Abstract
In an evolutionarily conserved signaling pathway, ‘soluble’ adenylyl cyclases (sACs) synthesize the ubiquitous second messenger cyclic adenosine 3′,5′-monophosphate (cAMP) in response to bicarbonate and calcium signals. Here, we present crystal structures of a cyanobacterial sAC enzyme in complex with ATP analogs, calcium and bicarbonate, which represent distinct catalytic states of the enzyme. The structures reveal that calcium occupies the first ion-binding site and directly mediates nucleotide binding. The single ion–occupied, nucleotide-bound state defines a novel, open adenylyl cyclase state. In contrast, bicarbonate increases the catalytic rate by inducing marked active site closure and recruiting a second, catalytic ion. The phosphates of the bound substrate analogs are rearranged, which would facilitate product formation and release. The mechanisms of calcium and bicarbonate sensing define a reaction pathway involving active site closure and metal recruitment that may be universal for class III cyclases.
The ubiquitous second messenger cAMP regulates a large variety of essential physiological processes such as gene expression, chromosome segregation and cellular metabolism. In mammalian cells, cAMP is synthesized by a family of nine transmembrane adenylyl cyclases (tmACs) and one sAC1. Unlike tmACs, which localize to the cellular membrane and respond to extracellular stimuli via heterotrimeric G proteins1, sAC is found in various intracellular compartments such as the mitochondria and the nucleus2. Its localization near intracellular cAMP targets is the impetus for current models of second messenger signal transduction, in which cAMP functions as a locally acting signaling molecule2–4.
sAC is insensitive to the tmAC regulators calmodulin and heterotrimeric G proteins as well as the nonphysiological activator forskolin; instead, sAC senses physiological levels of bicarbonate5. Aside from its role as a universal physiological buffer maintaining cellular and extracellular pH, bicarbonate functions as a signaling molecule3, regulating many biological processes in mammals such as fertility6, acid-base homeostasis, breathing rate, metabolism and fluid transport (reviewed in ref. 7). As the only known signaling enzyme sensitive to physiological fluctuations of bicarbonate5, sAC probably mediates each of these processes. Bicarbonate activation of sAC is essential for sperm motility8 as well as for pH-dependent acid secretion in the epididymis and possibly the kidney9. In addition to its bicarbonate sensitivity, sAC is synergistically activated by calcium10, and this potentiation seems to be important for sperm maturation11.
Previous work has revealed the overall structure of tmAC enzymes and suggested a two-metal ion mechanism for catalysis12,13. Despite their different regulation, mammalian sAC and tmACs are grouped into the nucleotidyl cyclase class III based on sequence similarities within their catalytic domains14. We set out to study the molecular basis for the unique regulation of sAC enzymes through bicarbonate and calcium. Here, we describe a series of high-resolution crystal structures of the sAC homolog CyaC from the cyanobacterium Spirulina platensis in complex with ATP analogs, magnesium, calcium or calcium analogs, and with or without bicarbonate. The structures show that calcium activates sAC enzymes by replacing an active site magnesium ion that coordinates the substrate ATP. In contrast, bicarbonate stimulates sAC activity by inducing a large conformational change that leads to a remodeling of the bound nucleotide. These structures of various enzyme states further suggest general mechanisms for the catalytic pathway of class III nucleotidyl cyclases and their activation.
RESULTS
CyaC as model system for mammalian sAC enzymes
The nucleotidyl cyclase class III includes many bacterial and all known eukaryotic adenylyl and guanylyl cyclases14. However, the catalytic domains of mammalian sAC are more closely related to bacterial class III ACs than to any other known mammalian cyclases15. We therefore studied the regulation of the catalytic domain of the sAC homolog CyaC from the cyanobacterium S. platensis (26% sequence identity), which carries all sequence properties specific for sAC enzymes14. Like the mammalian enzyme10, the catalytic domain of CyaC is stimulated by bicarbonate through an increase in its Vmax, potentiated by calcium through an increase in the low basal affinity for the substrate ATP typical for sAC enzymes, and is synergistically activated when both compounds are present (Supplementary Fig. 1 online). Therefore, coincident bicarbonate and calcium sensing via cAMP production seems to be an ancient and conserved biological mechanism, and CyaC can serve as a model system for studying sAC activation.
α,β-Me-ATP binds to an open enzyme state
To explain the molecular basis of sAC regulation, we determined a series of structures of the CyaC catalytic domain cocrystallized with the ATP analogs α,β-methylene-adenosine-5′-triphosphate (α,β-Me-ATP; Ki = 0.3 mM) or adenosine-5′-Rp-α-thio-triphosphate (Rp-ATPαS; Ki ≈ 0.1 mM), in the presence of magnesium as well as calcium or calcium analogs, and with or without bicarbonate, at a resolution of up to 1.9 Å (Table 1). Although sACs have unique regulatory properties, the overall structure and active site of CyaC (Fig. 1a,b) closely resemble the known structure of mammalian tmAC12. The only difference is that CyaC is a symmetrical homodimer of a single catalytic domain with two complete active sites, whereas tmACs are pseudo symmetrical heterodimers of structurally similar catalytic domains (C1 and C2), resulting in one active site and one degenerate pseudo active site.
Table 1.
Data collection and refinement statistics
| CyaC Rp-ATPαS | CyaC α,β-Me-ATP | CyaC α,β-Me-ATP + SrCl2 |
CyaC α,β-Me-ATP + EuCl3 |
CyaC α,β-Me-ATP + bicarbonate |
CyaC Rp-ATPαS + bicarbonate |
|
|---|---|---|---|---|---|---|
| Data collection | ||||||
| Space group | C2221 | P212121 | P212121 | P212121 | P21 | C2221 |
| Unit cell dimensions (Å) | ||||||
| a | 54.2 | 53.7 | 53.5 | 53.6 | 53.3 | 51.1 |
| b | 78.9 | 71.5 | 70.5 | 70.2 | 71.2 | 74.0 |
| c | 283.6 | 99.6 | 99.4 | 99.6 | 106.7 | 266.7 |
| β(°) | 90.0 | 90.0 | 90.0 | 90.0 | 95.8 | 90.0 |
| Resolution (Å) | 20.0–1.9 | 20.0–2.4 | 20.0–1.9 | 20.0–3.0 | 20.0–2.3 | 20.0–2.5 |
| Unique reflections | 48,991 | 15,574 | 30,126 | 8,038 | 33,968 | 14,233 |
| <I /σ> | 9.7 | 7.0 | 10.8 | 5.1 | 8.9 | 8.3 |
| Completeness (%)a | 99.6 (98.6) | 99.2 (99.8) | 98.8 (94.1) | 99.4 (99.2) | 95.0 (98.0) | 80.0 (69.0) |
| Rmerge (%)a | 7.2 (36.7) | 8.1 (34.4) | 5.6 (21.7) | 13.8 (36.2) | 7.0 (42.2) | 9.2 (27.7) |
| Refinement | ||||||
| Resolution (Å) | 15.0–1.93 | 15.0– 2.4 | 15.0–1.9 | 15.0–3.0 | 15.0–2.3 | 15.0–2.5 |
| Total reflections used | 40,595 | 12,704 | 25,021 | 5,601 | 26,793 | 11,732 |
| No. atoms | ||||||
| Protein | 4,495 | 3,042 | 3,025 | 3,041 | 4,510 | 4,447 |
| Ligand | 99 | 64 | 64 | 64 | 132 | 99 |
| Solvent | 385 | 141 | 227 | 2 | 99 | 86 |
| R.m.s. deviations | ||||||
| Bond lengths (Å) | 0.006 | 0.007 | 0.006 | 0.012 | 0.007 | 0.010 |
| Bond angles (°) | 1.2 | 1.4 | 1.2 | 1.6 | 1.2 | 1.5 |
| Average B-factor (Å2) | 30.8 | 33.9 | 28.8 | — | 39.0 | 29.2 |
| Final Rcryst / Rfree(%)b | 19.8 / 22.9 | 21.9 / 27.1 | 20.4 / 23.6 | 27.4 / 31.5 | 20.2/ 26.2 | 24.1 / 29.1 |
Values in parentheses are for the highest-resolution shell.
Rfree was calculated from 6–7% of measured reflections omitted from refinement.
Figure 1.
Open conformation of sAC in complex with α,β-Me-ATP and calcium. (a) Ribbon diagram of the sAC homodimer with the substrate analog α,β-Me-ATP and one metal ion bound to each active site. The two monomers are red and blue, respectively. (b) Active site of the sAC–α,β-Me-ATP complex with the two monomers colored red and blue, respectively. Positive Fo − Fc omit electron density after soaking with the calcium analog europium is overlaid (contoured at 8 σ), showing the single heavy atom bound at the ion B site. (c) Overlay of sAC–α,β-Me-ATP (blue) with the structure of Gαs-bound tmAC (yellow and red) without substrate analog. sAC helix α1 is shifted to a more open position to avoid clashes with the substrate analog. (d) Close-up view of the calcium-binding pocket in the high-resolution structure of the Sr2+-soaked sAC–α,β-Me-ATP complex showing the seven-fold coordination to the metal. The 2Fo − Fc omit electron density defining the ligands was contoured at 1.3 σ.
The cocrystal structures of the cyanobacterial sAC (CyaC; from now on referred to as sAC) differed depending on which ATP analog was used. Previously, a Gαs-bound tmAC structure in the absence of nucleotide revealed a more open state than in the presence of Rp-ATPαS, suggesting that substrate binding induces closure of the active site12,13,16. We observed a similar closed conformation for the sAC–Rp-ATPαS complex; however, the conformation of Rp-ATPαS was not suitable for the subsequent in-line reaction (see below), and this state therefore might not resemble the substrate-bound state. The closed enzyme conformation is similar to a tmAC–Gαs complex soaked with product analogs, and it has been speculated that it resembles a product complex and that the substrate complex adopts a different conformation12. Indeed, we found that in sAC in complex with α,β-Me-ATP (Fig. 1a), the ATP analog assumes a conformation in which all functional groups are arranged for the subsequent in-line reaction17 (Fig. 1b) and therefore likely resembles the conformation of the bound substrate ATP. The α-phosphate (Pα) is in minus synclinal position and oriented so that the 3′ hydroxyl, as the subsequent attacking group, and the bond to be broken between the α-phosphorus atom and the bridging function to the β-phosphate (Pβ), are arranged in a straight line. The nucleotide conformation also primes the enzyme for catalysis by arranging Pα in eclipsed position with Pβ, which repels the pyrophosphate to facilitate its release.
In contrast to the sAC–Rp-ATPαS complex, the state of the active site of the sAC–α,β-Me-ATP complex is even more open than in the tmAC–Gαs structure without nucleotide, manifested in a 3–4 Å outward shift of helix α1 (Fig. 1c). Modeling an ATP complex based on the sAC–α,β-Me-ATP structure revealed that ATP could adopt a conformation similar to the analog, which fits well into the open enzyme state (Supplementary Fig. 2 online). Therefore, we conclude that nucleotide binding to sAC does not necessitate active site closure; in fact, it seems to precipitate an open state poised for catalysis. The open substrate analog complex observed here for sAC might also apply to tmACs and would explain why soaking or cocrystallization of activated (Gαs-bound) tmAC with α,β-Me-ATP was not successful12: in contrast to the basally active sAC enzyme used here, binding of the activator Gαs to tmAC may have induced partial active site closure in the absence of an ATP analog.
Calcium replaces an active site magnesium
The open state has only a single bound metal ion, rather than the two metal ions required for catalysis16. This ion (positioned analogously to the ion B magnesium in tmAC structures) serves as an anchoring point for ATP by coordinating and stabilizing the Pβ and γ-phosphate (Pγ) of the ATP analog. Soaking with SrCl2 or EuCl3, which are both known to occupy calcium sites18,19, unambiguously identified this ion B site as a calcium-binding pocket (Fig. 1b). We found no allosteric binding sites for calcium, and soaking in the calcium analogs did not lead to any substantial conformational changes. Although calcium is not often found in active sites, the ion B site is a typical calcium-binding site formed by less flexible ligands and few solvent molecules19,20. The main chain carbonyl oxygen of Ile1018, the side chains of Asp1017 and Asp1061, the phosphate oxygen atoms of Pβ and Pγ of the substrate analog, and a single water molecule coordinate the ion B site calcium (Fig. 1d). The bidentate interaction with Asp1017, which results in a seven-fold coordination that is more favorable for calcium than for magnesium, probably explains the higher affinity of calcium for this site. As calcium has also been reported to have a higher affinity for ATP in solution21, this dual preference explains how calcium lowers the Km for ATP in both mammalian and bacterial sACs, an effect necessary for high sAC activity at physiological ATP concentrations10 because of the enzyme’s low basal substrate affinity (Km = 12.3 mM; Supplementary Fig. 1 online).
Bicarbonate induces active site closure
Unlike calcium’s ability to increase substrate affinity, bicarbonate stimulates substrate turnover. Cocrystallization of sAC with bicarbonate did not produce any crystals and soaking bicarbonate into preformed crystals dissolved them, suggesting that bicarbonate caused a structural change. Like sAC activation in solution5 (Supplementary Table 1 online), the bicarbonate effect on sAC crystals is specific and pH-independent and cannot be induced with other anions, such as nitrate or acetate (see Supplementary Fig. 3 and Supplementary Table 1 online). Flash soaking and freezing permitted us to catch a glimpse of the bicarbonate-induced conformational changes. Most markedly, bicarbonate induced closure of the active site and facilitated binding of the second metal ion (ion A), which serves as the catalytic metal (Fig. 2a,b and Supplementary Video 1 online).
Figure 2.
Bicarbonate induces active site closure. (a) Overlay of sAC structures in the open state (blue) and after bicarbonate addition (yellow and red). Major conformational changes include shifts of the α1 helix and the β7–β8 strands, a flip of the loop between β4 and β5 and a kinking of helix α3 (best seen in Supplementary Video 1 online). (b) Active site before (blue) and after (yellow) the bicarbonate-induced active site closure, showing the recruited second metal ion and the structural rearrangements described in the text. (c) Close-up view for the remodeling of Pβ and Pγ of the ATP analog upon bicarbonate-induced active site closure (blue before and yellow after bicarbonate addition).
Bicarbonate ‘closes’ the active site mainly by inducing a 4–5 Å movement of the β7–β8 loop toward the dimer center and a shift of the α1 helix in the same direction. This conformation is stabilized by a newly formed salt bridge between Arg1023 within the α1 helix and Asp1187* (asterisk indicates the partner monomer within the dimer) in the β7–β8 loop. The active site closure induced movements of a several active site residues conserved in all class III cyclases. The β7–β8 loop movement shifts Lys1184*, which in turn pushes Arg1150* of the neighboring α4 helix by 6 Å. Arg1150* is positioned by Asn1146* to be oriented toward the ATP ribose 3′ hydroxyl group and Pα such that it could stabilize the additional negative charge in the transition state22. The shift of the α1 helix rearranges the phosphate chain of the ATP analog, leading to a 180° flip of Pγ (Fig. 2c). The shift pushes Pγ out of its binding site and orients it toward Arg1117. This residue was previously thought to bind the substrate12 but now seems to attract the reaction product pyrophosphate to aid its exit. These same structural elements were found to be flexible in the Gαs-bound tmAC structures; therefore, we predict that Gαs facilitates similar conformational changes in tmACs as bicarbonate induces in sAC.
Rp-ATPαS binds nonproductively to sAC
As stated above, Rp-ATPαS binds in a nonproductive conformation to a more closed sAC state than α,β-Me-ATP (Fig. 3b). This intermediately closed enzyme state resembles Gαs-bound tmAC soaked with Rp-ATPαS16 and is slightly less closed than the bicarbonate-soaked sAC–α,β-Me-ATP structure. The sAC–Rp-ATPαS complex has two magnesium ions bound, and the electron density clearly reveals that instead of the pro-R oxygen being positioned for coordination of ion A, as observed in the α,β-Me-ATP structure (Fig. 1b,d) and as necessary for productive ATP binding, its replacement by sulfur in Rp-ATPαS forces Pα to turn such that the pro-S oxygen now coordinates ion A (Fig. 3c and Supplementary Fig. 4 online). This twisted conformation prevents the in-line arrangement of attacking and leaving group, that is, the ribose 3′ hydroxyl group and the bridging oxygen between the α- and β-phosphorus atoms. This observation rationalizes why Rp-ATPαS stereospecifically inhibits class III ACs17, whereas Sp-ATPαS, whose modification does not prevent binding in a productive, α,β-Me-ATP-like conformation, is a substrate for tmACs17 and likely for sAC enzymes (Supplementary Fig. 5 online).
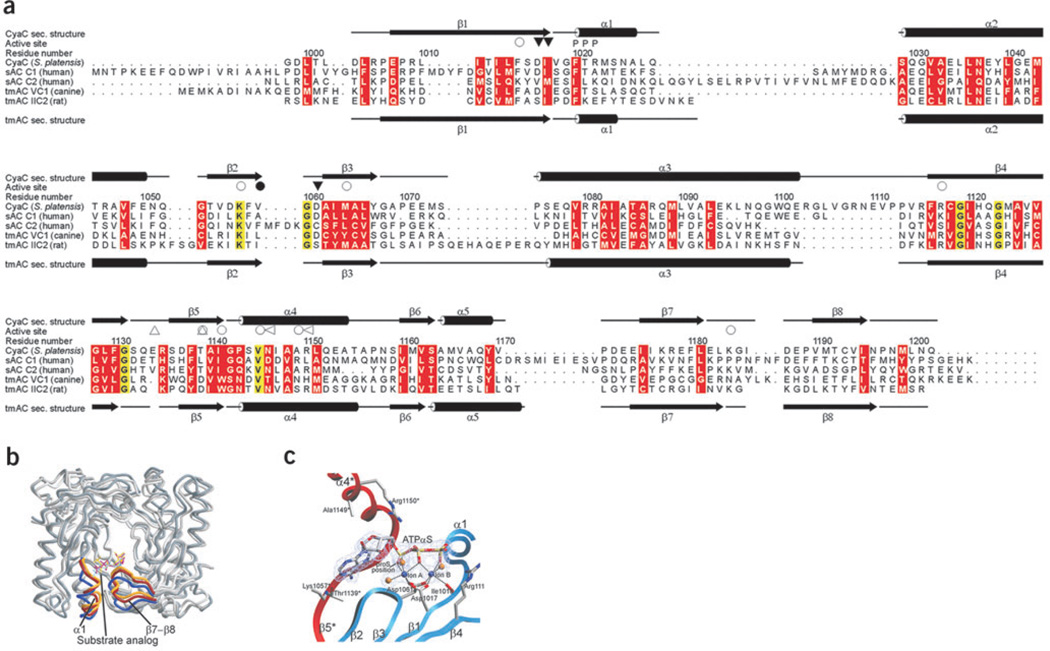
Figure 3.
Conformational states and comparison of AC enzymes. (a) Structure-based sequence alignment of bicarbonate responsive sAC enzymes and the G protein–regulated tmAC domains VC1 and IIC2 (PDB entry 1AZS). Secondary structure elements of sAC and IIC2 are indicated on top and bottom, respectively. Ion-binding residues (▽) and residues binding the substrate (○) or the transition state (◁) are labeled (filled and empty symbols label C1 and C2 residues, respectively). Thr1139* and the insertion characteristic for sAC enzymes are indicated (△). Conserved amino acids are shaded yellow, and residues with conserved physicochemical properties are shaded red. (b) Overlay of the sAC–α,β-Me-ATP structure (open state, darkest gray, with α1 helix and β7–β8 loop in blue), the sAC–Rp-ATPαS complex (partially closed, middle gray and red), and the bicarbonate-soaked Rp-ATPαS structure (closed, lightest gray and yellow). Structures were superimposed on sAC–α,β-Me-ATP by optimizing positional agreement for residues 1014–1018, 1056–1065, 1117–1126 and 1143–1167 in both subunits. (c) sAC active site in complex with Rp-ATPαS and two magnesium ions, with the two monomers colored red and blue, respectively. The dashed lines indicate the octahedral coordination of the ions through the ATP analog, protein residues and one and two water molecules (gold spheres), respectively. The 2Fo − Fc omit electron density for the ligands was contoured at 1.1 σ. In its tmAC complex, Pα of Rp-ATPαS was modeled differently but with limited electron density for the ribose and its link to the Pα16, and we speculate that this density might also be interpretable with the inhibitor conformation observed here for its sAC complex.
The sAC–Rp-ATPαS complex, in addition to being partially closed, has the second metal ion, essential for catalytic activity, bound to the ion A site. During catalysis this ion is recruited after binding of the substrate23, and this partially closed enzyme state with two bound ions therefore might resemble some features of an intermediate conformation of the enzyme during catalysis. This conclusion is consistent with the successful soaking of Rp-ATPαS into tmAC crystals already partially closed by Gαs16. Because of its artificial binding conformation, Rp-ATPαS induced a partially closed state of sAC in the absence of bicarbonate stimulation, and soaking bicarbonate into these crystals resulted in further closure into a structure similar to the bicarbonate-soaked structure of sAC in complex with α,β-Me-ATP (Fig. 3b).
Potential bicarbonate recognition sites
Bicarbonate induced these structural changes, but it was not detected in any of the soaked crystals. Its absence, along with the observations that bicarbonate (i) does not disrupt microcrystals of free sAC, and (ii) is very specific (Supplementary Table 1 online), yet requires high concentrations (physiological concentrations between 5 and 25 mM) to activate sAC5,10,24 (corresponding to an estimated off-rate of ~106 s−1, much higher than the turnover rate kcat of ~1 to 4 s−1; Supplementary Methods online), might suggest that bicarbonate interacts only very transiently with the enzyme during each catalytic cycle.
In the absence of a defined bicarbonate-binding site, sequence differences between tmACs and sACs provide the best insight into the unique bicarbonate regulation of sAC enzymes (Fig. 3a). One difference corresponds to sAC active site residue Thr1139*, which is conserved in the bicarbonate-responsive sACs but replaced by a conserved aspartate in bicarbonate-insensitive bacterial ACs and tmACs24. Mutating the analogous threonine to aspartate abolished bicarbonate responsiveness in CyaB1, a cyanobacterial sAC24, consistent with Thr1139* contributing to the bicarbonate stimulation of sACs. A potential scenario is that a single bicarbonate molecule could replace the two water molecules that coordinate ion A (Fig. 3c) and facilitate recruitment of this catalytic ion. TmACs with an aspartate replacing sAC Thr1139* would be insensitive to bicarbonate because of the negative charge that decreases the bicarbonate population near the ion A site. However, the effect of bicarbonate on the sAC–Rp-ATPαS complex indicates that ion recruitment can be only part of the bicarbonate function.
A second sequence variation between sACs and tmACs localizes to the β4–β5 loop14, in which bicarbonate-responsive sACs contain a single-residue insertion (Fig. 3b). The additional residue causes a different conformation of the β4–β5 strands, opening a hole in the back of the enzyme, which may be a conduit for bicarbonate access to the active site. However, we could not confirm the role of this insertion because shortening this loop by one amino acid abolished enzyme activity (data not shown).
DISCUSSION
The structures described here reveal two novel mechanisms of stimulating production of the ubiquitous second messenger cAMP by sAC enzymes. In contrast to its more common role as an allosteric regulator, calcium binds to the active site and directly contributes to substrate binding. We also show that bicarbonate, which was previously only known to serve as a substrate for enzymes, plays an allosteric role by inducing a stimulatory conformational change.
For a model for the dynamic catalytic pathway and the specific activation of all class III adenylyl and guanylyl cyclases (Fig. 4 and Supplementary Video 2 online), we assembled the structures presented here. In this model, substrate binding would lead to the formation of an enzyme–substrate complex in the open conformation, with one magnesium bound at the ion B site. In sAC enzymes, this ion can be replaced by calcium to increase substrate affinity. The next step of catalysis is binding of the second metal to the ion A site and the concomitant active site closure. This is the step facilitated by the physiological stimulators of ACs, either bicarbonate in the case of sAC enzymes or Gαs proteins for tmACs. Binding of the ion A magnesium enables transition state formation by contributing to the activation of the ribose 3′ hydroxyl group and by stabilizing the charges at the triphosphate. The force from the active site closure would shift Pγ and Pβ toward the front, where the products will ultimately exit. Unlike the phosphate flip observed for the α,β-Me-ATP substrate analog, Pγ and Pβ of ATP would move as a rigid body, thereby lengthening and weakening the bond to Pα (Fig. 4). The movement of Pγ and Pβ would force Pα to swing toward the 3′ hydroxyl, leading to ring formation and release of pyrophosphate. The closed conformation observed for the tmAC–Gαs–Rp-ATPαS complex and for the sAC complexes after bicarbonate addition probably corresponds to this product complex with one major exception: the positions of Pγ and Pβ are swapped compared with the product pyrophosphate (Fig. 4). This model is reinforced by the observation that both protein conformation and the position of pyrophosphate in a tmAC–Gαs–product analog structure12 match those observed in the Rp-ATPαS and bicarbonate-soaked structures. Dissociation of pyrophosphate finally enables the active site to open again by releasing the interactions of the phosphates with the α1 helix and β7–β8 loop.
Figure 4.
Model for catalysis by class III nucleotidyl cyclases. The model for catalysis (bottom pathway) is based on the conformational changes observed with the sAC–substrate analog complexes (top). The arrows at α1 and β7–β8 indicate the movements undergone by these protein parts. The individual catalytic states (open, intermediate and closed) are extrapolated from the different sAC structures presented in the text, with the protein conformation of the sAC–Rp-ATPαS complex being a speculative approximate intermediate state.
In summary, the high-resolution structures of different enzyme states presented here predict that class III cyclases might use the same reaction pathway, and that modulators, such as G proteins in tmACs and bicarbonate in sAC, might increase activity by promoting the catalytic cycle via active site closure and metal recruitment.
METHODS
Cloning, protein purification and activity assays
Residues 998–1202 and 1005–1202, respectively, which comprise the catalytic domain of CyaC from S. platensis (TrEMBL entry O32393)25, were cloned into the pET28a expression vector with an N-terminal His-tag and expressed in Escherichia coli BL21(DE3) for 18 h at 20 °C. The protein was purified using Ni-NTA affinity and Q-Sepharose ion exchange chromatography, followed by gel filtration in 20 mM Tris, pH 7.8. Adenylyl cyclase assays were carried out with purified CyaC(998–1202) as described10. Protein used for activity assays was stored at −20 °C in 50% (v/v) glycerol.
Crystallization and X-ray data collection
For crystallization of CyaC(998–1202) in complex with Rp-ATPαS, drops were mixed from 1 µl protein solution (8 mg ml−1 in 20 mM Tris, pH 7.8, 5 mM Rp-ATPαS, 7.5 mM MgCl2, 10 mM CaCl2) and 1 µl reservoir solution, equilibrated against 0.4 ml reservoir (100 mM HEPES, pH 7.2, 8% (w/v) isopropanol, 10% (w/v) PEG 4000), and microseeded after 24 h. CyaC(1005–1202) in complex with α,β-Me-ATP was crystallized by mixing 1 µl protein solution (6 mg ml−1 in 20 mM Tris, pH 7.8, 5 mM α,β-Me-ATP, 5 mM MgCl2, 5 mM CaCl2) and 1 µl reservoir solution and equilibration against 0.4 ml reservoir (100 mM cacodylate, pH 6.7, 6% (w/v) isopropanol, 11% (w/v) PEG 4000). Both crystal forms were frozen 60 s after addition of two-drop volumes cryoprotectant solution (25% (v/v) isopropanol in reservoir solution).
For identifying the calcium-binding site, CyaC(1005–1202)–α,β-Me-ATP crystals were soaked with 5 mM EuCl3 for 23 h and with 10 mM SrCl2 for 13 h, respectively. Bicarbonate soaking was done by adding 150 mM potassium bicarbonate, pH 7.5, to the cryoprotectant solution and freezing of the crystals after 15 to 25 s. Diffraction data were collected at 100 K at Brookhaven National Laboratory beamline X4A (Table 1) and indexed, scaled and merged with DENZO and SCALEPACK26.
Structure determination
Initial Patterson search trials with the CyaC–Rp-ATPαS data and with a polyalanine model of tmAC VC1 (PDB entry 1AZS; 20% identity with CyaC) or with conserved side chains included did not yield a prominent solution. We therefore generated a homology model of CyaC from the tmAC VC1 structure (HM-CyaC). Only after converting HM-CyaC to a polyalanine model were we able to obtain two of the three monomers in the asymmetric unit using MOLREP27: monomer A, which forms a dimer with its symmetry mate along a crystallographic two-fold rotation axis and an isolated monomer B. The missing monomer C expected from Matthews coefficient calculation, crystal packing analysis and the tmAC structure was generated manually by applying two-fold rotation symmetry on B resulting in a BC dimer. A fine grid search for C using Como28 then located the correct orientation and position of this monomer. For rigid body refinement and simulated annealing, all monomers had to be replaced with a tmAC VC1 model trimmed to include only the conserved core of the protein. Electron density quality was sufficient for model building after being markedly improved through three-fold noncrystallographic symmetry averaging and solvent flattening with DM29. Model building was done using O30, and the model was refined using CNS31 with an overall anisotropic B-factor, a bulk solvent correction and individual isotropic Debye-Waller factors. All further complex structures were solved by molecular replacement using MOLREP27. Structural figures were generated with MolScript32 and Raster3D33 (Figs. 1a,c, 2a and 3a), SETOR34 (Figs. 1b, 2b,c and 3b), and PyMOL (http://www.pymol.org) (Fig. 1d), and the alignment (Fig. 3c) with Alscript35.
Coordinates
The coordinates and structure factors have been deposited in the Protein Data Bank (accession codes: CyaC in complex with Rp-ATPαS, 1WC1; with α,β-Me-ATP, 1WC0, with α,β-Me-ATP and Sr2+, 1WC3; with α,β-Me-ATP and Eu3+, 1WC4; activated α,β-Me-ATP and Rp-ATPαS complexes after soaking with bicarbonate, 1WC5 and 1WC6, respectively).
Supplementary Material
ACKNOWLEDGMENTS
We thank K. Hess and N. Stephanou for technical assistance and R. Abramowitz and X. Yang for support at National Synchrotron Light Source beamline X4A. C.S. acknowledges support as Berger Fellow of the Damon-Runyon Cancer Research Foundation, and H.W. is a Pew Scholar of Biomedical Sciences and a Rita Allen Scholar. This work was supported by funds from the US National Institutes of Health (L.R.L. and J.B.), Hirschl Weill-Caulier Trust (L.R.L.) and the Ellison Medical Foundation (J.B.).
Footnotes
Note: Supplementary Information is available on the Nature Structural & Molecular Biology web site.
COMPETING INTERESTS STATEMENT
The authors declare that they have no competing financial interests.
References
- 1.Hanoune J, Defer N. Regulation and role of adenylyl cyclase isoforms. Annu. Rev. Pharmacol. Toxicol. 2001;41:145–174. doi: 10.1146/annurev.pharmtox.41.1.145. [DOI] [PubMed] [Google Scholar]
- 2.Zippin JH, et al. Compartmentalization of bicarbonate-sensitive adenylyl cyclase in distinct signaling microdomains. FASEB J. 2003;17:82–84. doi: 10.1096/fj.02-0598fje. [DOI] [PubMed] [Google Scholar]
- 3.Zippin JH, et al. Bicarbonate-responsive “soluble” adenylyl cyclase defines a nuclear cAMP microdomain. J. Cell Biol. 2004;164:527–534. doi: 10.1083/jcb.200311119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bundey RA, Insel PA. Discrete intracellular signaling domains of soluble adenylyl cyclase: camps of cAMP? Sci. STKE. 2004;2004:PE19. doi: 10.1126/stke.2312004pe19. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, et al. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science. 2000;289:625–628. doi: 10.1126/science.289.5479.625. [DOI] [PubMed] [Google Scholar]
- 6.Visconti PE, et al. Novel signaling pathways involved in sperm acquisition of fertilizing capacity. J. Reprod. Immunol. 2002;53:133–150. doi: 10.1016/s0165-0378(01)00103-6. [DOI] [PubMed] [Google Scholar]
- 7.Zippin JH, Levin LR, Buck J. CO(2)/HCO(3)(−)-responsive soluble adenylyl cyclase as a putative metabolic sensor. Trends Endocrinol. Metab. 2001;12:366–370. doi: 10.1016/s1043-2760(01)00454-4. [DOI] [PubMed] [Google Scholar]
- 8.Esposito G, et al. Mice deficient for soluble adenylyl cyclase are infertile because of a severe sperm-motility defect. Proc. Natl. Acad. Sci. USA. 2004;101:2993–2998. doi: 10.1073/pnas.0400050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pastor-Soler N, et al. Bicarbonate regulated adenylyl cyclase (sAC) is a sensor that regulates pH-dependent V-ATPase recycling. J. Biol. Chem. 2003;278:49523–49529. doi: 10.1074/jbc.M309543200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Litvin TN, Kamenetsky M, Zarifyan A, Buck J, Levin LR. Kinetic properties of “soluble” adenylyl cyclase. Synergism between calcium and bicarbonate. J. Biol. Chem. 2003;278:15922–15926. doi: 10.1074/jbc.M212475200. [DOI] [PubMed] [Google Scholar]
- 11.Jaiswal BS, Conti M. Calcium regulation of the soluble adenylyl cyclase expressed in mammalian spermatozoa. Proc. Natl. Acad. Sci. USA. 2003;100:10676–10681. doi: 10.1073/pnas.1831008100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tesmer JJ, Sunahara RK, Gilman AG, Sprang SR. Crystal structure of the catalytic domains of adenylyl cyclase in a complex with Gαs.GTPγS. Science. 1997;278:1907–1916. doi: 10.1126/science.278.5345.1907. [DOI] [PubMed] [Google Scholar]
- 13.Tesmer JJ, Sprang SR. The structure, catalytic mechanism and regulation of adenylyl cyclase. Curr. Opin. Struct. Biol. 1998;8:713–719. doi: 10.1016/s0959-440x(98)80090-0. [DOI] [PubMed] [Google Scholar]
- 14.Linder JU, Schultz JE. The class III adenylyl cyclases: multi-purpose signalling modules. Cell Signal. 2003;15:1081–1089. doi: 10.1016/s0898-6568(03)00130-x. [DOI] [PubMed] [Google Scholar]
- 15.Buck J, Sinclair ML, Schapal L, Cann MJ, Levin LR. Cytosolic adenylyl cyclase defines a unique signaling molecule in mammals. Proc. Natl. Acad. Sci. USA. 1999;96:79–84. doi: 10.1073/pnas.96.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tesmer JJ, et al. Two-metal-Ion catalysis in adenylyl cyclase. Science. 1999;285:756–760. doi: 10.1126/science.285.5428.756. [DOI] [PubMed] [Google Scholar]
- 17.Eckstein F, Romaniuk PJ, Heideman W, Storm DR. Stereochemistry of the mammalian adenylate cyclase reaction. J. Biol. Chem. 1981;256:9118–9120. [PubMed] [Google Scholar]
- 18.Fox BA, et al. Identification of the calcium binding site and a novel ytterbium site in blood coagulation factor XIII by X-ray crystallography. J. Biol. Chem. 1999;274:4917–4923. doi: 10.1074/jbc.274.8.4917. [DOI] [PubMed] [Google Scholar]
- 19.Pidcock E, Moore GR. Structural characteristics of protein binding sites for calcium and lanthanide ions. J. Biol. Inorg. Chem. 2001;6:479–489. doi: 10.1007/s007750100214. [DOI] [PubMed] [Google Scholar]
- 20.Katz AK, Glusker JP, Beebe SA, Bock CW. Calcium ion coordination: A comparison with that of beryllium, magnesium, and zinc. J. Am. Chem. Soc. 1996;118:5752–5763. [Google Scholar]
- 21.Mohan MS, Rechnitz GA. Ion-electrode study of the calcium-adenosine triphosphate system. J. Am. Chem. Soc. 1972;94:1714–1716. doi: 10.1021/ja00760a048. [DOI] [PubMed] [Google Scholar]
- 22.Yan S-Z, Huang Z-H, Shaw RS, Tang W-J. The conserved asparagine and arginine are essential for catalysis of mammalian adenylyl cyclase. J. Biol. Chem. 1997;272:12342–12349. doi: 10.1074/jbc.272.19.12342. [DOI] [PubMed] [Google Scholar]
- 23.Garbers DL, Johnson RA. Metal and metal-ATP interactions with brain and cardiac adenylate cyclase. J. Biol. Chem. 1975;250:8449–8456. [PubMed] [Google Scholar]
- 24.Cann MJ, Hammer A, Zhou J, Kanacher T. A defined subset of adenylyl cyclases is regulated by bicarbonate ion. J. Biol. Chem. 2003;278:35033–35038. doi: 10.1074/jbc.M303025200. [DOI] [PubMed] [Google Scholar]
- 25.Kasahara M, Yashiro K, Sakamoto T, Ohmori M. The Spirulina platensis adenylate cyclase gene, cyaC, encodes a novel signal transduction protein. Plant Cell Physiol. 1997;38:828–836. doi: 10.1093/oxfordjournals.pcp.a029241. [DOI] [PubMed] [Google Scholar]
- 26.Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 27.Vagin A, Teplyakov A. MOLREP: an automated program for molecular replacement. J. Appl. Crystallogr. 1997;30:1022–1025. [Google Scholar]
- 28.Jogl G, Tao X, Xu Y, Tong L. COMO: a program for combined molecular replacement. Acta Crystallogr. D. 2001;57:1127–1134. doi: 10.1107/s0907444901006783. [DOI] [PubMed] [Google Scholar]
- 29.Collaborative Computational Project, Number 4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D. 1994;50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- 30.Jones TA, Zou JY, Cowan SW, Kjeldgaard M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A. 1991;47:110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- 31.Brünger AT, et al. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr. Sect. D. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 32.Kraulis PJ. MOLSCRIPT: a program to produce both detailed and schematic plots of protein strucrures. J. Appl. Crystallogr. 1991;24:946–950. [Google Scholar]
- 33.Merrit EA, Murphy MEP. RASTER3D Version 2.0. A program for photorealistic molecular graphics. Acta Crystallogr. Sect. D. 1994;50:869–873. doi: 10.1107/S0907444994006396. [DOI] [PubMed] [Google Scholar]
- 34.Evans SV. SETOR: hardware lighted three-dimensional solid model representations of macromolecules. J. Mol. Graphics. 1993;11:134–138. doi: 10.1016/0263-7855(93)87009-t. [DOI] [PubMed] [Google Scholar]
- 35.Barton GJ. ALSCRIPT a tool to format multiple sequence alignments. Prot. Engineering. 1993;6:37–40. doi: 10.1093/protein/6.1.37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.