Google Scholar]
- 37.Ji C, Kaplowitz N. Betaine decreases hyperhomocysteinemia, endoplasmic reticulum stress, and liver injury in alcohol-fed mice. Gastroenterology. 2003;124(5):1488–1499. doi: 10.1016/s0016-5085(03)00276-2. [DOI] [PubMed] [Google Scholar]
- 38.Ji C, Chan C, Kaplowitz N. Predominant role of sterol response element binding proteins (SREBP) lipogenic pathways in hepatic steatosis in the murine intragastric ethanol feeding model. J Hepatol. 2006;45(5):717–724. doi: 10.1016/j.jhep.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 39.Esfandiari F, Villanueva JA, Wong DH, French SW, Halsted CH. Chronic ethanol feeding and folate deficiency activate hepatic endoplasmic reticulum stress pathway in micropigs. Am J Physiol Gastrointest Liver Physiol. 2005;289(1):G54–G63. doi: 10.1152/ajpgi.00542.2004. [DOI] [PubMed] [Google Scholar]
- 40.Chen HC, Smith SJ, Ladha Z, et al. Increased insulin and leptin sensitivity in mice lacking acyl CoA:diacylglycerol acyltransferase 1. J Clin Invest. 2002;109(8):1049–1055. doi: 10.1172/JCI14672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raabe M, Veniant MM, Sullivan MA, et al. Analysis of the role of microsomal triglyceride transfer protein in the liver of tissue-specific knockout mice. J Clin Invest. 1999;103(9):1287–1298. doi: 10.1172/JCI6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ji C, Shinohara M, Kuhlenkamp J, Chan C, Kaplowitz N. Mechanisms of protection by the betaine-homocysteine methyltransferase/betaine system in HepG2 cells and primary mouse hepatocytes. Hepatology. 2007;46(5):1586–1596. doi: 10.1002/hep.21854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mylonas C, Kouretas D. Lipid peroxidation and tissue damage. In Vivo. 1999;13(3):295–309. [PubMed] [Google Scholar]
- 44.Jaeschke H, Gores GJ, Cederbaum AI, Hinson JA, Pessayre D, Lemasters JJ. Mechanisms of hepatotoxicity. Toxicol Sci. 2002;65(2):166–176. doi: 10.1093/toxsci/65.2.166. [DOI] [PubMed] [Google Scholar]
- 45.Kaikaus RM, Chan WK, Lysenko N, Ray R, Ortiz de Montellano PR, Bass NM. Induction of peroxisomal fatty acid beta-oxidation and liver fatty acid-binding protein by peroxisome proliferators. Mediation via the cytochrome P-450IVA1 omega-hydroxylase pathway. J Biol Chem. 1993;268(13):9593–9603. [PubMed] [Google Scholar]
- 46.Schonfeld P, Wieckowski MR, Wojtczak L. Long-chain fatty acid-promoted swelling of mitochondria: further evidence for the protonophoric effect of fatty acids in the inner mitochondrial membrane. FEBS Lett. 2000;471(1):108–112. doi: 10.1016/s0014-5793(00)01376-4. [DOI] [PubMed] [Google Scholar]
- 47.Tang DG, La E, Kern J, Kehrer JP. Fatty acid oxidation and signaling in apoptosis. Biol Chem. 2002;383(3–4):425–442. doi: 10.1515/BC.2002.046. [DOI] [PubMed] [Google Scholar]
- 48.Bocher V, Pineda-Torra I, Fruchart JC, Staels B. PPARs: transcription factors controlling lipid and lipoprotein metabolism. Ann N Y Acad Sci. 2002;967:7–18. doi: 10.1111/j.1749-6632.2002.tb04258.x. [DOI] [PubMed] [Google Scholar]
- 49.Clarke SD, Gasperikova D, Nelson C, Lapillonne A, Heird WC. Fatty acid regulation of gene expression: a genomic explanation for the benefits of the mediterranean diet. Ann N Y Acad Sci. 2002;967:283–298. [PubMed] [Google Scholar]
- 50.Li Z, Berk M, McIntyre TM, Gores GJ, Feldstein AE. The lysosomal-mitochondrial axis in free fatty acid-induced hepatic lipotoxicity. Hepatology. 2008;47(5):1495–1503. doi: 10.1002/hep.22183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schaeffler A, Gross P, Buettner R, et al. Fatty acid-induced induction of Toll-like receptor-4/nuclear factor-kappaB pathway in adipocytes links nutritional signalling with innate immunity. Immunology. 2008 doi: 10.1111/j.1365-2567.2008.02892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reddy JK. Nonalcoholic steatosis, steatohepatitis. III Peroxisomal beta-oxidation, PPAR alpha, and steatohepatitis. Am J Physiol Gastrointest Liver Physiol. 2001;281(6):G1333–G1339. doi: 10.1152/ajpgi.2001.281.6.G1333. [DOI] [PubMed] [Google Scholar]
- 53.Macdonald GA, Prins JB. Peroxisomal fatty acid metabolism, peroxisomal proliferator-activated receptors and non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2004;19(12):1335–1337. doi: 10.1111/j.1440-1746.2004.03562.x. [DOI] [PubMed] [Google Scholar]
- 54.Reddy JK, Hashimoto T. Peroxisomal beta-oxidation and peroxisome proliferator-activated receptor alpha: an adaptive metabolic system. Annu Rev Nutr. 2001;21:193–230. doi: 10.1146/annurev.nutr.21.1.193. [DOI] [PubMed] [Google Scholar]
- 55.Ip E, Farrell G, Hall P, Robertson G, Leclercq I. Administration of the potent PPARalpha agonist, Wy-14,643, reverses nutritional fibrosis and steatohepatitis in mice. Hepatology. 2004;39(5):1286–1296. doi: 10.1002/hep.20170. [DOI] [PubMed] [Google Scholar]
- 56.Crabb DW, Galli A, Fischer M, You M. Molecular mechanisms of alcoholic fatty liver: role of peroxisome proliferator-activated receptor alpha. Alcohol. 2004;34(1):35–38. doi: 10.1016/j.alcohol.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 57.Nanji AA, Dannenberg AJ, Jokelainen K, Bass NM. Alcoholic liver injury in the rat is associated with reduced expression of peroxisome proliferator-alpha (PPARalpha)-regulated genes and is ameliorated by PPARalpha activation. J Pharmacol Exp Ther. 2004;310(1):417–424. doi: 10.1124/jpet.103.064717. [DOI] [PubMed] [Google Scholar]
- 58.Albano E. Oxidative mechanisms in the pathogenesis of alcoholic liver disease. Mol Aspects Med. 2008;29(1–2):9–16. doi: 10.1016/j.mam.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 59.Higuchi H, Adachi M, Miura S, Gores GJ, Ishii H. The mitochondrial permeability transition contributes to acute ethanol-induced apoptosis in rat hepatocytes. Hepatology. 2001;34(2):320–328. doi: 10.1053/jhep.2001.26380. [DOI] [PubMed] [Google Scholar]
- 60.Hruszkewycz AM, Bergtold DS. Oxygen radicals, lipid peroxidation and DNA damage in mitochondria. Basic Life Sci. 1988;49:449–456. doi: 10.1007/978-1-4684-5568-7_69. [DOI] [PubMed] [Google Scholar]
- 61.Caldwell SH, Swerdlow RH, Khan EM, et al. Mitochondrial abnormalities in non-alcoholic steatohepatitis. J Hepatol. 1999;31(3):430–434. doi: 10.1016/s0168-8278(99)80033-6. [DOI] [PubMed] [Google Scholar]
- 62.Mannaerts GP, Van Veldhoven PP, Casteels M. Peroxisomal lipid degradation via beta- and alpha-oxidation in mammals. Cell Biochem Biophys. 2000 Spring;32:73–87. doi: 10.1385/cbb:32:1-3:73. [DOI] [PubMed] [Google Scholar]
- 63.Hermesh O, Kalderon B, Bar-Tana J. Mitochondria uncoupling by a long chain fatty acyl analogue. J Biol Chem. 1998;273(7):3937–3942. doi: 10.1074/jbc.273.7.3937. [DOI] [PubMed] [Google Scholar]
- 64.Weltman MD, Farrell GC, Hall P, Ingelman-Sundberg M, Liddle C. Hepatic cytochrome P450 2E1 is increased in patients with nonalcoholic steatohepatitis. Hepatology. 1998;27(1):128–133. doi: 10.1002/hep.510270121. [DOI] [PubMed] [Google Scholar]
- 65.Chalasani N, Gorski JC, Asghar MS, et al. Hepatic cytochrome P450 2E1 activity in nondiabetic patients with nonalcoholic steatohepatitis. Hepatology. 2003;37(3):544–550. doi: 10.1053/jhep.2003.50095. [DOI] [PubMed] [Google Scholar]
- 66.Leclercq IA, Farrell GC, Field J, Bell DR, Gonzalez FJ, Robertson GR. CYP2E1 and CYP4A as microsomal catalysts of lipid peroxides in murine nonalcoholic steatohepatitis. J Clin Invest. 2000;105(8):1067–1075. doi: 10.1172/JCI8814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Garcia-Ruiz I, Rodriguez-Juan C, Diaz-Sanjuan T, et al. Uric acid and anti-TNF antibody improve mitochondrial dysfunction in ob/ob mice. Hepatology. 2006;44(3):581–591. doi: 10.1002/hep.21313. [DOI] [PubMed] [Google Scholar]
- 68.Ardies CM, Lasker JM, Lieber CS. Characterization of the cytochrome P-450 monooxygenase system of hamster liver microsomes Effects of prior treatment with ethanol and other xenobiotics. Biochem Pharmacol. 1987;36(21):3613–3619. doi: 10.1016/0006-2952(87)90010-4. [DOI] [PubMed] [Google Scholar]
- 69.Castillo T, Koop DR, Kamimura S, Triadafilopoulos G, Tsukamoto H. Role of cytochrome P-450 2E1 in ethanol-, carbon tetrachloride- and iron-dependent microsomal lipid peroxidation. Hepatology. 1992;16(4):992–996. doi: 10.1002/hep.1840160423. [DOI] [PubMed] [Google Scholar]
- 70.Thiele GM, Freeman TL, Klassen LW. Immunologic mechanisms of alcoholic liver injury. Semin Liver Dis. 2004;24(3):273–287. doi: 10.1055/s-2004-832940. [DOI] [PubMed] [Google Scholar]
- 71.Garcia-Ruiz C, Morales A, Ballesta A, Rodes J, Kaplowitz N, Fernandez-Checa JC. Effect of chronic ethanol feeding on glutathione and functional integrity of mitochondria in periportal and perivenous rat hepatocytes. J Clin Invest. 1994;94(1):193–201. doi: 10.1172/JCI117306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Garcia-Ruiz C, Morales A, Colell A, et al. Feeding S-adenosyl-L-methionine attenuates both ethanol-induced depletion of mitochondrial glutathione and mitochondrial dysfunction in periportal and perivenous rat hepatocytes. Hepatology. 1995;21(1):207–214. doi: 10.1002/hep.1840210133. [DOI] [PubMed] [Google Scholar]
- 73.Mato JM, Camara J, Fernandez de Paz J, et al. S-adenosylmethionine in alcoholic liver cirrhosis: a randomized, placebo-controlled, double-blind, multicenter clinical trial. J Hepatol. 1999;30(6):1081–1089. doi: 10.1016/s0168-8278(99)80263-3. [DOI] [PubMed] [Google Scholar]
- 74.Garcia-Ruiz C, Colell A, Morales A, Kaplowitz N, Fernandez-Checa JC. Role of oxidative stress generated from the mitochondrial electron transport chain and mitochondrial glutathione status in loss of mitochondrial function and activation of transcription factor nuclear factor-kappa B: studies with isolated mitochondria and rat hepatocytes. Mol Pharmacol. 1995;48(5):825–834. [PubMed] [Google Scholar]
- 75.Mansouri A, Fromenty B, Berson A, et al. Multiple hepatic mitochondrial DNA deletions suggest premature oxidative aging in alcoholic patients. J Hepatol. 1997;27(1):96–102. doi: 10.1016/s0168-8278(97)80286-3. [DOI] [PubMed] [Google Scholar]
- 76.Rolla R, Vay D, Mottaran E, et al. Detection of circulating antibodies against malondialdehyde-acetaldehyde adducts in patients with alcohol-induced liver disease. Hepatology. 2000;31(4):878–884. doi: 10.1053/he.2000.5373. [DOI] [PubMed] [Google Scholar]
- 77.Cao Q, Mak KM, Lieber CS. Cytochrome P4502E1 primes macrophages to increase TNF-alpha production in response to lipopolysaccharide. Am J Physiol Gastrointest Liver Physiol. 2005;289(1):G95–G107. doi: 10.1152/ajpgi.00383.2004. [DOI] [PubMed] [Google Scholar]
- 78.Boden G, She P, Mozzoli M, et al. Free fatty acids produce insulin resistance and activate the proinflammatory nuclear factor-kappaB pathway in rat liver. Diabetes. 2005;54(12):3458–3465. doi: 10.2337/diabetes.54.12.3458. [DOI] [PubMed] [Google Scholar]
- 79.Wheeler MD, Kono H, Yin M, et al. The role of Kupffer cell oxidant production in early ethanol-induced liver disease. Free Radic Biol Med. 2001;31(12):1544–1549. doi: 10.1016/s0891-5849(01)00748-1. [DOI] [PubMed] [Google Scholar]
- 80.Thakur V, Pritchard MT, McMullen MR, Wang Q, Nagy LE. Chronic ethanol feeding increases activation of NADPH oxidase by lipopolysaccharide in rat Kupffer cells: role of increased reactive oxygen in LPS-stimulated ERK1/2 activation and TNF-alpha production. J Leukoc Biol. 2006;79(6):1348–1356. doi: 10.1189/jlb.1005613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kono H, Rusyn I, Yin M, et al. NADPH oxidase-derived free radicals are key oxidants in alcohol-induced liver disease. J Clin Invest. 2000;106(7):867–872. doi: 10.1172/JCI9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Magnusson C, Vaux DL. Signalling by CD95 and TNF receptors: not only life and death. Immunol Cell Biol. 1999;77(1):41–46. doi: 10.1046/j.1440-1711.1999.00800.x. [DOI] [PubMed] [Google Scholar]
- 83.Leist M, Gantner F, Bohlinger I, Germann PG, Tiegs G, Wendel A. Murine hepatocyte apoptosis induced in vitro and in vivo by TNF-alpha requires transcriptional arrest. J Immunol. 1994;153(4):1778–1788. [PubMed] [Google Scholar]
- 84.Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336(15):1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 85.Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275(4):2247–2250. doi: 10.1074/jbc.275.4.2247. [DOI] [PubMed] [Google Scholar]
- 86.Brenner DA, O’Hara M, Angel P, Chojkier M, Karin M. Prolonged activation of jun and collagenase genes by tumour necrosis factor-alpha. Nature. 1989;337(6208):661–663. doi: 10.1038/337661a0. [DOI] [PubMed] [Google Scholar]
- 87.Lieber CS, Leo MA, Cao Q, et al. The Combination of S-adenosylmethionine and Dilinoleoylphosphatidylcholine Attenuates Non-alcoholic Steatohepatitis Produced in Rats by a High-Fat Diet. Nutr Res. 2007;27(9):565–573. doi: 10.1016/j.nutres.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Feo F, Pascale R, Garcea R, et al. Effect of the variations of S-adenosyl-L-methionine liver content on fat accumulation and ethanol metabolism in ethanol-intoxicated rats. Toxicol Appl Pharmacol. 1986;83(2):331–341. doi: 10.1016/0041-008x(86)90310-8. [DOI] [PubMed] [Google Scholar]
- 89.Stewart S, Prince M, Bassendine M, et al. A randomized trial of antioxidant therapy alone or with corticosteroids in acute alcoholic hepatitis. J Hepatol. 2007;47(2):277–283. doi: 10.1016/j.jhep.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 90.Nanji AA, Yang EK, Fogt F, Sadrzadeh SM, Dannenberg AJ. Medium chain triglycerides and vitamin E reduce the severity of established experimental alcoholic liver disease. J Pharmacol Exp Ther. 1996;277(3):1694–1700. [PubMed] [Google Scholar]
- 91.Choi SS, Sicklick JK, Ma Q, et al. Sustained activation of Rac1 in hepatic stellate cells promotes liver injury and fibrosis in mice. Hepatology. 2006;44(5):1267–1277. doi: 10.1002/hep.21375. [DOI] [PubMed] [Google Scholar]
- 92.Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 1999;13(10):1211–1233. doi: 10.1101/gad.13.10.1211. [DOI] [PubMed] [Google Scholar]
- 93.Kaufman RJ. Orchestrating the unfolded protein response in health and disease. J Clin Invest. 2002;110(10):1389–1398. doi: 10.1172/JCI16886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rutkowski DT, Kaufman RJ. A trip to the ER: coping with stress. Trends Cell Biol. 2004;14(1):20–28. doi: 10.1016/j.tcb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 95.Gentile CL, Pagliassotti MJ. The role of fatty acids in the development and progression of nonalcoholic fatty liver disease. J Nutr Biochem. 2008;19(9):567–576. doi: 10.1016/j.jnutbio.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Feng B, Yao PM, Li Y, et al. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat Cell Biol. 2003;5(9):781–792. doi: 10.1038/ncb1035. [DOI] [PubMed] [Google Scholar]
- 97.Bird GL, Sheron N, Goka AK, Alexander GJ, Williams RS. Increased plasma tumor necrosis factor in severe alcoholic hepatitis. Ann Intern Med. 1990;112(12):917–920. doi: 10.7326/0003-4819-112-12-917. [DOI] [PubMed] [Google Scholar]
- 98.Tilg H, Diehl AM. Cytokines in alcoholic and nonalcoholic steatohepatitis. N Engl J Med. 2000;343(20):1467–1476. doi: 10.1056/NEJM200011163432007. [DOI] [PubMed] [Google Scholar]
- 99.Iimuro Y, Gallucci RM, Luster MI, Kono H, Thurman RG. Antibodies to tumor necrosis factor alfa attenuate hepatic necrosis and inflammation caused by chronic exposure to ethanol in the rat. Hepatology. 1997;26(6):1530–1537. doi: 10.1002/hep.510260621. [DOI] [PubMed] [Google Scholar]
- 100.Nanji AA, Khettry U, Sadrzadeh SM. Lactobacillus feeding reduces endotoxemia and severity of experimental alcoholic liver (disease) Proc Soc Exp Biol Med. 1994;205(3):243–247. doi: 10.3181/00379727-205-43703. [DOI] [PubMed] [Google Scholar]
- 101.Adachi Y, Moore LE, Bradford BU, Gao W, Thurman RG. Antibiotics prevent liver injury in rats following long-term exposure to ethanol. Gastroenterology. 1995;108(1):218–224. doi: 10.1016/0016-5085(95)90027-6. [DOI] [PubMed] [Google Scholar]
- 102.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6(10):772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 103.Lancaster JR, Jr., Laster SM, Gooding LR. Inhibition of target cell mitochondrial electron transfer by tumor necrosis factor. FEBS Lett. 1989;248(1–2):169–174. doi: 10.1016/0014-5793(89)80454-5. [DOI] [PubMed] [Google Scholar]
- 104.Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440(7086):944–948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- 105.Fox-Robichaud A, Kubes P. Molecular mechanisms of tumor necrosis factor alpha-stimulated leukocyte recruitment into the murine hepatic circulation. Hepatology. 2000;31(5):1123–1127. doi: 10.1053/he.2000.6961. [DOI] [PubMed] [Google Scholar]
- 106.Boetticher NC, Peine CJ, Kwo P, et al. A Randomized, Double-Blinded, Placebo-Controlled Multicenter Trial of Etanercept in the Treatment of Alcoholic Hepatitis. Gastroenterology. 2008 doi: 10.1053/j.gastro.2008.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Naveau S, Chollet-Martin S, Dharancy S, et al. A double-blind randomized controlled trial of infliximab associated with prednisolone in acute alcoholic hepatitis. Hepatology. 2004;39(5):1390–1397. doi: 10.1002/hep.20206. [DOI] [PubMed] [Google Scholar]
- 108.Akriviadis E, Botla R, Briggs W, Han S, Reynolds T, Shakil O. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119(6):1637–1648. doi: 10.1053/gast.2000.20189. [DOI] [PubMed] [Google Scholar]
- 109.Adams LA, Zein CO, Angulo P, Lindor KD. A pilot trial of pentoxifylline in nonalcoholic steatohepatitis. Am J Gastroenterol. 2004;99(12):2365–2368. doi: 10.1111/j.1572-0241.2004.40064.x. [DOI] [PubMed] [Google Scholar]
- 110.Fernandes JL, de Oliveira RT, Mamoni RL, et al. Pentoxifylline reduces pro-inflammatory and increases anti-inflammatory activity in patients with coronary artery disease--a randomized placebo-controlled study. Atherosclerosis. 2008;196(1):434–442. doi: 10.1016/j.atherosclerosis.2006.11.032. [DOI] [PubMed] [Google Scholar]
- 111.Houglum K, Buck M, Adir V, Chojkier M. LAP (NF-IL6) transactivates the collagen alpha 1(I) gene from a 5’ regulatory region. J Clin Invest. 1994;94(2):808–814. doi: 10.1172/JCI117400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ramond MJ, Poynard T, Rueff B, et al. A randomized trial of prednisolone in patients with severe alcoholic hepatitis. N Engl J Med. 1992;326(8):507–512. doi: 10.1056/NEJM199202203260802. [DOI] [PubMed] [Google Scholar]
- 113.Mathurin P, Mendenhall CL, Carithers RL, Jr., et al. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis (AH): individual data analysis of the last three randomized placebo controlled double blind trials of corticosteroids in severe AH. J Hepatol. 2002;36(4):480–487. doi: 10.1016/s0168-8278(01)00289-6. [DOI] [PubMed] [Google Scholar]
- 114.Aithal GP, Thomas JA, Kaye PV, et al. Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology. 2008;135(4):1176–1184. doi: 10.1053/j.gastro.2008.06.047. [DOI] [PubMed] [Google Scholar]
- 115.Belfort R, Harrison SA, Brown K, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355(22):2297–2307. doi: 10.1056/NEJMoa060326. [DOI] [PubMed] [Google Scholar]
- 116.Nakano S, Nagasawa T, Ijiro T, et al. Bezafibrate prevents hepatic stellate cell activation and fibrogenesis in a murine steatohepatitis model, and suppresses fibrogenic response induced by transforming growth factor-beta1 in a cultured stellate cell line. Hepatol Res. 2008;38(10):1026–1039. doi: 10.1111/j.1872-034X.2008.00363.x. [DOI] [PubMed] [Google Scholar]
- 117.Bergheim I, Guo L, Davis MA, et al. Metformin prevents alcohol-induced liver injury in the mouse: Critical role of plasminogen activator inhibitor-1. Gastroenterology. 2006;130(7):2099–2112. doi: 10.1053/j.gastro.2006.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Marchesini G, Brizi M, Bianchi G, Tomassetti S, Zoli M, Melchionda N. Metformin in non-alcoholic steatohepatitis. Lancet. 2001;358(9285):893–894. doi: 10.1016/s0140-6736(01)06042-1. [DOI] [PubMed] [Google Scholar]
- 119.Zhou G, Myers R, Li Y, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108(8):1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Caligiuri A, Bertolani C, Guerra CT, et al. Adenosine monophosphate-activated protein kinase modulates the activated phenotype of hepatic stellate cells. Hepatology. 2008;47(2):668–676. doi: 10.1002/hep.21995. [DOI] [PubMed] [Google Scholar]
- 121.Dong JM, Leung T, Manser E, Lim L. cAMP-induced morphological changes are counteracted by the activated RhoA small GTPase and the Rho kinase ROKalpha. J Biol Chem. 1998;273(35):22554–22562. doi: 10.1074/jbc.273.35.22554. [DOI] [PubMed] [Google Scholar]
- 122.Lalor PF, Faint J, Aarbodem Y, Hubscher SG, Adams DH. The role of cytokines and chemokines in the development of steatohepatitis. Semin Liver Dis. 2007;27(2):173–193. doi: 10.1055/s-2007-979470. [DOI] [PubMed] [Google Scholar]
- 123.Minokoshi Y, Kim YB, Peroni OD, et al. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415(6869):339–343. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- 124.Guebre-Xabier M, Yang S, Lin HZ, Schwenk R, Krzych U, Diehl AM. Altered hepatic lymphocyte subpopulations in obesity-related murine fatty livers: potential mechanism for sensitization to liver damage. Hepatology. 2000;31(3):633–640. doi: 10.1002/hep.510310313. [DOI] [PubMed] [Google Scholar]
- 125.Saxena NK, Ikeda K, Rockey DC, Friedman SL, Anania FA. Leptin in hepatic fibrosis: evidence for increased collagen production in stellate cells and lean littermates of ob/ob mice. Hepatology. 2002;35(4):762–771. doi: 10.1053/jhep.2002.32029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ikejima K, Takei Y, Honda H, et al. Leptin receptor-mediated signaling regulates hepatic fibrogenesis and remodeling of extracellular matrix in the rat. Gastroenterology. 2002;122(5):1399–1410. doi: 10.1053/gast.2002.32995. [DOI] [PubMed] [Google Scholar]
- 127.Kamada Y, Tamura S, Kiso S, et al. Enhanced carbon tetrachloride-induced liver fibrosis in mice lacking adiponectin. Gastroenterology. 2003;125(6):1796–1807. doi: 10.1053/j.gastro.2003.08.029. [DOI] [PubMed] [Google Scholar]
- 128.Bjarnason I, Peters TJ, Wise RJ. The leaky gut of alcoholism: possible route of entry for toxic compounds. Lancet. 1984;1(8370):179–182. doi: 10.1016/s0140-6736(84)92109-3. [DOI] [PubMed] [Google Scholar]
- 129.Mathurin P, Deng QG, Keshavarzian A, Choudhary S, Holmes EW, Tsukamoto H. Exacerbation of alcoholic liver injury by enteral endotoxin in rats. Hepatology. 2000;32(5):1008–1017. doi: 10.1053/jhep.2000.19621. [DOI] [PubMed] [Google Scholar]
- 130.Cani PD, Bibiloni R, Knauf C, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57(6):1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 131.Farhadi A, Gundlapalli S, Shaikh M, et al. Susceptibility to gut leakiness: a possible mechanism for endotoxaemia in non-alcoholic steatohepatitis. Liver Int. 2008;28(7):1026–1033. doi: 10.1111/j.1478-3231.2008.01723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bode C, Kugler V, Bode JC. Endotoxemia in patients with alcoholic and non-alcoholic cirrhosis and in subjects with no evidence of chronic liver disease following acute alcohol excess. J Hepatol. 1987;4(1):8–14. doi: 10.1016/s0168-8278(87)80003-x. [DOI] [PubMed] [Google Scholar]
- 133.Bhagwandeen BS, Apte M, Manwarring L, Dickeson J. Endotoxin induced hepatic necrosis in rats on an alcohol diet. J Pathol. 1987;152(1):47–53. doi: 10.1002/path.1711520107. [DOI] [PubMed] [Google Scholar]
- 134.Yin M, Bradford BU, Wheeler MD, et al. Reduced early alcohol-induced liver injury in CD14-deficient mice. J Immunol. 2001;166(7):4737–4742. doi: 10.4049/jimmunol.166.7.4737. [DOI] [PubMed] [Google Scholar]
- 135.Uesugi T, Froh M, Arteel GE, Bradford BU, Thurman RG. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. Hepatology. 2001;34(1):101–108. doi: 10.1053/jhep.2001.25350. [DOI] [PubMed] [Google Scholar]
- 136.Li Z, Yang S, Lin H, et al. Probiotics and antibodies to TNF inhibit inflammatory activity and improve nonalcoholic fatty liver disease. Hepatology. 2003;37(2):343–350. doi: 10.1053/jhep.2003.50048. [DOI] [PubMed] [Google Scholar]
- 137.Pappo I, Bercovier H, Berry EM, Haviv Y, Gallily R, Freund HR. Polymyxin B reduces total parenteral nutrition-associated hepatic steatosis by its antibacterial activity and by blocking deleterious effects of lipopolysaccharide. JPEN J Parenter Enteral Nutr. 1992;16(6):529–532. doi: 10.1177/0148607192016006529. [DOI] [PubMed] [Google Scholar]
- 138.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 139.Hritz I, Mandrekar P, Velayudham A, et al. The critical role of toll-like receptor (TLR) 4 in alcoholic liver disease is independent of the common TLR adapter MyD88. Hepatology. 2008;48(4):1224–1231. doi: 10.1002/hep.22470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Liu S, Gallo DJ, Green AM, et al. Role of toll-like receptors in changes in gene expression and NF-kappa B activation in mouse hepatocytes stimulated with lipopolysaccharide. Infect Immun. 2002;70(7):3433–3442. doi: 10.1128/IAI.70.7.3433-3442.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Paik YH, Schwabe RF, Bataller R, Russo MP, Jobin C, Brenner DA. Toll-like receptor 4 mediates inflammatory signaling by bacterial lipopolysaccharide in human hepatic stellate cells. Hepatology. 2003;37(5):1043–1055. doi: 10.1053/jhep.2003.50182. [DOI] [PubMed] [Google Scholar]
- 142.Cope K, Risby T, Diehl AM. Increased gastrointestinal ethanol production in obese mice: implications for fatty liver disease pathogenesis. Gastroenterology. 2000;119(5):1340–1347. doi: 10.1053/gast.2000.19267. [DOI] [PubMed] [Google Scholar]
- 143.Nair S, Cope K, Risby TH, Diehl AM. Obesity and female gender increase breath ethanol concentration: potential implications for the pathogenesis of nonalcoholic steatohepatitis. Am J Gastroenterol. 2001;96(4):1200–1204. doi: 10.1111/j.1572-0241.2001.03702.x. [DOI] [PubMed] [Google Scholar]
- 144.Svegliati-Baroni G, Inagaki Y, Rincon-Sanchez AR, et al. Early response of alpha2(I) collagen to acetaldehyde in human hepatic stellate cells is TGF-beta independent. Hepatology. 2005;42(2):343–352. doi: 10.1002/hep.20798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Potter JJ, Rennie-Tankersley L, Mezey E. Leptin deficiency prevents the activation of the murine alpaha 2(I) collagen promoter by acetaldehyde. Arch Biochem Biophys. 2004;426(1):73–77. doi: 10.1016/j.abb.2004.03.031. [DOI] [PubMed] [Google Scholar]
- 146.Szczepiorkowski ZM, Dickersin GR, Laposata M. Fatty acid ethyl esters decrease human hepatoblastoma cell proliferation and protein synthesis. Gastroenterology. 1995;108(2):515–522. doi: 10.1016/0016-5085(95)90081-0. [DOI] [PubMed] [Google Scholar]