Abstract
The purpose of this study was to determine the potential of platelet-rich fibrin (PRF) membranes used for guided bone and tissue regeneration. A patient with insufficient alveolar ridge width in aesthetic zone was enrolled. The patient's blood was centrifuged to obtain PRF membranes. Autogenous bone graft was mixed with bovine hydroxyapatite, PRF particles and applied to fill the defect. Five PRF membranes were placed over the bone mix. After 4 months a cone-beam CT was performed to evaluate bone regeneration. The use of PRF as cover membrane permitted a rapid epithelisation and represented an effective barrier versus epithelial cell penetration. After 4 months the site appeared precociously healed and the bone volume increased. This new approach represents a predictable method of augmenting deficient alveolar ridges. Guided bone regeneration with PRF showed limitation compared with guided bone regeneration using collagen membrane in terms of bone gain. The association of collagen membrane and PRF could be a good association.
Background
Platelet-rich fibrin (PRF) is a new regenerative material that contain growth factors.1 2 PRF is an immune and platelet concentrate collecting on a single fibrin membrane all constituents of a blood sample favourable to healing and immunity.3 4 The use of platelet concentrates in oral and maxillofacial surgery, particularly in implant dentistry, is a current and interesting trend.5 PRF production process is completely natural, with no use of anticoagulant during blood harvest nor bovine thrombine and calcium chloride for platelet activation and fibrin polymerisation.1–3 Literature demonstrated that PRF membrane was able to accelerate healing of soft tissues.2 6
Case presentation
Patient selection
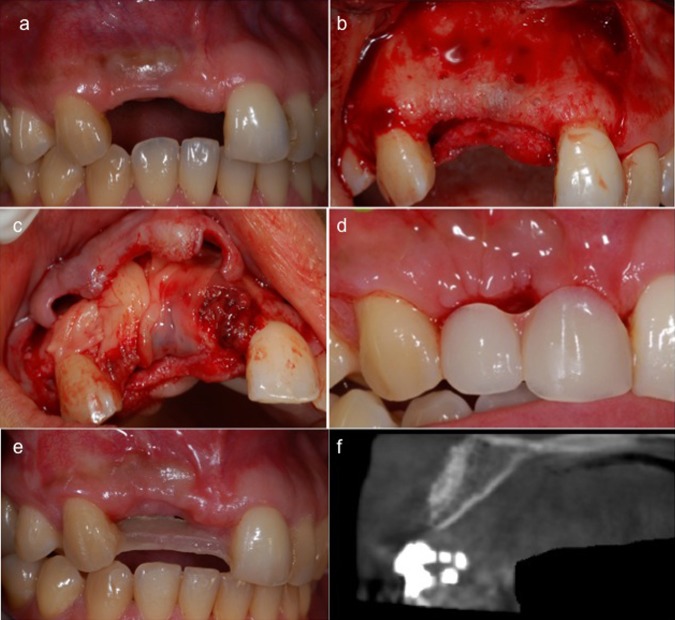
A patient with insufficient alveolar ridge width for implant placement in aesthetic zones (1.1 and 1.2) was enrolled in the present study (figure 2A). The patient was in good health and had no contraindications to surgical therapy with absence of local inflammation and absence of mucosal disease.3 Presurgical preparation included extensive oral hygiene instructions and treatment in order to obtain a periodontal good health.
Figure 2.
Insufficient alveolar ridge width in aesthetic zone of the patient enrolled in the present study (A). Elevation and mobilisation of facial and lingual mucoperiosteal flaps in surgical area (B). PRF membranes placed over the mix of autologous bone, PRF particles and bovine hydroxyapatite in order to cover all the graft and create a recontouring of the bone architecture (C). Tissue healing after 14 days (D). Tissue healing (E) and bone increasing (F) after 4 months evaluated clinically and with cone-beam CT.
Figure 1.
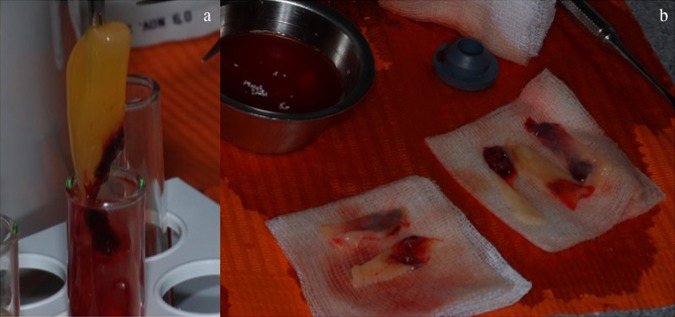
Platelet-rich fibrin (PRF) clot of PRF in the middle (A). PRF membranes can be obtained by squeezing out the fluids in the fibrin clot using sterile compresses (B).
Preparation of PRF
The advantages of PRF over platelet-rich plasma (PRP) are its simplified preparation and lack of biochemical handling of the blood.7 The required blood quantity (about 60 ml) was drawn into six 10 ml test tubes without anticoagulants and centrifuged immediately. Blood was centrifuged using a tabletop centrifuge for 12 min at 2700 rpm. The absence of anticoagulant induced the activation of platelet thus triggering a coagulation cascade. The resultant products consisted of the following three layers:
Topmost layer consisting of acellular platelet-poor plasma
PRF clot in the middle
Red blood cells at the bottom
Because of the absence of anticoagulant, blood begins to coagulate as soon as it comes in contact with the glass surface. Therefore, for successful preparation of PRF, speedy blood collection and immediate centrifugation, before the clotting cascade started, is absolutely essential.
Surgical technique
Prior to start the surgery, the patient was given 2 g of amoxicillin and rinsed with a 0.2% aqueous solution of chlorhexidine for 1 min. Anaesthesia is achieved through standard local infiltration and nerve block methods. However, during infiltration, special care is taken not to unduly expand the facial vestibular soft tissues by using excessive pressure or amounts of anaesthetic solution, because this unnecessarily complicates the periosteal separation incision. Access to the surgical site is obtained by a crestal incision and by mesial and distal vertical releasing incisions both facially and lingually. Vertical releasing incisions were placed distal to the 2.1 and mesial to the 2.2 providing the flap with a large base and allowing access to the defect. Facial and lingual mucoperiosteal flaps are elevated and further mobilised (figure 2B). The mobility of the flaps is then tested to ensure that primary closure of the wound can be achieved through tension-free suturing. Following flap reflection, any residual soft tissue was removed with curettes. Autogenous corticocancellous bone graft material was collected using bone scraper mixed with bovine hydroxyapatite (50%) and applied on the bone surface in order to fill the defect. Fibrin clots were squeezed in order to obtain six PRF membranes. One PRF membrane was cut into small pieces and mixed with the graft. Five PRF membranes were placed over the mix of autologous bone, PRF particles and bovine hydroxyapatite in order to cover all the graft and recontour the bone architecture (figure 2C). Finally the suture was carried out and the patient was dismissed. After 4 months a cone-beam CT (CBCT) was performed to evaluate bone regeneration in the surgical site.
Postoperative management
Medications prescribed included chlorhexidine rinses twice daily for 14 days, 1 g amoxicillin twice daily for 6 days, 600 mg ibuprofen three times daily as needed for pain. The sutures were removed 6 days after surgery (figure 2D).
Outcome and follow-up
The use of PRF as cover membrane permitted a rapid epithelisation of the surface of the site and represented an effective barrier versus epithelial cell penetration inside the bone defect.4–6 In fact only after 4 months the site appeared healed and the bone volume increased (figure 2E) as confirmed by CBCT (figure 2F). This fact demonstrated that, when PRF was used as graft and membrane during guided bone regeneration in order to fill bone defect, the physiological healing phenomenon was accelerated. The present study demonstrated that PRF membrane was able to reduce the healing period and improve bone regeneration. Moreover gingival tissues appeared to be not only in good health, but also they showed precociously a good maturation. However, authors think that PRF is a good material for guided tissue regeneration in particular for gingival augmentation thanks to its intrinsic property of improving healing and maturation.7 Authors think moreover that guided bone regeneration with PRF showed limitation compared with guided bone regeneration using collagen membrane in terms of bone gain. In fact PRF is rapidly resorbed in a few days after its placement on bone defect and it is only partially able to maintain a space and a barrier against epithelial cells penetration.
Discussion
During GBR procedures, it is crucial to create a space that is properly isolated from the surrounding soft tissues and can be maintained for an appropriate period of time to ensure osteogenesis.8 9 In addition to space maintenance, the membrane plays a role in clot stabilisation while simultaneously preventing migration of non-osteogenic tissues into the area.10 11 PRF has to be considered as a fibrin biomaterial. Its molecular structure with low thrombin concentration is an optimal matrix for migration of endothelial cells and fibroblasts.1 2 It permits a rapid angiogenesis and an easier remodeling of fibrin in a more resistant connective tissue.12 The matrix carries all the favourable constituents present in a blood sample. Because the PRF fibrin matrix is better organised, it was able to more efficiently direct stem cell harnessing and the healing program.13 Direct interactions between fibrin and osseous cells during healing are insufficiently documented. In conclusion, guided tissue regeneration using PRF as grafting material and membrane enriched with growth factors can induce and improve bone formation. The new approach described in this report has proven to be a safe and predictable method of augmenting deficient alveolar ridges in preparation for endosseous implant placement. Despite a reduced residual bone thickness14 a good bone volume was obtained after the application of bone graft and PRF membranes. Moreover after 4 months gingival tissues appeared to be not only in good health, but also they showed precociously a good maturation. Authors think that guided bone regeneration with PRF showed limitation compared with guided bone regeneration using collagen membrane in terms of bone gain.11 15 The association of collagen membrane and PRF could be a good idea in order to obtain good bone regeneration in less time and with a great healing of soft tissues. More investigations are needed about PRF and bone regenerations and the association with collagen membranes.
Learning points.
Present study demonstrated that platelet-rich fibrin (PRF) membrane was able to reduce the healing period and improve bone regeneration.
Use of PRF as a cover membrane permitted a rapid epithelisation of the surface of the site and represented an effective barrier versus epithelial cell penetration inside the bone defect.
Using of PRF membrane as regenerative barriers gingival tissues appeared to be not only in good health, but also they showed precociously a good maturation.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Dohan DM, Choukroun J, Diss A, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part I: technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006;2013:37–44 [DOI] [PubMed] [Google Scholar]
- 2.Dohan DM, Choukroun J, Diss A, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part II: platelet-related biologic features. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006;2013:45–50 [DOI] [PubMed] [Google Scholar]
- 3.Dohan DM, Choukroun J, Diss A, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part III: leucocyte activation: a new feature for platelet concentrates? Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006;2013:e51–5 [DOI] [PubMed] [Google Scholar]
- 4.Choukroun J, Diss A, Simonpieri A, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part V: histologic evaluations of PRF effects on bone allograft maturation in sinus lift. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006;2013:299–303 [DOI] [PubMed] [Google Scholar]
- 5.Mazor Z, Horowitz RA, Del Corso M, et al. Sinus floor augmentation with simultaneous implant placement using Choukroun's PRF (platelet-rich fibrin) as sole grafting material: a radiological and histological study at 6 months. J Periodontol 2009;2013:2056–64 [DOI] [PubMed] [Google Scholar]
- 6.Diss A, Dohan D, Mouhyi J, et al. Osteotome sinus floor elevation using Choukroun's platelet-rich fibrin as grafting material: a 1-year prospective pilot study with microthreaded implants. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2008;2013:572–9 [DOI] [PubMed] [Google Scholar]
- 7.Choukroun J, Diss A, Simonpieri A, et al. Platelet-rich fibrin (PRF): a second generation platelet concentrate. Part IV: clinical effects on tissue healing. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006;2013:56–60 [DOI] [PubMed] [Google Scholar]
- 8.Dahlin C, Andersson L, Linde A. Bone augmentation at fenestrated implants by an osteopromotive membrane technique: a controlled clinical study. Clin Oral Implants Res 1991;2013:159–65 [DOI] [PubMed] [Google Scholar]
- 9.Zitzmann NU, Naef R, Scharer P. Resorbable versus nonresorbable membranes in combination with Bio-Oss for guided bone regeneration. Int J Oral Maxillofac Implants 1997;2013:844–52 [PubMed] [Google Scholar]
- 10.Machtei EE. The effect of membrane exposure on the outcome of regenerative procedures in humans: a meta- analysis. J Periodontol 2001;2013:512–16 [DOI] [PubMed] [Google Scholar]
- 11.Simion M, Baldoni M, Rossi P, et al. A comparative study of the effectiveness of e-PTFE membranes with and without early exposure during the healing period. Int J Periodontics Restorative Dent 1994;2013:166–80 [PubMed] [Google Scholar]
- 12.Sclar AG. The Bio-Col technique. In: Soft tissue and esthetic considerations in implant therapy. Carol Stream, IL: Quintessence, 2003:75–112 [Google Scholar]
- 13.Soffer E, Ouhayoun JP, Anagnostou F. Fibrin sealants and platelet preparations in bone and periodontal healing. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2003;2013:521–8 [DOI] [PubMed] [Google Scholar]
- 14.Cawood JI, Howell RA. A classification of the edentulous jaws. Int J Oral Maxillofac Surg 1988;2013:232–6 [DOI] [PubMed] [Google Scholar]
- 15.Seibert JS. Reconstruction of deformed, partially edentulous ridges, using full thickness onlay grafts. Part I. Technique and wound healing. Compend Contin Educ Dent 1988;2013:437–53 [PubMed] [Google Scholar]