Abstract
Background and Purpose
We used transcranial Doppler (TCD) ultrasonography to examine the cerebral blood flow (CBF) response to orthostatic stress in the middle and posterior cerebral circulations and to determine the effects of healthy aging on regional CBF regulation.
Methods
Continuous simultaneous middle (MCA) and posterior (PCA) cerebral artery blood flow velocities (BFV) and mean arterial pressure (MAP) were measured in response to standing from a sitting position in 13 young (30 ± 7 years) and 13 older (73 ± 4 years) healthy subjects.
Results
The older subjects had a significantly larger decline in MAP (−31% ± 3 in the old and −21% ± 2 in the young) and smaller increase in heart rate (15 bpm ±1 in the old, 24bpm ±2 in the young) during the posture change. Despite a larger decline in MAP, the older subjects had a very similar decline in their BFVs in both vascular territories. This was associated with a significantly larger vasodilatory response in the MCA and PCA vascular territories of the older subjects. There were no regional differences of the cerebrovascular resistance and BFV responses to orthostasis in the young subjects. However, in the older subjects, there was a significantly larger BFV decline and a smaller vasodilatory response in the PCA as compared to the MCA territory.
Conclusions
Healthy aging is associated with preserved cerebrovascular adaptation to orthostatic hypotension. However, in the older subjects, the PCA territory blood flow may be more vulnerable to reduced perfusion during orthostatic stress.
Keywords: Cerebral Autoregulation, Aging, Transcranial Doppler Ultrasound, Middle Cerebral Artery, Posterior Cerebral Artery
Introduction
Cerebral autoregulation serves to maintain constant blood flow to the brain over a wide range of cerebral perfusion pressures. Syncope, which is often associated with upright posture, can be provoked by any condition that threatens cerebral blood flow (CBF). Upright posture challenges cerebral autoregulation by reducing arterial pressure, cardiac output and cerebral perfusion pressure. Although syncope is thought to be due to a global decrease in CBF, the initial prodromal symptoms are often referable to the posterior cerebral circulation. Most patients initially describe nausea, sweating, dizziness, blurred vision, and tingling of the ears, with progression to yawning, hyperventilation and pupillary dilation before losing consciousness. However, it is not known whether there is a disproportionate reduction in posterior cerebral blood flow during conditions that provoke syncope.
Transcranial Doppler (TCD) ultrasonography is a non-invasive tool frequently used to measure cerebral blood flow (CBF) velocity which can be used to measure instantaneous changes in CBF in response to a variety of stimuli. Given the high prevalence of orthostatic hypotension in elderly people [1], we aimed to determine if healthy aging altered regional CBF regulation during orthostatic stress.
We used TCD to simultaneously study CBF regulation in two vascular territories, the middle (MCA) and posterior cerebral arteries (PCA), in response to step changes in systemic blood pressure in healthy young and older volunteers. Numerous methods have been used to estimate cerebral autoregulation. These include the thigh cuff test, valsalva maneuver, lower body negative pressure, antihypertensive medication administration, and most recently, the sit-to-stand protocol [2–6]. We chose the sit-to-stand protocol [4], a similar but more physiological stimulus than the thigh cuff test because it is much better tolerated by elderly subjects and simulates a common activity of daily living (ADL) that potentially threatens cerebral perfusions.
Subjects and Methods
Subjects
Seventeen healthy young subjects and 13 healthy older subjects volunteered to participate in the study. Subjects were recruited from among laboratory personnel and members of the Harvard Cooperative Program on Aging subject registry. All subjects were carefully screened with a medical history, physical examination, and electrocardiogram to exclude any acute or chronic medical conditions. Subjects were asked to refrain from alcohol or nicotine for at least 12-hours. Files for 4 young subjects were excluded from analysis due to poor TCD signal quality. Exclusion criteria were as follows: ΔMAP<10 mmHg, a prolonged MAP drop lasting >5seconds after standing, or an unstable state of MAP or BFV. The study was approved by the Hebrew Rehabilitation Center for Aged institutional review board, and followed institutional guidelines.
Experimental Protocol
Instrumentation
Subjects reported to the cardiovascular laboratory in the post-absorptive state, ≥2 hours after their last meal. Instrumentation for heart rate (HR electrocardiogram) and beat-to-beat arterial pressure monitoring (MAP, Finapres, Ohmeda Monitoring Systems, Englewood, CO) were as previously described [4]. End-tidal CO2 was measured using a Vacumed CO2 Analyzer (Ventura, CA).
TCD ultrasonography (MultiDop X4, DWL-Transcranial Doppler Systems Inc., Sterling, VA) was used to measure simultaneous changes in MCA and PCA blood flow velocity (BFV, reported as mean flow velocity) in response to: 1) blood pressure changes during a sit-to-stand protocol and 2) end-tidal CO2 changes (CO2 Analyzer, Vacumed, Ventura, CA). The MCA and PCA signals were identified according to the criteria of Aaslid et al [7] and recorded at a depth of 50 to 65 mm. A Mueller-Moll probe fixation device was used to stabilize the Doppler probes for the duration of the study. The envelope of the velocity waveform, derived from a fast-Fourier analysis of the Doppler frequency signal, was digitized at 500Hz, displayed simultaneously with the MAP, ECG, and end-tidal CO2 signals, and stored for later off-line analysis.
Standing Protocol
The active sit-to-stand procedure, which produces immediate orthostatic hypotension without altering the spatial relation between the Doppler probe and the insonated vessels, was developed in our laboratory and previously described in detail [4]. After instrumentation, subjects sat in a straight-backed chair with their legs elevated at 90 degrees in front of them on a stool. For each of 2 active stands, subjects rested in the sitting position for 5 minutes, then stood upright for 1 minute. The initiation of standing was timed from the moment both feet touched the floor. Data were collected continuously during the final1-minute of sitting and 1-minute of standing. The autoregulatory response to transient orthostatic hypotension was assessed by determining the absolute and percent changes in cerebrovascular resistance (CVR=MAP/BFV) for each vessel from the sitting position (average of 50 seconds data) to the BP nadir during standing (average of 5 values). Rate of Regulation (RoR) was calculated from the slope of the recovery of blood flow according to the method of Aaslid et al [8].
CO2 Reactivity Protocol
Changes in MCA and PCA BFV were measured during alterations in end-tidal CO2[9] to determine whether differences in regional autoregulation correlated with cerebrovascular reactivity to CO2 (VR) in that vascular territory. In this technique cerebral BFVs in the MCA and PCA were measured continuously while subjects inspired a gas mixture of 5% CO2, 21% O2, and balance nitrogen for 2 minutes and then mildly hyperventilated to an end-tidal CO2 of approximately 25 mmHg for 2 minutes. To determine cerebrovascular reactivity using this technique, percent change in MCA or PCA blood flow velocities were plotted against end tidal CO2 in response to room air, breathing 5% CO2 and mild hyperventilation. Cerebrovascular reactivity was measured as the slope of this relationship and expressed as percent change in cerebral blood flow per mmHg change in end-tidal CO2.
Data Processing
All data were displayed and digitized in real time at 500 Hz with commercially available data acquisition software (Windaq, Dataq Instruments). BFV and BP waveforms were re-sampled at 1Hz using a MATLAB program. Beat-to-beat R-R interval, MAP and BFV (reported as mean flow velocity) were determined from the R wave of the ECG and the maximum and minimum of the arterial pressure or BFV waveforms.
Beat-to-beat values for BFV and MAP during the sit-to-stand protocol were averaged across all trials for each individual. Cerebrovascular resistance (CVR) was calculated as the ratio of MAP to BFV. To compare group responses, percent change in BFV, CVR and MAP were calculated as the difference between standing and sitting values, divided by sitting values. Individual percent changes were then averaged to obtain percent changes in BFV, CVR and MAP for each group.
Statistical Analysis
Group averages in BFV, CVR, and MAP were compared using a Student’s t-test. Significance was set at p<0.05. Linear regression was used to compute the slope of the relationship between end-tidal CO2 and BFV. Data are presented as mean ± SD.
Results
Subject Characteristics
Demographic and baseline data for the young (n=13) and older groups (n=13) are shown in Table 1. Baseline MAP and HR were not significantly different between the two groups. The older subjects had lower baseline BFV in the MCA (p<0.05).
Table 1.
Subject Characteristics
| YOUNG | OLD | |
|---|---|---|
| N | 13 | 13 |
| M:F ratio | 6:7 | 9:4 |
| Age, y | 30(7) | 73(4) |
| Mean Arterial Pressure, mmHg | 88(17) | 85(12) |
| Baseline heart rate, bpm | 70(12) | 63(4) |
| MCA Baseline CBFV, cm/s | 70(14) | 50(17)* |
| PCA Baseline CBFV, cm/s | 40(14) | 36(14) |
All values are mean (SD).
MCA= Middle Cerebral Artery, PCA=Posterior Cerebral Artery
Significant difference between two groups (p<0.05)
Hemodynamic Response to Posture Change
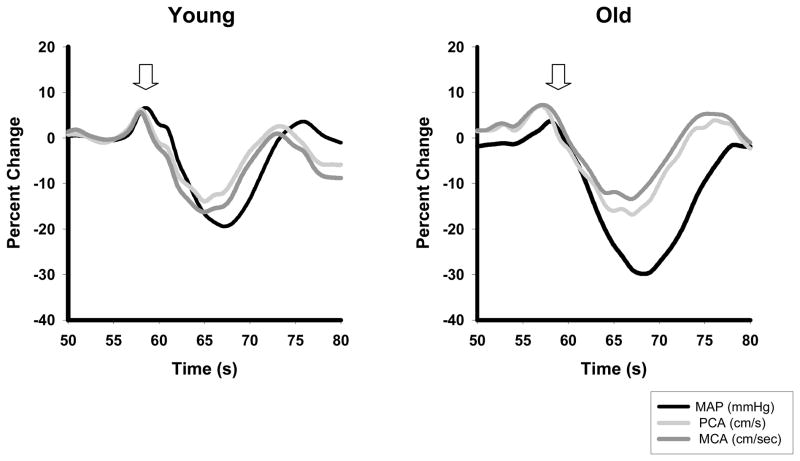
Mean MAP and BFV responses in the left MCA and the right PCA during the sit-to-stand protocol for the young and old groups are shown in Figure 1. This figure shows effective cerebral autoregulation in both vascular territories of the young and older groups. With the rapid decline in MAP, there is a simultaneous decline in cerebral BFV, which rapidly begins to recovers in both vessels at a time when the MAP is still declining.
Figure 1.
Relative percent changes over time (sec) in mean arterial pressure (MAP) (black line), middle (MCA, dark gray line) and posterior (PCA, light gray line) cerebral artery blood flow velocities during the sit-to-stand protocol in the young and older subjects. Arrow indicates standing.
Hemodynamic responses to standing are summarized in Table 2. The orthostatic blood pressure decline was significantly higher in the older subjects compared to the young. The older subjects also had a smaller increase in HR compared to young subjects. Despite a larger decline in their MAP, the older group had a very similar decline in BFV in both vascular territories compared to young. This was accomplished by a significantly larger reduction in CVR in the MCA and PCA vascular territories of the older subjects.
Table 2.
Hemodynamic Responses to Standing
| Sitting | YOUNG | OLD |
|---|---|---|
| MAP, mmHg | 88(17) | 85(12) |
| MCA MFV, cm/s | 70(14)† | 50(18)*† |
| PCA MFV, cm/s | 40(14) | 36(14) |
| MCA CVR, mmHg/cm/s | 1.3(0.5)† | 2.1(1.2)* |
| PCA CVR, mmHg/cm/s | 2.5(0.9) | 2.8(1.5) |
| HR, bpm | 70(12) | 63(4) |
| Standing | ||
| MAP, mmHg | 70(17) | 59(13) |
| MCA MFV, cm/s | 57(14)† | 43(18)† |
| PCA MFV, cm/s | 33(15) | 30(16) |
| MCA CVR, mmHg/cm/s | 1.3(0.5)† | 1.7(1.0) |
| PCA CVR, mmHg/cm/s | 2.4(0.8) | 2.5(1.3) |
| HR, bpm | 94(9) | 77(7)* |
| Hemodynamic Response | ||
| % Change MAP | −21(7) | −31(11)* |
| % Change MCA MFV | −20(7) | −16(12)*† |
| % Change PCA MFV | −18(13) | −19(15) |
| % Change MCA CVR | −0.8(10) | −17(8)*† |
| % Change PCA CVR | −2.0(16) | −13(9)* |
| Change HR | 24(8) | 15(5)* |
| Rate of Recovery (RoR) | ||
| MCA ROR (sec−1) | 0.27(0.39) | 0.12(0.05) |
| PCA ROR (sec−1) | 0.29(0.33) | 0.13(0.05) |
All values represent Means (SD).
Significant differences between young and old.
Significant differences between MCA and PCA.
Comparing middle and posterior cerebrovascular territories, there were no statistically significant differences in the CVR and BFV responses to orthostasis in the young subjects. However, in the older subjects, there was a larger decline in BFV and a smaller decline in CVR in the PCA as compared to the MCA territory.
The rate of recovery (RoR), or rate of autoregulation for the PCA and MCA blood flow velocities were generally higher in the young compared to the older subjects, but these differences did not reach statistical significance.
CO2 Reactivity
Table 3 shows the values for CO2 reactivity in the PCA and MCA territory for both groups of subjects. In the older subjects there were no significant regional differences in vasoreactivity. However, in the young subjects vasoreactivity was significantly higher the MCA than the PCA territory.
Table 3.
Vasoreactivity
All values represent Means (SD).
MCA= Middle Cerebral Artery, PCA= Posterior Cerebral Artery
Significant difference between young and old (p<0.02)
Significant differences between MCA and PCA (p<0.04)
Discussion
To our knowledge, this is the first study comparing cerebral hemodynamics in the PCA and MCA territories of healthy young and old volunteers. Recently, two studies compared MCA and PCA CBF regulation, but the effects of aging were not explored [10, 11]. Rosengarten and Kaps [11] showed that in healthy young volunteers (aged 26.7±0.3 years), the PCA BFV decline in response to thigh cuff deflation recovered 900 ms faster than the MCA response. However, they attributed this accelerated recovery to a smaller initial BFV decline in the PCA and concluded that cerebral autoregulation in the MCA and PCA territories did not differ. In contrast, Haubrich et al [10] studied thirty older adults (mean age 65 ± 10 years) without cerebrovascular disease or dysautonomia. They showed that in older subjects during supine rest and passive tilt, transfer function gains were significantly higher in the MCA than the PCA territory. They concluded that the PCA territory of older subjects permitted greater transmission of arterial blood pressure oscillations into CBF, a finding suggestive of impaired posterior autoregulation.
Our study demonstrates that healthy aging is associated with a remarkable preservation of cerebral autoregulation. Our older subjects had a significantly larger decline in MAP and a smaller increase in HR during postural change, most likely secondary to an impaired baroreflex function [12]. Despite the larger orthostatic stress, there was a significantly higher cerebral vasodilatory response in both vascular territories in the older subjects, indicating a preserved autoregulatory response. However, among elderly subjects BFV declined to a greater extent in the PCA territory compared to the MCA. While the magnitude of this difference was small and not associated with posterior circulatory symptoms, the PCA circulation may be more vulnerable to hypoperfusion. This finding is supported by Haubrich et al [10], which showed that the PCA territory CBF is more sensitive to beat-to-beat blood pressure variations.
The effect of aging on cerebral autoregulation has been the subject of several recent studies. Contrary to animal studies, which have shown age related alterations in cerebral autoregulation, human studies report preserved cerebral autoregulatory capacity of the MCA with age[4]. Our study, suggests that while healthy aging is associated with preserved cerebral autoregulatory capacity, there may be an increased vulnerability of the posterior cerebral circulation to blood pressure fluctuations. This finding may explain the vulnerability of the PCA territory to syncope in elderly subjects..
Finally, we compared CO2 reactivity in the two vascular territories in young and elderly subjects to determine if there are regional differences in vasoreactivity associated with aging. In the young subjects MCA vasoreactivity was significantly higher than the PCA. With aging, both the MCA and PCA vasoreactivity declined. Albeit the differences between MCA and PCA vasoreactivity did not reach statistical significance in the elderly (p=0.07), the lowest vasoreactivity was seen in the PCA territory. Thus, vasomotor responsiveness to alterations in both perfusion pressure and CO2 may be down-regulated in the posterior circulation.
It is important to note that the PCA may receive collaterals from the anterior circulation via the posterior communicating arteries and may therefore be a poor surrogate for the basilar artery, which is the main supply to the brain stem. Unfortunately, simultaneous continuous monitoring of the basilar and MCA arteries during the sit-to-stand protocol was not possible using our TCD device. One previous study compared regional cerebral autoregulation and vasoreactivity in the basilar and MCA arteries in healthy young subjects using TCD [14]. The study was performed in the supine position with the basilar probe held manually. Autoregulatory and reactivity measures were similar in the basilar artery and the MCA. It is hoped that future technical developments will allow us to extend our study to the basilar artery in healthy older subjects and those with vascular disease.
In conclusion, we have shown that healthy aging is associated with overall preserved autoregulation in response to posture change. However, in the older subjects the PCA territory blood flow may be more vulnerable to hypoperfusion during orthostatic stress. The lower vasoreactivity of the PCA territory may be one possible mechanism for the lower vasodilatory response.
Acknowledgments
This work was supported by a generous donation from Mr. and Mrs. Robert Krakoff at the Hebrew Rehabilitation Center for Aged and by grants AG04390, AG08812, and AG05134 from the National Institute on Aging, Bethesda, MD.
Dr. Sorond is the recipient of Mentored Clinical Scientist K12 Award (AG00294) from the National Institute on Aging. Dr. Lipsitz holds the Irving and Edyth S. Usen and Family Chair in Geriatric Medicine.
References
- 1.Lipsitz LA. Orthostatic hypotension in the elderly. N Engl J Med. 1989;321(14):952–7. doi: 10.1056/NEJM198910053211407. [DOI] [PubMed] [Google Scholar]
- 2.Newell DW, et al. Comparison of flow and velocity during dynamic autoregulation testing in humans. Stroke. 1994;25(4):793–7. doi: 10.1161/01.str.25.4.793. [DOI] [PubMed] [Google Scholar]
- 3.Tiecks FP, et al. Comparison of static and dynamic cerebral autoregulation measurements. Stroke. 1995;26(6):1014–9. doi: 10.1161/01.str.26.6.1014. [DOI] [PubMed] [Google Scholar]
- 4.Lipsitz LA, et al. Dynamic regulation of middle cerebral artery blood flow velocity in aging and hypertension. Stroke. 2000;31(8):1897–903. doi: 10.1161/01.str.31.8.1897. [DOI] [PubMed] [Google Scholar]
- 5.Balldin UI, et al. Cerebral artery blood flow velocity changes following rapid release of lower body negative pressure. Aviat Space Environ Med. 1996;67(1):19–22. [PubMed] [Google Scholar]
- 6.Tiecks FP, et al. Effects of the valsalva maneuver on cerebral circulation in healthy adults. A transcranial Doppler Study. Stroke. 1995;26(8):1386–92. doi: 10.1161/01.str.26.8.1386. [DOI] [PubMed] [Google Scholar]
- 7.Aaslid R, Markwalder TM, Nornes H. Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J Neurosurg. 1982;57(6):769–74. doi: 10.3171/jns.1982.57.6.0769. [DOI] [PubMed] [Google Scholar]
- 8.Aaslid R, et al. Cerebral autoregulation dynamics in humans. Stroke. 1989;20(1):45–52. doi: 10.1161/01.str.20.1.45. [DOI] [PubMed] [Google Scholar]
- 9.Maeda H, et al. Reactivity of cerebral blood flow to carbon dioxide in hypertensive patients: evaluation by the transcranial Doppler method. J Hypertens. 1994;12(2):191–7. [PubMed] [Google Scholar]
- 10.Haubrich C, et al. Dynamic autoregulation testing in the posterior cerebral artery. Stroke. 2004;35(4):848–52. doi: 10.1161/01.STR.0000120729.99039.B6. [DOI] [PubMed] [Google Scholar]
- 11.Rosengarten B, Kaps M. Cerebral autoregulation in middle cerebral artery territory precedes that of posterior cerebral artery in human cortex. Cerebrovasc Dis. 2002;13(1):21–5. doi: 10.1159/000047741. [DOI] [PubMed] [Google Scholar]
- 12.Ferrari AU. Modifications of the cardiovascular system with aging. Am J Geriatr Cardiol. 2002;11(1):30–3. doi: 10.1111/1467-8446.00044-i1. [DOI] [PubMed] [Google Scholar]
- 14.Park CW, et al. Autoregulatory response and CO2 reactivity of the basilar artery. Stroke. 2003;34(1):34–9. doi: 10.1161/01.str.0000047122.42591.b3. [DOI] [PubMed] [Google Scholar]