Abstract
Enteroviral infection in pregnancy is common and there is growing evidence relating it to congenital anomalies and neonatal mortality. Neonatal disease may range from unapparent infection to overwhelming systemic illness. Passively acquired maternal serotype specific antibodies determine the severity of the disease in the newborn. A fatal case of congenital echovirus 21 infection, confirmed by PCR in the patient's blood and positive culture of the mother's stools, is reported. A sibling had symptoms of respiratory tract infection and their mother had fever, which prompted iatrogenic delivery that same day. The newborn presented with bradycardia and hypotonia in the first minutes of life and later developed respiratory distress, disseminated intravascular coagulopathy, fulminant hepatitis, acute renal failure and necrotising enterocolitis. Death occurred on the 8 day of life. This case highlights the potential severity of Enteroviral infection in the newborn. Since only supportive treatment is available, prevention is paramount.
Background
Enteroviral (EV) infections late in pregnancy are common and most of them are not associated with significant maternal or neonatal disease. However, there is growing evidence that relates maternal EV infection to increased neonatal mortality and congenital anomalies.1–9
Vertical transmission of enteroviruses is relatively common and may occur through contact with blood or maternal secretions during vaginal delivery or upper respiratory tract secretions. Transplacental viraemia before delivery has been occasionally reported.1–5 8
Neonatal infection may range from clinically asymptomatic to overwhelming systemic illness and death or long-term sequelae. Common clinical syndromes associated with neonatal EV infections are meningoencephalitis, pneumonia, myocarditis and hepatitis.1–5 8
The severity of perinatally acquired infection is influenced by several factors, including the virus strain, the mode of transmission and the presence of passively acquired serotype-specific maternal antibodies, the later playing the most important role.1 2 8
The exceptional feature of this case is the unfortunate timing of the delivery, targeting an immunological gap that allowed for the virus to cause mild disease in the mother and sibling but overwhelming hepatic failure in the neonate. It also highlights the need for timely differential diagnosis between treatable and untreatable causes of severe neonatal hepatic disease.
Case presentation
A male newborn was admitted to the neonatal intensive care unit (NICU) in the fifth day of life.
An older sibiling showed signs of upper respiratory tract infection a few days earlier and their mother had fever and begun spontaneous labour at 37 weeks of gestation. Given the hypothesis of on-going chorioamnionitis, an elective caesarean section was performed. The Apgar Scores were 8, 8 and 9 at 1, 5 and 10 min respectively, but the newborn presented with bradycardia and hypotonia in the first minutes of life, with good response to oxygen supplementation. On the second day, tachypnea persisted and he was assisted with nasal continuous positive airway pressure (CPAP) for 24 h, after which he seemed clinically stable and was started on oral feeding. On the third day, he became icteric and had body temperature of 38°C. Poor peripheral perfusion, hypotonia and feeding intolerance were evident on the fourth day, and at the fifth day he was transferred to a tertiary level NCU.
On admission, he presented poor peripheral perfusion and pallor. Slight icterus, haemorrhagic diastasis (purpuric rash and active haemorrhage from venous punctures, gastric tube and urine) and hepatomegaly (6–7 cm below the rib cage) were also evident. Finally, he was hypo reactive, had diminished reflexes and discrete dystonic hand movements.
As neither viral nor bacterial sepsis could be excluded, wide spectrum triple antibiotic therapy was started. Laboratory evaluation revealed lactic acidaemia, anaemia, thrombocytopenia, hypofibrinogenaemia and high ferritin, leading to the hypothesis of haemophagocytic syndrome. Faced with neurological signs and progressive renal insufficiency, thrombotic thrombocytopenic purpura was also addressed. Moreover, mitochondrial disease and haemochromatosis had to be excluded (Vide Differencial Diagnosis).
The clinical, analytical and radiological findings on the following 2 days indicated further deterioration of hepatic and renal function despite antibiotics, immunoglobulin and intensive support therapy. Disseminated intravascular coagulopathy developed as a consequence of irreversible hepatic failure, with uncontrollable haemorrhagic diastasis, subcortical cerebral haemorragic infarctions, necrotising enterocolitis and anuric renal failure. On the 8 day of life, he suffered a cardiac arrest irresponsive to resuscitation.
The parents did not consent to the autopsy. However, they gave consent to postmortem hepatic, cutaneous and muscular biopsies. The former revealed pan-lobular haemorrhagic hepatic necrosis, suggestive of EV infection4 (figures 1 and 2).
Figure 1.
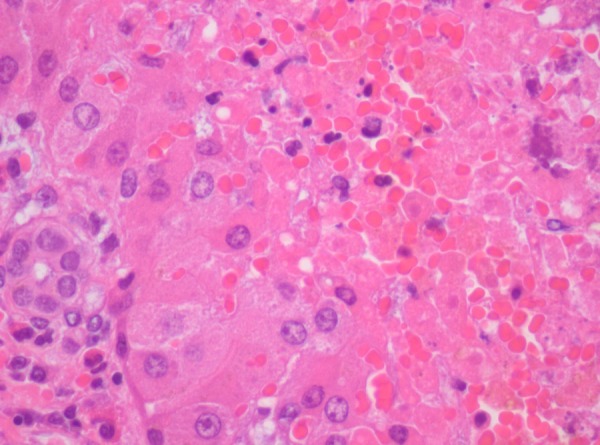
Liver biopsy histology, haematoxylin colouration: panlobular necrosis of the haemorrhagic type. There is residual periportal parenchima, with associated focal haematopoiesis.
Figure 2.
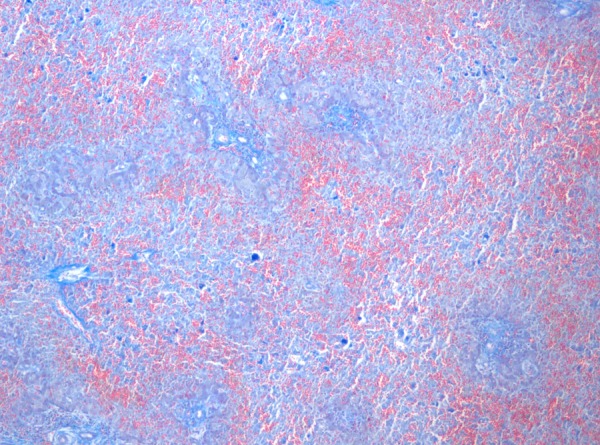
Liver biopsy histology, aniline blue chromotrope colouration: erythrocytes are red coloured and show the severe haemorrhage of the liver.
Investigations
General evaluation: Progressive anaemia (minimum Hb 5.8 g/dl), thrombocytopenia (minimum 16 000/µl), hepatic insufficiency (maximum aspartate aminotransferase of 9005 U/l, alanine transaminase 1015 U/l, ferritin 146 240 ng/ml), haemorrhagic diastasis (maximum activated partial thromboplastin time (aPTT) 109 s, international normalised ratio (INR) 5.7, D-dimers 41 744 mcg/l, fibrinogen (0.8 g/l) and renal failure (creatine 1.83 mg/dl).
Chest radiogram and echocardiogram were normal. The abdominal ultrasound at admission in the NICU showed enlarged liver with granular parenchyma and free peritoneal fluid in small quantity. On the eighth day, it showed vascular intestinal (sigmoid) compromise with abundant ascites, suggesting necrotising enterocolitis, confirmed on MRI. Body MRI showed no abnormal iron deposits.
Cranial ultrasound showed thalamo-striate vasculitis and diffuse subcortical hyperecogenicity. EEG revealed sporadic bitemporal abrupt waves.
Aetiological evaluation: positive reverse-transcription PCR for enterovirus in the newborn's blood and culture of the mother's stools. Serotype identification of Echovirus 21. The remaining studies, including PCR for CMV and herpes virus, ADAMTS-13 (protease enzyme), soluble CD25 perforins (pore-forming proteins) study, lymphocytic populations, alfa1-antitrispsin and metabolic screening were normal. Cutaneous and muscular biopsies were not analysed because the final diagnosis was achieved in the meanwhile.
Differential diagnosis
Several diagnostic hypothesis had to be considered to rule out treatable causes as rapidly as possible.
Bacterial sepsis was a possibility and antibiotics were started, although indirect inflammatory parameters were only slightly elevated and blood cultures were sterile.
Several findings lead to the hypothesis of haemophagocytic syndrome, mainly the simultaneous presence of anaemia, thrombocytopenia, hypofibrinogenaemia and elevated ferritin; it usually responds to aggressive treatment with immunomodulators. However, plasma triglycerides, CD25 and perforins were normal. Bone marrow aspirate was not performed due to haemodynamic instability.
Given the neurological compromise associated to progressive renal insufficiency, thrombotic thrombocytopenic purpura was also considered but ADAMTS 13 antigen and activity were normal.
Due to very elevated ferritin, MRI was done to exclude haemochromatosis. This exclusion was important for its implications in future pregnancies.
Mitochondrial disease was early dismissed because lactic acidosis was transient and ammonia was only moderately elevated. Metabolic screening revealed normal results. Nevertheless, cofactors were started on admission.
Disseminated viral infection had to be assessed, given the familiar context of upper respiratory tract infection, slightly elevated inflammatory parameters, hepatic disease and thalamic vasculitis. This was the reason for immunoglobulin perfusion. PCR for Enterovirus was positive. PCR for herpes virus and cytomegalovirus (CMV) were negative.
Treatment
The infant was on escalating life-support, including: respiratory support with oxygen supplementation, nasal CPAP, moving to invasive mechanical ventilation with sedation (fentanyl); renal support with bicarbonate, furosemide, albumin and aminophylline perfusions; haemodynamic support with dopamine and dobutamine, erythrocyte and platelet concentrates, frozen plasma and cryoprecipitate transfusions.
Therapy was tried with ampicillin, cefotaxime (in meningitis dosages) and gentamicin, hydrocortisone and immunoglobulin.
Metabolic disease empirical therapy was started: carnitine, thiamine, pyridoxine, vitamin B12 and coenzyme Q10.
Discussion
This case points out the potential severity for pregnant women and neonates of otherwise usually mild community-acquired infections. Enterovirus is frequently linked to community outbreaks. For this reason, infection may occur in late pregnancy. To prevent its transmission to the neonate, it is fundamental to keep a high index of suspicion in face of a case of maternal fever followed by neonatal pneumonia, myocarditis and/or hepatitis, with slightly elevated C reactive protein and progressive deterioration despite antibiotic therapy.1 3
If the lack of laboratorial evidence of maternal bacterial infection and the signs of fetal wellbeing had been taken into account, maybe the delivery would not have been anticipated. If the pregnancy had have lasted a few more days, the transplacental passage of maternal specific antibodies would probably have taken place and helped in the control of fetal infection.1 2 8
Although several cases of severe neonatal EV infection have been reported, few references to Echovirus 21 are found in the literature. Echovirus 11 and Coxsackievirus B are the serotypes most frequently associated with fulminant hepatitis.6 8 Few reported cases have documented horizontal intrafamilial transmission leading to vertical mother-to-child transmission of Enterovirus. In this case, transplacental viraemia is likely to have occurred, since the symptoms began immediately after birth.4 6 7
There are currently no approved antivirals for EV infection. Large doses of immunoglobulin have been used in these situations but its effectiveness has not yet been completely proven. Pleconaril appeared as a promising drug for the treatment of EV infection but its efficacy has also not been definitively established. Presently, hygienic measures of prevention of the infection late in pregnancy and avoidance of unnecessary, deleterious iatrogenic delivery are mandatory.2 7 8–11
Learning points.
Echovirus infection in the newborn can lead to overwhelming hepatic disease and death.
Diagnosis requires a high level of suspicion and rapid exclusion of treatable causes of hepatic failure.
There is no specific treatment to Enterovirus infection.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Farcy C, Mirand A, Marque JS, et al. Enterovirus nosocomial infections in a neonatal care unit: From diagnosis to evidence, from a clinical observation of a central nervous system infection. Arch Pediatr 2012;2013:921–6 [DOI] [PubMed] [Google Scholar]
- 2.Tebruegge M, Curtis N. Enterovirus infections in neonates. Semin Fetal Neonatal Med 2009;2013:222–7 [DOI] [PubMed] [Google Scholar]
- 3.Verboon-Maciolek MA, Krediet TG, Gerards LJ, et al. Severe neonatal parechovirus infection and similarity with enterovirus infection. Pediatr Infect Dis J 2008;2013:241–5 [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention Increased detections and severe neonatal disease associated with coxsackievirus B1 infection—United States, 2007. Morb Mortal Wkly Rep 2008;2013:553–6 [PubMed] [Google Scholar]
- 5.Cheng LL, Ng PC, Chan PK, et al. Probable intra-familial transmission of coxsackievirus B3 with vertical transmission, severe early-onset neonatal hepatitis, and prolonged viral RNA shedding. Pediatrics 2006;2013:e929–33 [DOI] [PubMed] [Google Scholar]
- 6.Khetsuriani N, Lamonte A, Oberste MS, et al. Neonatal enterovirus infections reported to the national enterovirus surveillance system in the United States, 1983–2003. Pediatr Infect Dis J 2006;2013:889–93 [DOI] [PubMed] [Google Scholar]
- 7.Rentz AC, Libbey JE, Fujinami RS, et al. Investigation of treatment failure in neonatal echovirus 7 infection. Pediatr Infect Dis J 2006;2013:259–62 [DOI] [PubMed] [Google Scholar]
- 8.Bendig JW, Franklin OM, Hebden AK, et al. Coxsackievirus B3 sequences in the blood of a neonate with congenital myocarditis, plus serological evidence of maternal infection. J Med Virol 2003;2013:606–9 [DOI] [PubMed] [Google Scholar]
- 9.Brunetti L, DeSantis ER. Treatment of viral myocarditis caused by coxsackievirus B. Am J Health Syst Pharm 2008;2013:132–7 [DOI] [PubMed] [Google Scholar]
- 10.Desmond RA, Accortt NA, Talley L, et al. Enteroviral meningitis: natural history and outcome of pleconaril therapy. Antimicrob Agents Chemother 2006;2013:2409–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goland S, Czer LS, Siegel RJ, et al. Intravenous immunoglobulin treatment for acute fulminant inflammatory cardiomyopathy: series of six patients and review of literature. Can J Cardiol 2008;2013:571–4 [DOI] [PMC free article] [PubMed] [Google Scholar]


