Abstract
BACKGROUND
Although originally isolated from the bone marrow, mesenchymal stem cells (MSCs) have recently been detected in other tissues. However, little is known about MSCs in the brain.
OBJECTIVE
To determine the extent to which cells with the features of MSCs exist in normal brain tissue and to determine the location of these cells in the brain.
METHODS
Single-cell suspensions from mouse brains were cultured according to the same methods used for culturing bone marrow–derived MSCs (BM-MSCs). These brain-derived cells were analyzed by fluorescence-activated cell sorting for surface markers associated with BM-MSCs (stem cell antigen 1 [Sca-1+], CD9+, CD45−, CD11b−, and CD31−). Brain-derived cells were exposed to mesenchymal differentiation conditions. To determine the locations of these cells within the brain, sections of normal brains were analyzed by immunostaining for Sca-1, CD31, and nerve/glial antigen 2.
RESULTS
Cells morphologically similar to mouse BM-MSCs were identified and called brain-derived MSCs (Br-MSCs). Fluorescence-activated cell sorting indicated that the isolated cells had a surface marker profile similar to BM-MSCs, ie, Sca-1+, CD9+, CD45−, and CD11b−. Like BM-MSCs, Br-MSCs were capable of differentiation into adipocytes, osteocytes, and chondrocytes. Immunostaining indicated that Sca-1+ Br-MSCs are located around blood vessels and may represent progenitor cells that serve as a source of mesenchymal elements (eg, pericytes) within the brain.
CONCLUSION
Our results indicate that cells similar to BM-MSCs exist in the brain. These Br-MSCs appear to be located within the vascular niche and may provide the mesenchymal elements of this niche. Because MSCs may be part of the cellular response to tissue injury, Br-MSCs may represent targets in the therapy of pathological processes such as stroke, trauma, and tumorigenesis.
Keywords: Brain-derived cells, Endothelial cells, Mesenchymal stem cells, Pericytes, Perivascular location, Stem cell antigen-1
Bone marrow is a complex tissue and is a source of several stem/progenitor cell populations. In addition to hematopoietic stem cells and endothelial progenitors,1–4 the bone marrow is also a source of a less well-characterized stem cell/progenitor population referred to as mesenchymal stem cells (MSCs). This cell population was originally identified in the 1970s by Friedenstein et al,1,5 who placed whole bone marrow in plastic culture dishes and, after removing the nonadherent (hematopoietic) cells, was left with an adherent cell population that had the ability to differentiate into colonies resembling bone or cartilage. Castro-Malaspina et al6 used similar methods to isolate adherent bone marrow cells, which others showed could be induced to differentiate into a variety of mesenchymal elements, including fat, bone, cartilage, and bone marrow stroma.7–10 Most recently, Pittenger and Marshak4 and Pittenger et al9 isolated a bone marrow population, referred to as MSCs, that grew as adherent cells on plastic, could be expanded ex vivo to high passage numbers, and could be differentiated into multiple mature connective tissue cell types, particularly adipocytes, chondrocytes, and osteocytes. Although there is no specific surface marker that defines MSCs, human MSCs are negative for CD45 and CD31 and express CD105, CD73, CD90, CD44, and CD166.11 In the mouse, there is less consensus regarding the defining surface markers,12–14 but several studies indicate that mouse MSCs are negative for CD45, CD11b, and CD31 and positive for stem cell antigen-1 (Sca-1), and CD9.14,15 Physiologically, these cells are thought to give rise to the bone marrow stromal elements that provide the microenvironment and supporting structure necessary for marrow homeostasis.1,4,16–19
For the neurosurgeon, MSCs are of interest because of their potential use in the therapy of a variety of neurological disorders.12 In the field of neuro-oncology, we and others have reported that ex vivo expanded human bone marrow–derived MSCs (BM-MSCs) selectively localize to human gliomas after systemic administration and can be engineered to deliver antiglioma therapies to experimental human glioblastomas such as interferon-β and tumor necrosis factor-α–related apoptosis-inducing ligand.20,21 Exogenous MSCs also localize to areas of stroke,22 traumatic brain injury,23 and intracerebral hemorrhage,24 suggesting a potential role in these pathological processes.
MSCs are also of interest because of the potential involvement of endogenous MSCs in normal physiological responses to injury and inflammation and because they may comprise the stroma of solid tumors, the milieu of which is much like a nonhealing wound.25 Recent evidence suggests that endogenous MSCs may be an important component of glioblastoma multiforme, the most common adult malignant brain tumor. Specifically, cells resembling MSCs can be isolated from fresh glioma specimens obtained at surgery.26,27 Like BM-MSCs, these tumor derived-MSCs grow as adherent cells in culture; differentiate into adipocytes, chondrocytes, and osteocytes; and express the requisite MSC surface markers.26,27 These tumor-derived MSCs are thought to contribute to the stroma of human gliomas.
Although it is postulated that MSCs may be recruited into areas of inflammation, injury, and tumors from a pool of circulating BM-MSCs originating from the bone marrow, to date there is conflicting evidence supporting the notion that BM-MSCs actually circulate in the bloodstream. However, an alternative source of MSCs may be the local tissues within peripheral organs. Cells similar to MSCs have been reported to be found in tissues other than the bone marrow, including fat, dermis, muscle, liver, pancreas, lung, endometrium, kidney, vessels, and brain.28–36 It is suggested that MSCs may reside throughout the body in most postnatal organs.28,32 Although Rutka et al37 reported in the 1980s that nonglial, mesenchymal cells can be cultured from normal brain tissue, the isolation of cells with the specific characteristics of MSCs from the brain has only begun to be explored, and the existence of brain-derived MSCs (Br-MSCs) and their location within the brain remains unclear. Identifying MSCs in normal brain tissue would provide new insight into the capacity of central nervous system tissues to respond to inflammation and tumor formation.
In this context, we sought to determine the extent to which cells with the features of MSCs exist in normal brain tissue and to determine the location of these cells in the brain. Consequently, we isolated and cultured cells from normal mouse brain using the same methods used to isolate BM-MSCs and thereby identified cells that were similar to BM-MSCs in terms of morphology, surface markers, and differentiation pattern, which we called brain-derived MSCs (Br-MSCs). Histological studies suggest that these Br-MSCs reside within the perivascular niche. We propose that these Br-MSCs may represent a pool of local stem cells that maintain normal homeostasis of the perivascular niche and are capable of responding to tissue stresses within the brain.
MATERIALS AND METHODS
Animals
Four- to 8-week-old male severe combined immune-deficient (SCID) mice (Jackson Laboratory, Bar Harbor, Maine), athymic nude mice (University of Texas M. D. Anderson Cancer Center, Houston, Texas), and C57/BL6 mice (M. D. Anderson Cancer Center) were used in these studies. Mice were housed in microisolator cages under sterile conditions and observed for at least 1 week before study initiation to ensure proper health. Lighting, temperature, and humidity were controlled centrally. All experiments were approved by the Institutional Animal Care and Use Committee at M. D. Anderson.
Isolation and Culture of Mouse Br-MSCs
To isolate Br-MSCs, SCID (n = 12) and C57/BL6 (n = 4) mice were anesthetized with a ketamine/xylazine solution (1.16 g ketamine and 2.3 g xylazine per 1 kg body weight) delivered intraperitoneally. Whole brains from 4 mice were dissected and minced with a scalpel in minimal essential medium-α (MEM-α; Mediatech, Herndon, Virginia); the resulting cell suspension was combined and washed twice in serum-free MEM-α; and single-cell suspensions were plated in a 10-cm2 cell culture dish at a density of 2 × 106 cell/cm2. These cells were cultured in complete MSC medium consisting of MEM-α, 10% fetal bovine serum (FBS; Lonza, Basel, Switzerland), 2 mmol/L l-glutamine (Mediatech), and penicillin/streptomycin (50 U/mL and 50 mg/mL, respectively; Flow Laboratories, Rockville, Maryland). After 24 hours, nonadherent cells were removed by 2 washes with phosphate-buffered saline (PBS; Mediatech), and adherent cells were cultured until they reached confluence. Cells were then trypsinized (0.25% trypsin with 0.1% EDTA) and subcultured at a density of 5000 cells/cm2. These cells were cultured continuously for 12 months through 60 passages, consistent with their role as progenitor/stem cells. Cell cultures were observed with an inverted phase-contrast microscope (Axiovert 200, Zeiss, Hallbergmoos, Germany) to see their morphology. Photographs of cells were taken with a digital camera (AxioCam MRc, Zeiss) using Xcap-Plus version 2.1 software (Epix Inc, Buffalo Grove, Illinois) at each passage. On the other hand, mouse BM-MSCs were harvested as previously described.14
Flow Cytometry
To investigate whether the Br-MSCs have properties similar to those of BM-MSCs, cells were trypsinized and counted in a Vi-Cell machine (version 1.01, Beckman Coulter Inc, Fullerton, California). They were washed in PBS, and the cell pellet was resuspended in fluorescence-activated cell sorting (FACS) buffer (PBS with 10% FBS) at a concentration of 5 × 105 cells per 100 µL. These single-cell suspensions were incubated at 4°C for 30 minutes with phycoerythrin-, FITC-, Alexa Fluor 647-, or allophycocyanin-conjugated antibodies against mouse Sca-1 (eBioscience, San Diego, California), CD9 (eBioscience), CD45 (eBioscience), CD11b (eBioscience), CD31 (BD Biosciences, San Jose, California), and IgG (eBioscience). For the detection of nerve/glial antigen 2 (NG2) proteoglycan, an FITC-conjugated secondary antibody (Millipore, Billerica, Massachusetts) was used after primary antibody incubation (NG2 antibody; Santa Cruz Biotechnology, Inc, Santa Cruz, California). FACS analysis was performed with a FACScalibur (BD Biosciences) flow cytometer equipped with BD CellQuest Pro version 5.1.1 software (Apple, Cupertino, California), with 20 000 events recorded for each sample. Surface antigens in mouse BM-MSCs were also analyzed in the same manner. We performed the FACS analyses > 3 times.
In Vitro Differentiation
To determine the mesenchymal differentiation potential of Br-MSCs, we used a trilineage differentiation test identical to that described for BM-MSCs. After primary culture in complete MSC medium as described above, we tested the capacity of single Br-MSC clones to differentiate along adipogenic, osteogenic, and chondrogenic lineages.
For adipogenic differentiation, Br-MSCs were seeded at a density of 2 × 104 cells/cm2 in a 6-well plate with complete MSC medium. At confluence, cell differentiation was induced with adipogenic differentiation medium, which contained MEM-α, 10% FBS, 1 µmol/L dexamethasone (American Reagent Laboratories, Inc, Shirley, New York), 100 µg/mL 3-isobutyl-methyl-xanthine (Sigma, St Louis, Missouri), 60 µmol/L indomethacin (Sigma), 5 µg/mL bovine insulin (Life Technologies, Carlsbad, California), 2 mmol/L l-glutamine, and penicillin/streptomycin (50 U/mL and 50 mg/mL, respectively). These cells were fed this fresh medium every 3 to 4 days for 3 weeks. In control experiments, cells were incubated for the same period of time in complete MSC medium. On days 22 to 28, the cells were washed in PBS and fixed in 10% formalin (Fisher Scientific, Fair Lawn, New Jersey) for 1 hour at room temperature. After fixation, the cells were rinsed with deionized water several times, followed by the addition of 60% isopropanol (Pharmco-AAPER, Brookfield, Connecticut) and allowed to sit for 5 minutes. Oil Red O (Sigma) solution was added to each well. After 5 minutes, the cells were rinsed with deionized water and briefly counterstained with hematoxylin (Fisher Scientific).
For osteogenic differentiation, Br-MSCs were plated at a density of 3 × 103 cells/cm2 in a 6-well plate. The next day, the medium was replaced with osteogenic differentiation medium, which is composed of MEM-α, 10% FBS, 0.1 µmol/L dexamethasone, 10 mmol/L β-glycerophosphate (Sigma), 50 µg/mL ascorbic acid-2-phosphate (Sigma), and penicillin/streptomycin (50 U/mL and 50 mg/mL, respectively). These cells were fed this fresh medium every 3 to 4 days for 4 weeks. In control experiments, cells were incubated for the same period of time in complete MSC medium. On day 28, cell cultures were washed twice with PBS and fixed in 70% ice-cold ethanol (Pharmco-AAPER) for 1 hour, followed by washing with deionized water. The cells were stained with 40 mmol/L (pH 4.2) Alizarin Red (Sigma) for 10 minutes at room temperature with rotation, followed by washing with deionized water 5 times.
For chondrogenic differentiation, a modification of previous MSC protocols was used.38 Br-MSCs were trypsinized and washed in serum-containing medium. Aliquots of 250 000 cells suspended in 0.5 mL of medium were placed in 15-mL conical polypropylene tubes (Becton Dickinson, Franklin Lakes, New Jersey). Then, the cells were gently centrifuged for 5 minutes at 150g and left at the bottom of the tubes, which were placed in an incubator with caps loosened to permit gas exchange. The cells formed small pellets that were cultured for 4 weeks in chondrogenic differentiation medium, which was composed of Dulbecco’s modified Eagle’s medium/Nutrient Mixture F-12 (DMEM/F12, Mediatech), 1 mmol/L sodium pyruvate (Sigma), 0.17 mmol/L ascorbic acid-2-phosphate (Fluka, Bucks, Germany), 0.1 µmol/L dexamethasone, and 20 µg/mL transforming growth factor-β3 (Ontogeny Research Products, Cambridge, Massachusetts). Every 3 to 4 days, the cells were fed fresh medium. In control experiments, the cells were incubated for the same period of time in complete MSC medium. These pellets were fixed in 10% formalin for 1 hour at room temperature and then embedded in paraffin sections stained with Safranin O (Sigma) for glycosaminoglycans.
Immunohistochemistry and Immunofluorescent Labeling
To determine the locations of Br-MSCs, we studied normal mouse brains using immunohistochemical analysis and double-immunofluorescent labeling. For immunohisto-chemical analyses, male athymic (nude) mice were anesthetized and euthanized by intracardiac perfusion of PBS (2 mL) followed by 4% paraformaldehyde (EMS, Hatfield, PA). Brains were removed and fixed in 10% formalin. Paraffin sections were prepared by the usual method. These sections were processed for immunohisto-chemical analysis using goat anti-mouse Sca-1 (R&D Systems, Inc, Minneapolis, Minnesota). Biotinylated horse anti-goat IgG antibody was used as a secondary antibody. Vectastain ABC kit (Vector Laboratories, Burlingame, California) and DAB substrate (SK 4100, Vector Laboratories) were used for color development. For immunofluorescence analyses, the brains of C57BL6 mice were collected, immediately frozen with optimal cutting temperature compound (Sakura Finetek USA Inc, Torrance, California) by acetone with dry ice, and stored at −80°C until use. Frozen sections (5 µm) were fixed with 4% paraformaldehyde for 5 minutes, washed, blocked with 5% bovine serum albumin in PBS for 30 minutes at room temperature, and incubated with goat anti-mouse Sca-1 antibodies (15 µg/mL, R&D Systems, Inc) with blocking solution at 4°C overnight. For visualization, the sections were incubated with Alexa Fluor 488 donkey anti-goat IgG (Molecular Probes, Inc, Eugene, Oregon) at 1:200 dilution for 45 minutes at room temperature. After washing, the sections were incubated with rabbit anti-NG2 antibodies (1:200, Millipore Corporate Headquarters, Billerica, Massachusetts) or rat anti-mouse CD31 antibodies (1:40, Abcam Inc, Cambridge, Massachusetts) for double staining at room temperature for 2 hours, followed by incubation with Alexa Fluor 594 goat anti-rabbit IgG or Alexa Fluor 594 goat antirat IgG (Molecular Probes, Inc) at 1:200 dilution for 45 minutes at room temperature. DAPI (Vectashield H-1500, Vector Laboratories) was used for nuclear staining. A fluorescence microscope (Axiovert 200M, Zeiss) and Axiovision version 4.5 software (Zeiss) were used for observation.
Statistics
Statistically significant differences (P < .05) were estimated with the Mann-Whitney U test, and data are expressed as mean ± standard deviation. Statistical analyses were performed with SPSS version 12.01 software (SPSS Inc, Chicago, Illinois).
RESULTS
Isolation of MSC-Like Cells From Normal Mouse Brains
To determine the extent to which cells with the features of MSCs could be isolated from normal brain, the brains of mice (n = 4 per culture) were rapidly removed and minced, and a single-cell suspension was plated in 10% MSC-certified FBS on uncoated plastic culture dishes using methods identical to those used for isolating BM-MSCs (See Materials and Methods). Four independent cultures were established (3 from 12 SCID mice and 1 from 4 BL6/C57 mice). In each case, initial cultures contained highly heterogeneous populations of cells (Figure 1A), which became increasingly homogeneous, and by passage 10 to 15, a uniform population of Br-MSCs (Figure 1B) was evident. These Br-MSCs were capable of long-term culture and have been maintained for up to 60 passages without changing their morphology. Each of the cultures progressed at similar rates. These Br-MSCs had a morphology and doubling time (~36 h) similar to those of BM-MSCs (Figure 1C) obtained from C57/BL6 mice.
FIGURE 1.
The morphology of brain-derived cells at different passages. A, after 1 week, isolated cells from the brain were subconfluent and had heterogeneous morphology. Tube-forming cells were seen (arrow) (magnification × 200). B, at passage 28, cells had a more homogenous appearance (magnification × 200). C, the morphology of bone marrow–derived mouse mesenchymal stem cells is shown for comparison (original magnification × 200).
Flow Cytometry for MSC Markers
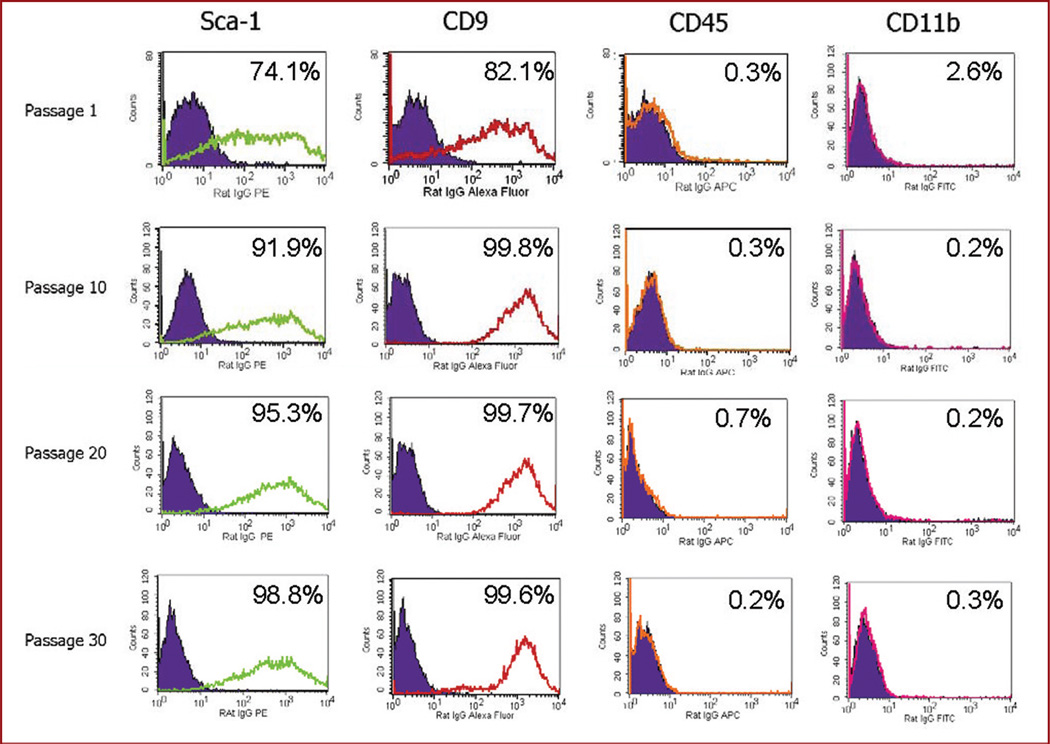
Although there is no pathognomonic marker, it is generally agreed that mouse BM-MSCs are positive for Sca-1 and CD9 and negative for CD45 and CD11b. Consequently, cultured brain cells were analyzed by flow cytometry for these markers. Consistent with their identity as MSCs, cultured cells from the brains were Sca-1+, CD9+, CD45−, and CD11b− (Figure 2 and Table 1). As expected, most sca-1 cells coexpressed CD9 (Table 1). This profile was identical to that of MSCs isolated from the bone marrow of mice (Table 1). Consistent with the initial heterogeneity of the cells, the percentage of Sca-1+ and CD9+ cells increased with each passage; by passage 15, 95% of the cultured Br-MSCs expressed Sca-1 and CD9 (Figure 2). After passage 15, the surface expression of these markers remained stable over multiple subsequent passages.
FIGURE 2.
Flow cytometric analysis for mesenchymal (stem cell antigen 1 [Sca-1], CD9) and hematopoietic (CD45, CD11b) surface markers of brain-derived cells obtained at increasing passage. Each column represents the specific marker tested. Each row represents the labeled passage number. Within each graph, the control antibody is shown as the filled curve, and the tested antibody is shown as the unfilled curve. The percentage of cells expressing the marker is also shown in each graph. Cells were positive for Sca-1 and CD9 and negative for CD45 and CD11b, consistent with known markers of bone marrow–derived mesenchymal stem cells. The marker profile remained stable over time.
TABLE 1.
Expression of Surface Markers on Cultured Mouse Brain-Derived Mesenchymal Stem Cells and Mouse Bone Marrow--Derived Mesenchymal Stem Cellsa
| Marker | Brain-Derived Mesenchymal Stem Cells (n = 9), % |
Bone Marrow-Derived Mesenchymal Stem Cells (n = 4), % |
P Value |
|---|---|---|---|
| Sca-1 | 97.9 ± 2.0 | 99.4 ± 1.0 | NS |
| CD9 | 99.5 ± 0.3 | 99.2 ± 1.1 | NS |
| Sca-1/CD9 | 96.7 ± 2.1 | 99.2 ± 1.0 | NS |
| CD45 | 1.3 ± 1.4 | 0.6 ± 0.8 | NS |
| CD11b | 0.7 ± 0.2 | 0.5 ± 1.1 | NS |
Sca-1, stem cell antigen 1.
Brain- or bone marrow–derived mesenchymal stem cells were cultured. Cells were collected at passage 15 or greater and analyzed by flow cytometry using antibodies against the specific antigens. Results are expressed as mean ± standard deviation for the particular antigen. N is the number of cultures tested. Statistical significance was evaluated with the Mann-Whitney U test.
In Vitro Differentiation
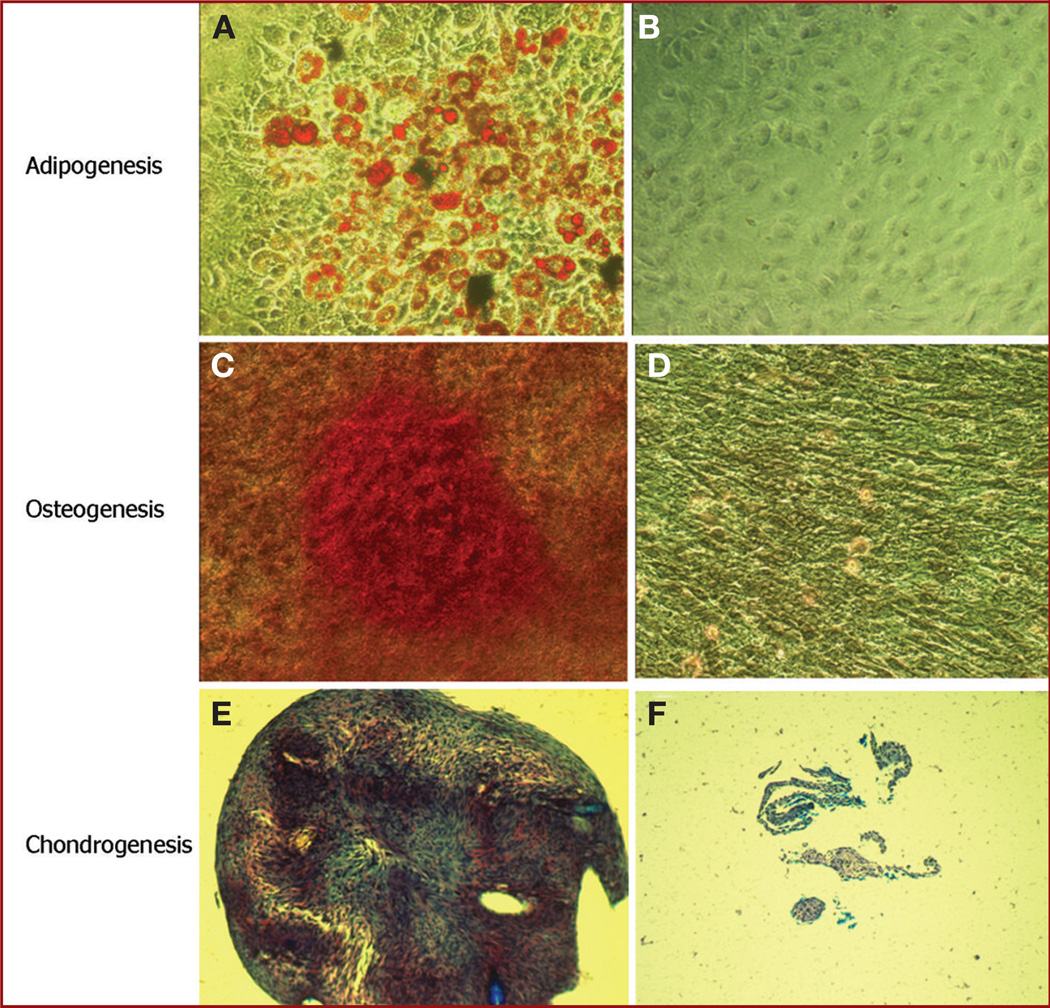
A defining feature of BM-MSCs is the ability to differentiate into multiple mesenchymal cell types, specifically adipocytes, osteocytes, and chondrocytes. Consequently, we evaluated the mesenchymal differentiation potential of 2 Br-MSCs cultures at several passages. During adipogenic induction, cells filled with lipid droplets appeared by day 3 and increased in number by day 22. At the end of the induction period, cells with lipid droplets were consistently evident, with individual cells showing the typical characteristics of adipocytes with lipid droplets filling the cytoplasm by Oil Red O staining (Figure 3A). In the control experiments (Figure 3B), rare spontaneous adipogenesis was detected in a few cells in noninduced medium, suggesting intrinsic spontaneous adipogenesis. During osteogenic induction, the cells changed from a cuboidal appearance to a rounder shape. After a 4-week induction period, calcium deposits were visible in the osteogenesis induction culture, which was confirmed by Alizarin Red staining (Figure 3C). In the control experiments, most cells in noninduced medium did not have calcium deposits (Figure 3D), but in a few cells, spontaneous osteogenesis was also detected. Finally, Br-MSCs pellets were cultured in chondrogenesis differentiation medium for 4 weeks, after which time Safranin O staining revealed deposition of glycosaminoglycan in the pellet matrix (Figure 3E), which was consistent with differentiation into chondrocytes. No chondrogenesis was detected in control cultures in noninduced medium, and only disorganized cells were seen (Figure 3F). Thus, the cells isolated from the brain were capable of trilineage mesenchymal differentiation, a cardinal feature of MSCs.
FIGURE 3.
Photomicrographs of brain-derived mesenchymal stem cells (Br-MSCs) demonstrating trilineage mesenchymal differentiation. A, differentiation of Br-MSCs into adipocytes was confirmed by Oil Red O staining (orange) for lipid formation (magnification × 320). B, corresponding control for A in which cells were grown in noninducing medium. Control cultures were negative for adipogenesis (magnification × 320). C, differentiation of Br-MSCs into osteocytes was confirmed by staining with Alizarin Red (red color) for calcium formation (magnification × 200). D. corresponding control for B in which noninduced Br-MSCs were negative for Alizarin Red staining (original magnification × 200). E, differentiation of Br-MSCs into chondrocytes was confirmed by pellet culture with chondrogenic medium as described in Materials and Methods. Pellets were positive for Safranin O staining (reddish or purple), which revealed glycosaminoglycan (magnification × 100). F, corresponding control for E in which cells were grown in pellet culture noninduced medium. Only small, poorly organized pellets (consistent with lack of cartilage matrix) that were negative for Safranin staining were evident (magnification × 100).
Immunohistochemical Analysis for Sca-1, NG2, and CD31
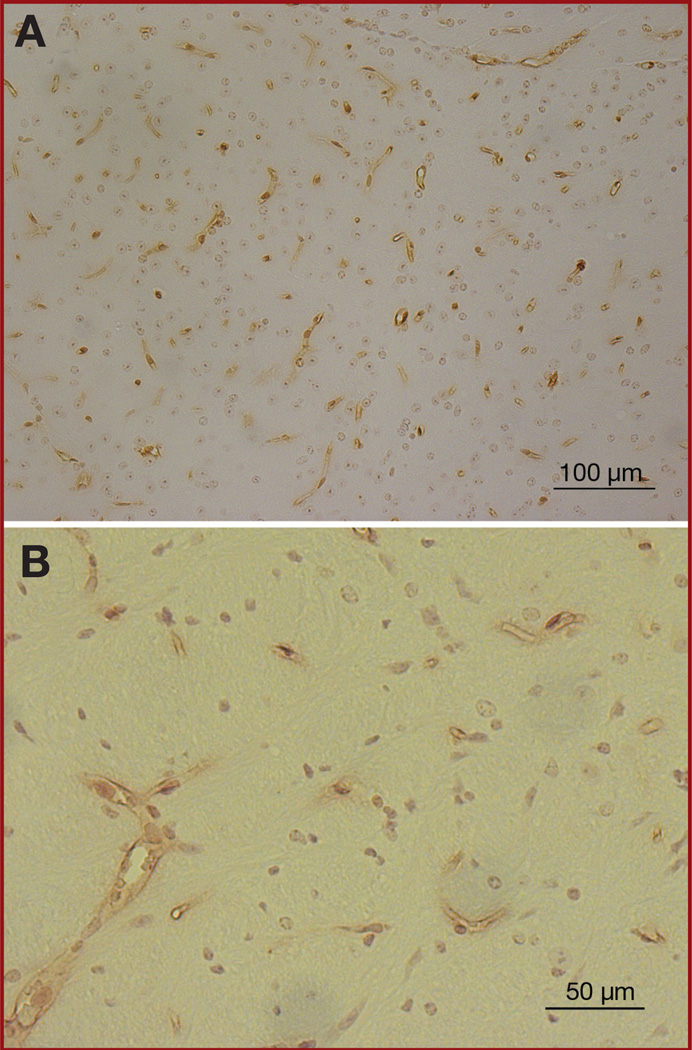
To determine the location of Br-MSCs in the brain, we analyzed paraffin sections of normal brains of mice by immunohistochemical staining with Sca-1 antibody. Although Sca-1 is not exclusive to Br-MSCs, its high surface expression on Br-MSCs makes it a good screening agent for Br-MSCs in the brain. In this analysis, Sca-1+ cells were found within blood vessels, both large and small, within the parenchyma of the brain (Figure 4), suggesting that Br-MSCs may be located within the vascular niche.
FIGURE 4.
Photomicrographs of immunohistochemical staining with anti-stem cell antigen 1 (Sca-1) antibody in the normal mouse brain. A, Sca-1–positive cells (brown) were distributed throughout the brain and were located almost exclusively in the vasculature (magnification × 200). B, high-powered view of Sca-1+ cells verifying location within the vasculature (magnification × 400).
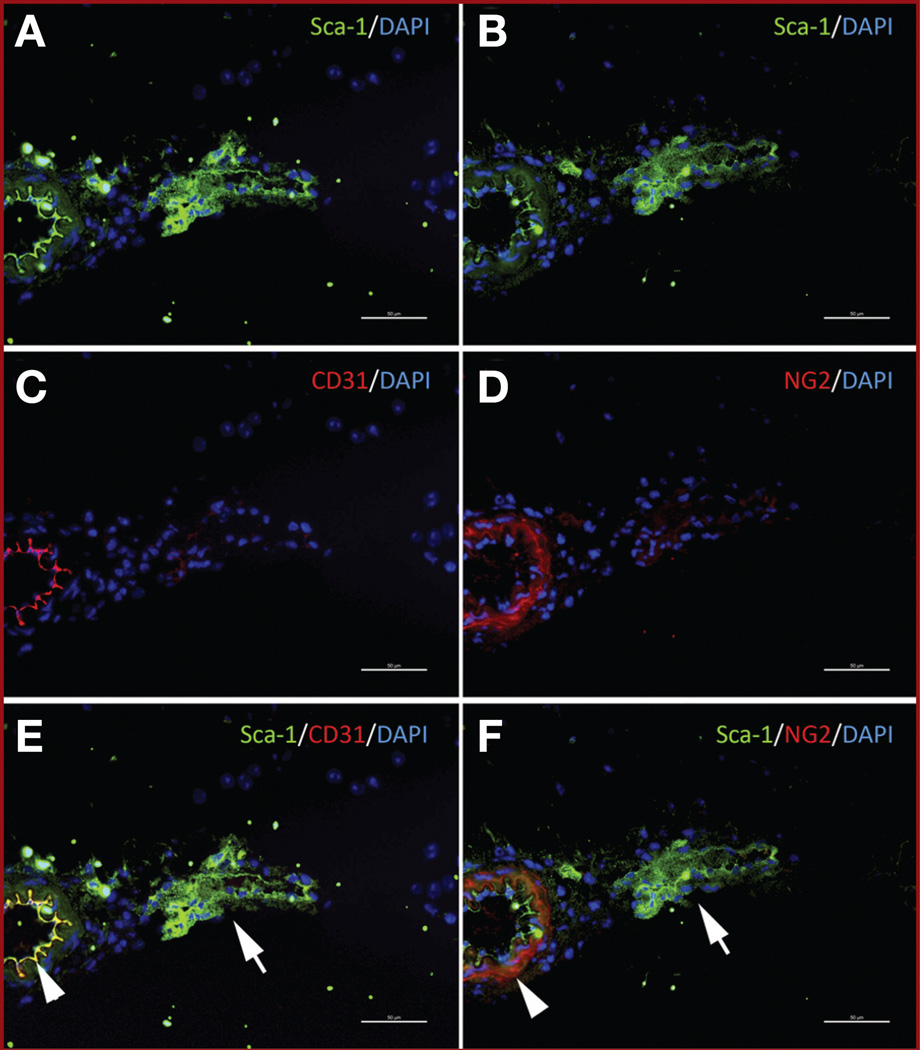
Intracerebral vessels are composed of endothelial cells and surrounding pericytes. To determine whether the Sca-1+ cells were distinct from endothelial cells and pericytes, we performed double-immunofluorescent staining using antibodies against Sca-1 (to identify stem cells) and CD31, a known marker of mature endothelial cells (Figure 5A, 5C, and 5E), and for Sca-1 and NG2, we used a known marker of mature pericytes (Figure 5B, 5D, and 5F). Merged fluorescent images indicated that a population of cells within blood vessels stained only for Sca-1+ (stained green in Figure 5E and 5F) and did not stain for either CD31 or NG2, suggesting that cells distinct from mature pericytes and endothelial cells and staining with the MSC marker Sca-1 are located in the perivascular niche. However, a population of Sca-1 cells also costained for CD31 (yellow cells within vessels; Figure 5E), suggesting that mature endothelial cells may derive from Sca-1+ cells and thus possibly MSCs. Similarly, a population of the Sca-1+ cells also stained for NG2 (yellow cells within vessels; Figure 5F), indicating that mature pericytes may also derive from Br-MSCs. Thus, Br-MSCs may be important stem cells that serve to repopulate the mesenchymal cells within the perivascular niche.
FIGURE 5.
Photomicrographs of normal mouse brain after double-immunofluorescent labeling. A, C, and E were stained with anti-stem cell antigen 1 (Sca-1) antibody (secondary antibody Alexa Fluor 488, green) and anti-CD31 antibody (secondary antibody Alexa Fluor 594, red). A and C are unmerged images, and E is the merged image. Arrow indicated Sca-1+/CD31+ cells. B, D, and F were stained with anti-Sca-1 antibody (secondary antibody Alexa Fluor 488, green) and anti-nerve/glial antigen 2 (NG2) antibody (secondary antibody Alexa Fluor 594, red). B and D are unmerged images, and F is the merged image. Arrow indicates Sca-1+/NG2+ cells. Merged images also reveal Sca-1+/NG2−/CD31− cells (green only).
FACS Analysis of Sca-1, NG2, and CD31
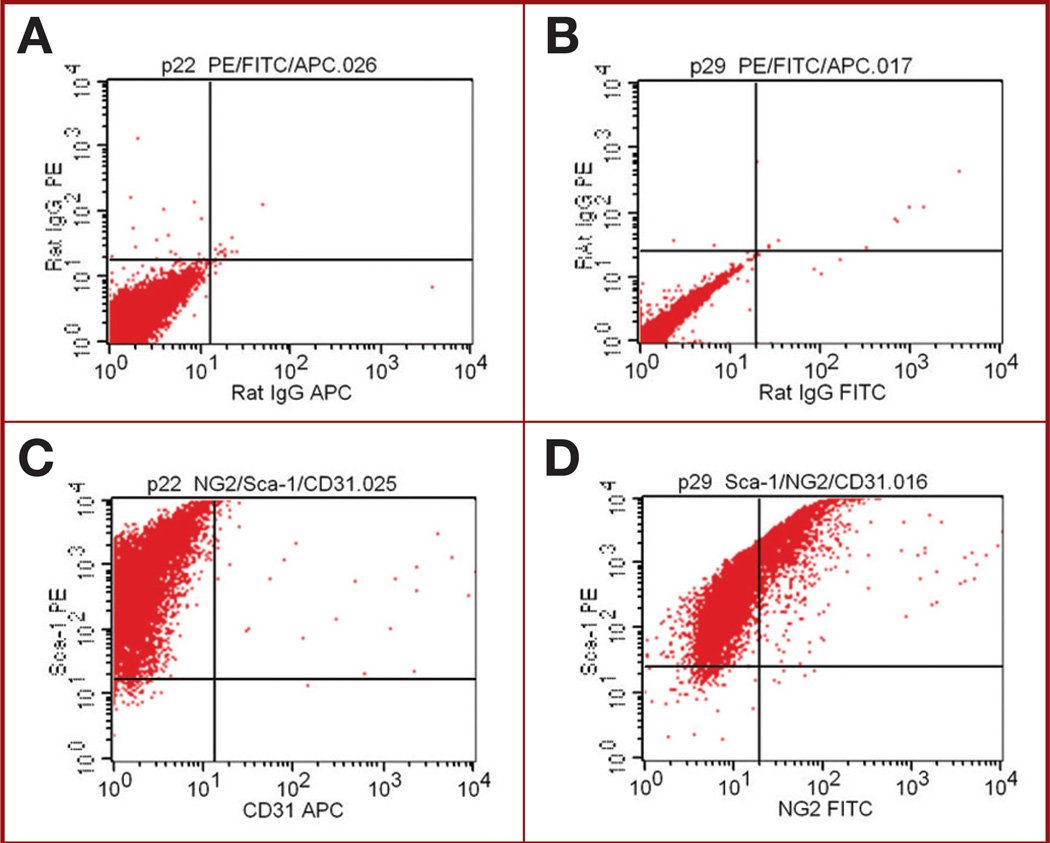
Because of the immunofluorescent staining results, we next determined whether the Br-MSCs isolated from the brains expressed endothelial and/or pericyte markers. In vitro cultured Br-MSCs were analyzed by FACS for coexpression of Sca-1 and CD31 and for coexpression of Sca-1 and NG2. These Br-MSCs were negative for the endothelial marker CD31 (Figure 6 and Table 2), as reported for BM-MSCs.15 In contrast, a small population of Br-MSCs were positive for the pericytes marker NG2 (Figure 6 and Table 2). Most of the Sca-1 cells in the culture also expressed CD9, indicating that these cells were similar to Br-MSCs (Table 2).
FIGURE 6.
Flow cytometric analysis of cultured brain-derived mesenchymal stem cells (Br-MSCs) after labeling with antibodies to stem cell antigen 1 (Sca-1) and nerve/glial antigen 2 (NG2) or CD31. A, plot showing isotype control for IgG phycoerythrin and IgG allophycocyanin, which is control for graph of C. B, plot showing isotype control for IgG phycoerythrin and IgG FITC, which is control for graph of D. C, plot showing result after simultaneous staining of Br-MSCs for Sca-1 and CD31. Sca-1+ cells are negative for CD31. D, plot showing result after simultaneous staining of Br-MSCs for Sca-1 and NG2. A population of Sca-1+ cells also stain for NG-2 (10%).
TABLE 2.
Coexpression of Stem Cell Antigen 1 and CD9, CD31, or Nerve/Glial Antigen 2 on Brain-Derived Mesenchymal Stem Cellsa
| Sample | Sca-1+/ CD9+, % |
Sca1+/ NG2+, % |
Sca-1+/ CD31+, % |
|---|---|---|---|
| Fresh (preculture) | 25.0 | 4.1 | 5.3 |
| Br-MSC passage 15 | 95.5 | 13.4 | 0.3 |
| Br-MSC passage 20 | 98.8 | 10.7 | 0.3 |
| Br-MSC passage 30 | 98.4 | 17.6 | 0.3 |
Br-MSC, brain-derived mesenchymal stem cell; NG2, nerve/glial antigen 2; Sca-1, stem cell antigen 1.
The lack of expression of CD31 in these cultured cells contrasted with the histological findings (see Figure 5) in which a population of Sca-1+ cells also expressed CD31. This suggested that the Sca-1+/CD31+ population of cells may have been lost over time under the in vitro culture conditions for isolating Br-MSCs. To test this hypothesis, we analyzed a freshly procured suspension of normal brain cells (ie, before culturing in MSC media) for Sca-1, CD31, and NG2. Interestingly, 25% of the cells from this fresh specimen were Sca-1+/CD9+, and after culturing for 15 passages, the percentage of this population of Sca-1+/CD9+ cells increased to 95.5%, consistent with the progressive isolation of Br-MSCs under these culture conditions. Simultaneous staining for Sca-1 and CD31 showed that 5.3% of the cells from the fresh (preculture) specimen were Sca-1+/CD31+, but this population was no longer present by passage 15 (0.3%) when cultured in MSC media. In contrast, Sca-1+/NG2+ cells represented 4.11% of the cells in the preculture specimen, and this population increased to 13% to 17% of the cells in the culture at passages 15 to 30. These results support the notion that the culture conditions used to isolate Br-MSCs select for a population of MSCs that also express NG-2 but are negative for CD31.
DISCUSSION
We show that cells similar to BM-MSCs, called Br-MSCs, can be isolated from normal mouse brain. Immunohistochemical staining for Sca-1, one of the surface markers of MSCs, suggests that these Br-MSCs are a component of the vascular niche. Double-immunofluorescent and FACS analyses suggest that mouse Br-MSCs may be the primary source of vascular pericytes.
Although the exact definition of MSCs is controversial, it is generally agreed and endorsed by the International Society for Cellular Therapy that cells referred to as MSCs (1) grow as adherent cells on plastic in vitro, (2) express specific positive and negative surface markers, and (3) can differentiate into adipocytes, chondrocytes, and osteocytes.11 In this context, the cells isolated from mouse brains in this study met all three of these criteria. With regard to in vitro growth, BM-MSCs were originally isolated on the basis of their ability to adhere to plastic surfaces,39 and we applied this method to the initial isolation of Br-MSCs. Although more recently MSCs have been isolated by positive40 or negative selection,14 which can result in more homogeneous cell populations, the original adherence-based approach has a lower risk of losing important cell populations, even though it may require a longer time for selection of a homogenous cell population. However, a shortcoming of this approach is that the chosen in vitro culture condition may cause loss of or may select for MSC subpopulations with specific properties. With regard to surface markers, the International Society for Cellular Therapy proposed minimal criteria for the definition of human MSCs11; however, there is currently no universally agreed-on surface marker profile of mouse MSCs. Nevertheless, the pattern of surface markers that we used (ie, Sca-1+, CD9+, CD45−, CD11b−) have been used in several other studies as markers of mouse BM-MSCs.14,15 In this context, the cells isolated from the normal mouse brain had the surface marker profile similar to that of mouse BM-MSCs and therefore met the second criterion for classifying them as MSCs. Lastly, a critical feature of MSCs is the potential for tri-lineage (adipogenic, osteogenic, and chondrogenic) differentiation. This functional definition of MSCs is probably the most critical attribute of these cells because it defines their stem/progenitor cell phenotype as mesenchymal. Thus, the capacity for trilineage differentiation of the cells isolated in this study provides strong evidence that they resemble MSCs. Importantly, this differentiation pattern was a property of single-cell clones, indicating that a single cell possessed true trilineage differentiation potential and that the result was not caused by progenitor cells with unilineage differential potential growing in the same culture dish.
Our results confirm and extend the findings of da Silva Meirelles et al,32 who showed that MSCs could be isolated from most peripheral organs, including the brain. In addition to culturing MSC-like cells, our study is one of the first to attempt to determine the location of MSCs in the brain. Immunohistochemical staining with Sca-1 antibody, which is highly expressed on cultured mouse MSCs, demonstrated that Sca-1+ cells were located exclusively within the brain vasculature (see Figure 4). This distribution of Sca-1+ cells around blood vessels has been described in several other peripheral organs41 and is of interest because Crisan et al42 recently suggested that MSCs reside as pericytes within the vascular niche and may represent precursor cells for mature vascular pericytes. On the basis of this information, we performed double-immunofluorescent staining for Sca-1 and NG-2, a recognized marker of pericytes, and found that both Sca-1+/NG-2− cells and Sca-1+/NG2+ cells exist around brain vessels. Consistent with this finding, FACS analysis showed that 10% to 15% of cultured Sca-1+ Br-MSCs were also positive for NG2 in vitro. Taken together, these results support the notion that Br-MSCs reside primarily within the vascular niche and may represent progenitors of mature pericytes.28,32,42
Immunohistochemical staining also showed that a population of Sca-1+ cells coexpressed CD31, a known endothelial marker. This finding is consistent with the finding of Kotton et al43 of Sca-1 staining on the surface of endothelial cells in normal lung tissue. In contrast to this in vivo staining pattern, we found that cultured in vitro Br-MSCs do not express CD31 on their surface on the basis of FACS analysis. This lack of CD31 staining is consistent with other reports showing that cultured BM-MSCs do not express endothelial markers.11,15 It is interesting to note that MSCs are defined by their in vitro characteristics, and the precise in vivo counterpart of these cultured cells has not been fully defined. In this context, we found that when cells isolated from fresh brain specimens were analyzed before culturing in MSC medium, a population of Sca-1+/CD31+ could be identified. However, this population was lost when the cells were cultured in MSC medium (see Table 2). Several possibilities may reconcile the finding that Sca-1+/CD31+ cells exist in the brain but are not found in the cultures of Br-MSCs. One possibility is that Sca-1+/CD31+ cells may represent an endothelial population of cells distinct from Br-MSCs. Whereas Br-MSCs tend to differentiate into pericytes (see above), these Sca-1+ cells are exclusive precursors for endothelial cells. Therefore, attempts to culture them in MSC medium are unsuccessful. Alternatively, Sca-1+/CD31+ cells may represent a population of cells derived from MSCs that differentiate along an endothelial lineage. Because pericytelike cells (Sca-1+/NG2+) also exist in the brain (see above), MSCs (Sca-1+/NG2−/CD31−) may represent a mesenchymal precursor that can differentiate into either pericytes or endothelial cells. However, the in vitro culture conditions used to isolate MSCs (and that define MSCs) simply favor and therefore select for pericytelike cells rather than the endotheliumlike cells. Further studies that track the in vivo differentiation of cells within the vascular niche in early development are needed to sort out these alternative hypotheses. Nevertheless, these studies provide evidence that cells resembling MSCs reside within the vascular niche of the brain and may represent a precursor of more mature mesenchy-mal cells (ie, pericytes/endothelial cells).
Finding Br-MSCs in normal mouse brain supports the notion that MSCs exist in a wide range of peripheral organs other than the bone marrow.28,30,32,33,36,42,44 Although Br-MSCs are morphologically and phenotypically similar to BM-MSCs, it is unclear whether Br-MSCs actually originate from BM-MSCs. Although some authors have shown that circulating MSCs are capable of adhering to endothelial cells in different organs,45,46 we think it is unlikely that the large number of Br-MSCs seen in the brains of the mice in our studies were a result of migration from the bone marrow to the brain. First, the notion that MSCs actually circulate has been disputed. In addition, the mobilization of bone marrow stem cells is typically in response to tissue injury or stress,47,48 which was not present in our normal specimens. Ultimately, analyses of embryonic brains may address the role of innate versus circulating MSCs as the source of MSCs in adult peripheral organs. However, if currently available data are considered together, it is likely that Br-MSCs are intrinsic to the brain and are resident local mesenchymal precursors.
The finding that MSCs exist in the brain has important implications for neurosurgeons’ understanding of normal brain homeostasis and of the response of the brain to tissue injury and stress. The identification of MSCs in the brain vasculature suggests that these stem cells may maintain the normal integrity of brain blood vessels, at least in part by repopulating the pericyte population, which stabilizes endothelial cells. In addition, increasing evidence suggests that MSCs have important functions in response to tissue injury and stress.22–25 Indeed, BM-MSCs have been shown to be capable of populating peripheral organs, including the brain, in times of severe tissue injury.49,50 Ex vivo expanded MSCs can improve the outcome of stroke and head injury.22,23 The finding that MSCs exist in the brain suggests that local Br-MSCs may participate in the response to tissue injury, potentially as “first responders,” and that BM-MSCs may represent a secondary source of responding MSCs. Interestingly, in addition to trilineage differentiation, it has been shown that MSCs are capable of differentiating into neurons and astrocytes.1 It is possible that Br-MSCs may participate in the response of the brain to ischemia and trauma by providing secreted growth factors needed for tissue repair, by acting as a source of newly differentiated neural elements that could play a role in restoring damaged neuronal circuits, or by providing astrocytic cells that could help reestablish degenerated neural components. Increased knowledge of the biology of these cells may provide insight into ways of manipulating these cells for therapeutic advantage.
The finding that MSCs exist in the brain is also of interest in light of recent findings in the field of neuro-oncology that human MSCs have a tropism for gliomas and that gliomas express a wide range of mesenchymal gene products.51–53 Most important and consistent with other studies,53,54 we have shown that cells similar to BM-MSCs can be isolated and cultured from fresh glioma specimens obtained at surgery.26,27 Evidence suggests that these tumor-derived MSCs may contribute to the stroma of human gliomas, potentially by modifying the vascular niche. Although it has been presumed that tumor-derived MSCs arise from a circulating pool of BM-MSCs, the finding that there are local MSCs intrinsic to the brain suggests that the source of MSCs in gliomas may be Br-MSCs rather than circulating BM-MSCs. It is possible that pharmacological manipulation of Br-MSCs may provide a new therapeutic approach to brain tumors.
CONCLUSION
We demonstrate that cells similar to BM-MSCs in terms of in vitro growth, surface markers, and trilineage mesenchymal differentiation can be isolated from the brains of normal mice. These Br-MSCs are located within the vascular niche and may represent a population of nonneural progenitor/stem cells that act as a source of mesenchymal elements (eg, pericytes and possibly endothelial cells) within the brain. They may also participate in the response of the brain to tissue injury and stress such as in stroke, trauma, and tumorigenesis.
Acknowledgments
This study was supported by grants from the National Cancer Institute (R01 CA115729 and P50 CA 127001 to Dr Lang; CA-1094551 and CA-116199 to Dr Marini; and CA-55164, CA-16672, and CA-49639 to Dr Andreeff); the M. D. Anderson Center for Targeted Therapy, the National Brain Tumor Foundation, the Elias Family Fund for Brain Tumor Research, the Gene Pennebaker Brain Cancer Fund, the Brian McCullough Research Fund, and the Run for the Roses Foundation to Dr Lang; the Susan G. Komen Breast Cancer Foundation to Dr Marini; and the Paul and Mary Haas Chair in Genetics to Dr Andreeff.
ABBREVIATIONS
- BM-MSC
bone marrow–derived human mesenchymal stem cell
- Br-MSC
brain-derived human mesenchymal stem cell
- FACS
fluorescence-activated cell sorting
- FBS
fetal bovine serum
- MEM
minimal essential medium
- MSC
mesenchymal stem cell
- NG2
nerve/glial antigen 2
- PBS
phosphate-buffered saline
- Sca-1
stem cell antigen 1
- SCID
severe combined immune-deficient
Footnotes
Disclosure
The authors have no personal financial or institutional interest in any of the drugs, materials, or devices described in this article.
Contributor Information
Seok-Gu Kang, Department of Neurosurgery, and Brain Tumor Center, University of Texas M. D. Anderson Cancer Center, Houston, Texas; and Department of Neurosurgery, Catholic University of Korea College of Medicine, Seoul, Korea.
Naoki Shinojima, Department of Neurosurgery, and Brain Tumor Center, University of Texas M. D. Anderson Cancer Center, Houston, Texas.
Anwar Hossain, Department of Neurosurgery, and Brain Tumor Center, University of Texas M. D. Anderson Cancer Center, Houston, Texas.
Joy Gumin, Department of Neurosurgery, and Brain Tumor Center, University of Texas M. D. Anderson Cancer Center, Houston, Texas.
Raymund L. Yong, Department of Neurosurgery, and Brain Tumor Center, University of Texas M. D. Anderson Cancer Center, Houston, Texas.
Howard Colman, Department of Neuro-oncology, and Brain Tumor Center, University of Texas M. D. Anderson Cancer Center, Houston, Texas.
Frank Marini, Department of Blood and Marrow Transplantation, University of Texas M. D. Anderson Cancer Center, Houston, Texas.
Michael Andreeff, Department of Blood and Marrow Transplantation, University of Texas M. D. Anderson Cancer Center, Houston, Texas.
Frederick F. Lang, Departments of Neurosurgery and Blood and Marrow Transplantation, University of Texas M. D. Anderson Cancer Center, Houston, Texas.
REFERENCES
- 1.Friedenstein AJ, Chailakhyan RK, Gerasimov UV. Bone marrow osteogenic stem cells: in vitro cultivation and transplantation in diffusion chambers. Cell Tissue Kinet. 1987;20(3):263–272. doi: 10.1111/j.1365-2184.1987.tb01309.x. [DOI] [PubMed] [Google Scholar]
- 2.Orkin SH. Hematopoietic stem cells: molecular diversification and developmental interrelationships. In: Marshak DR, Gardner RL, Gottlieb D, editors. Stem Cell Biology. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2001. pp. 289–306. [Google Scholar]
- 3.Keller G. The hemangioblast. In: Marshak DR, Gardner RL, Gottlieb D, editors. Stem Cell Biology. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2001. pp. 329–348. [Google Scholar]
- 4.Pittenger MF, Marshak DR. Mesenchymal stem cells of human adult bone marrow. In: Marshak DR, Gardner RL, Gottlieb D, editors. Stem Cell Biology. Vol. 16. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2001. pp. 349–373. [Google Scholar]
- 5.Friedenstein AJ. Marrow stromal fibroblasts. Calcif Tissue Int. 1995;56:S17. [Google Scholar]
- 6.Castro-Malaspina H, Gay RE, Resnick G, et al. Characterization of human bone marrow fibroblast colony-forming cells (CFU-F) and their progeny. Blood. 1980;56(2):289–301. [PubMed] [Google Scholar]
- 7.Grigoriadis AE, Heersche JN, Aubin JE. Differentiation of muscle, fat, cartilage, and bone from progenitor cells present in a bone-derived clonal cell population: effect of dexamethasone. J Cell Biol. 1988;106(6):2139–2151. doi: 10.1083/jcb.106.6.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cassiede P, Dennis JE, Ma F, Caplan AI. Osteochondrogenic potential of marrow mesenchymal progenitor cells exposed to TGF-beta 1 or PDGF-BB as assayed in vivo and in vitro. J Bone Miner Res. 1996;11(9):1264–1273. doi: 10.1002/jbmr.5650110911. [DOI] [PubMed] [Google Scholar]
- 9.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 10.Caplan AI. The mesengenic process. Clin Plast Surg. 1994;21(3):429–435. [PubMed] [Google Scholar]
- 11.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells: the International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 12.Baksh D, Song L, Tuan RS. Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy. J Cell Mol Med. 2004;8(3):301–316. doi: 10.1111/j.1582-4934.2004.tb00320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Javazon EH, Beggs KJ, Flake AW. Mesenchymal stem cells: paradoxes of passaging. Exp Hematol. 2004;32(5):414–425. doi: 10.1016/j.exphem.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Tropel P, Noel D, Platet N, Legrand P, Benabid AL, Berger F. Isolation and characterisation of mesenchymal stem cells from adult mouse bone marrow. Exp Cell Res. 2004;295(2):395–406. doi: 10.1016/j.yexcr.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 15.Deng J, Petersen BE, Steindler DA, Jorgensen ML, Laywell ED. Mesenchymal stem cells spontaneously express neural proteins in culture and are neurogenic after transplantation. Stem Cells. 2006;24(4):1054–1064. doi: 10.1634/stemcells.2005-0370. [DOI] [PubMed] [Google Scholar]
- 16.Minguell JJ, Erices A, Conget P. Mesenchymal stem cells. Exp Biol Med (Maywood) 2001;226(6):507–520. doi: 10.1177/153537020122600603. [DOI] [PubMed] [Google Scholar]
- 17.Caplan AI, Bruder SP. Mesenchymal stem cells: building blocks for molecular medicine in the 21st century. Trends Mol Med. 2001;7(6):259–264. doi: 10.1016/s1471-4914(01)02016-0. [DOI] [PubMed] [Google Scholar]
- 18.Conget PA, Minguell JJ. Phenotypical and functional properties of human bone marrow mesenchymal progenitor cells. J Cell Physiol. 1999;181(1):67–73. doi: 10.1002/(SICI)1097-4652(199910)181:1<67::AID-JCP7>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 19.Bianco P, Riminucci M, Gronthos S, Robey PG. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001;19(3):180–192. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- 20.Nakamizo A, Marini F, Amano T, et al. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005;65(8):3307–3318. doi: 10.1158/0008-5472.CAN-04-1874. [DOI] [PubMed] [Google Scholar]
- 21.Yang B, Wu X, Mao Y, et al. Dual-targeted antitumor effects against brainstem glioma by intravenous delivery of tumor necrosis factor-related, apoptosis-inducing, ligand-engineered human mesenchymal stem cells. Neurosurgery. 2009;65(3):610–624. doi: 10.1227/01.NEU.0000350227.61132.A7. [DOI] [PubMed] [Google Scholar]
- 22.Zhang J, Li Y, Zhang ZG, et al. Bone marrow stromal cells increase oligodendrogenesis after stroke. J Cereb Blood Flow Metab. 2009;29(6):1166–1174. doi: 10.1038/jcbfm.2009.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y, Chopp M. Marrow stromal cell transplantation in stroke and traumatic brain injury. Neurosci Lett. 2009;456(3):120–123. doi: 10.1016/j.neulet.2008.03.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seyfried DM, Han Y, Yang D, et al. Localization of bone marrow stromal cells to the injury site after intracerebral hemorrhage in rats. J Neurosurg. 2010;112(2):329–335. doi: 10.3171/2009.2.JNS08907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kidd S, Spaeth E, Klopp A, Andreeff M, Hall B, Marini FC. The (in) auspicious role of mesenchymal stromal cells in cancer: be it friend or foe. Cytotherapy. 2008;10(7):657–667. doi: 10.1080/14653240802486517. [DOI] [PubMed] [Google Scholar]
- 26.Lang F, Amano T, Hata N, Gumin J, Aldape K, Colman H. Bone marrow-derived mesenchymal stem cells are recruited to and alter the growth of human gliomas [abstract] Neuro Oncol. 2007;9:596. [Google Scholar]
- 27.Lang F, Gumin J, Amano T, et al. Tumor-derived mesenchymal stem cells in human gliomas: isolation and biological properties [abstract] J Clin Oncol. 2008;26(15):2512–2518. [Google Scholar]
- 28.da Silva Meirelles L, Caplan AI, Nardi NB. In search of the in vivo identity of mesenchymal stem cells. Stem Cells. 2008;26(9):2287–2299. doi: 10.1634/stemcells.2007-1122. [DOI] [PubMed] [Google Scholar]
- 29.Hochedlinger K, Jaenisch R. Nuclear reprogramming and pluripotency. Nature. 2006;441(7097):1061–1067. doi: 10.1038/nature04955. [DOI] [PubMed] [Google Scholar]
- 30.Martin J, Helm K, Ruegg P, Varella-Garcia M, Burnham E, Majka S. Adult lung side population cells have mesenchymal stem cell potential. Cytotherapy. 2008;10(2):140–151. doi: 10.1080/14653240801895296. [DOI] [PubMed] [Google Scholar]
- 31.Even-Ram S, Artym V, Yamada KM. Matrix control of stem cell fate. Cell. 2006;126(4):645–647. doi: 10.1016/j.cell.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 32.da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119(pt 11):2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 33.Brachvogel B, Moch H, Pausch F, et al. Perivascular cells expressing annexin A5 define a novel mesenchymal stem cell-like population with the capacity to differentiate into multiple mesenchymal lineages. Development. 2005;132(11):2657–2668. doi: 10.1242/dev.01846. [DOI] [PubMed] [Google Scholar]
- 34.Bieback K, Kern S, Kocaomer A, Ferlik K, Bugert P. Comparing mesenchymal stromal cells from different human tissues: bone marrow, adipose tissue and umbilical cord blood. Biomed Mater Eng. 2008;18(1) suppl:S71–S76. [PubMed] [Google Scholar]
- 35.Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7(2):211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 36.Brachvogel B, Pausch F, Farlie P, et al. Isolated Anxa5+/Sca-1+ perivascular cells from mouse meningeal vasculature retain their perivascular phenotype in vitro and in vivo. Exp Cell Res. 2007;313(12):2730–2743. doi: 10.1016/j.yexcr.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 37.Rutka JT, Kleppe-Hoifodt H, Emma DA, et al. Characterization of normal human brain cultures: evidence for the outgrowth of leptomeningeal cells. Lab Invest. 1986;55(1):71–85. [PubMed] [Google Scholar]
- 38.Mackay AM, Beck SC, Murphy JM, Barry FP, Chichester CO, Pittenger MF. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng. 1998;4(4):415–428. doi: 10.1089/ten.1998.4.415. [DOI] [PubMed] [Google Scholar]
- 39.Delorme B, Charbord P. Culture and characterization of human bone marrow mesenchymal stem cells. Methods Mol Med. 2007;140:67–81. doi: 10.1007/978-1-59745-443-8_4. [DOI] [PubMed] [Google Scholar]
- 40.Nadri S, Soleimani M. Isolation murine mesenchymal stem cells by positive selection. In Vitro Cell Dev Biol Anim. 2007;43(8–9):276–282. doi: 10.1007/s11626-007-9041-5. [DOI] [PubMed] [Google Scholar]
- 41.van de Rijn M, Heimfeld S, Spangrude GJ, Weissman IL. Mouse hematopoietic stem-cell antigen Sca-1 is a member of the Ly-6 antigen family. Proc Natl Acad Sci U S A. 1989;86(12):4634–4638. doi: 10.1073/pnas.86.12.4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 43.Kotton DN, Summer RS, Sun X, Ma BY, Fine A. Stem cell antigen-1 expression in the pulmonary vascular endothelium. Am J Physiol Lung Cell Mol Physiol. 2003;284(6):L990–L996. doi: 10.1152/ajplung.00415.2002. [DOI] [PubMed] [Google Scholar]
- 44.Dimitrov R, Timeva T, Kyurkchiev D, et al. Characterization of clonogenic stromal cells isolated from human endometrium. Reproduction. 2008;135(4):551–558. doi: 10.1530/REP-07-0428. [DOI] [PubMed] [Google Scholar]
- 45.Solanilla A, Grosset C, Duchez P, et al. Flt3-ligand induces adhesion of haematopoietic progenitor cells via a very late antigen (VLA)-4- and VLA-5-dependent mechanism. Br J Haematol. 2003;120(5):782–786. doi: 10.1046/j.1365-2141.2003.04155.x. [DOI] [PubMed] [Google Scholar]
- 46.Segers VF, Van Riet I, Andries LJ, et al. Mesenchymal stem cell adhesion to cardiac microvascular endothelium: activators and mechanisms. Am J Physiol Heart Circ Physiol. 2006;290(4):H1370–H1377. doi: 10.1152/ajpheart.00523.2005. [DOI] [PubMed] [Google Scholar]
- 47.Gutova M, Najbauer J, Frank RT, et al. Urokinase plasminogen activator and urokinase plasminogen activator receptor mediate human stem cell tropism to malignant solid tumors. Stem Cells. 2008;26(6):1406–1413. doi: 10.1634/stemcells.2008-0141. [DOI] [PubMed] [Google Scholar]
- 48.Picinich SC, Mishra PJ, Mishra PJ, Glod J, Banerjee D. The therapeutic potential of mesenchymal stem cells: cell- and tissue-based therapy. Expert Opin Biol Ther. 2007;7(7):965–973. doi: 10.1517/14712598.7.7.965. [DOI] [PubMed] [Google Scholar]
- 49.Mansilla E, Marin GH, Drago H, et al. Bloodstream cells phenotypically identical to human mesenchymal bone marrow stem cells circulate in large amounts under the influence of acute large skin damage: new evidence for their use in regenerative medicine. Transplant Proc. 2006;38(3):967–969. doi: 10.1016/j.transproceed.2006.02.053. [DOI] [PubMed] [Google Scholar]
- 50.Kucia M, Ratajczak J, Reca R, Janowska-Wieczorek A, Ratajczak MZ. Tissue-specific muscle, neural and liver stem/progenitor cells reside in the bone marrow, respond to an SDF-1 gradient and are mobilized into peripheral blood during stress and tissue injury. Blood Cells Mol Dis. 2004;32(1):52–57. doi: 10.1016/j.bcmd.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 51.Phillips HS, Kharbanda S, Chen R, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9(3):157–173. doi: 10.1016/j.ccr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 52.Hall B, Andreeff M, Marini F. The participation of mesenchymal stem cells in tumor stroma formation and their application as targeted-gene delivery vehicles. Handb Exp Pharmacol. 2007;180:263–283. doi: 10.1007/978-3-540-68976-8_12. [DOI] [PubMed] [Google Scholar]
- 53.Stagg J. Mesenchymal stem cells in cancer. Stem Cell Rev. 2008;4(2):119–124. doi: 10.1007/s12015-008-9030-4. [DOI] [PubMed] [Google Scholar]
- 54.Tso CL, Shintaku P, Chen J, et al. Primary glioblastomas express mesenchymal stem-like properties. Mol Cancer Res. 2006;4(9):607–619. doi: 10.1158/1541-7786.MCR-06-0005. [DOI] [PubMed] [Google Scholar]