Abstract
Since approximately 5–10% of the ~50,000 Tuberous Sclerosis Complex (TSC) patients in the US are “MRI-negative,” our goal was to test the hypothesis that they nevertheless exhibit metabolic abnormalities. To test this, we used proton-MR spectroscopy to obtain and compare gray and white matter (GM, WM) levels of the neuronal marker N-acetylaspartate (NAA); the glial marker, myo-inositol (mI) and its associated creatine (Cr) and choline (Cho), between two “MRI-negative” female TSC patients (5- and 43-year-olds) and their matched controls. The NAA, Cr, Cho and mI concentrations in the pediatric control: 9.8, 6.3, 1.4 and 5.7 millimolar, were similar to the patient’s; whereas the adult patient revealed a 17% WM NAA decrease and 16% WM Cho increase from their published means for healthy adults - both outside their respective 90% prediction intervals. These findings suggest that longer disease duration and/or TSC2 gene mutation may cause axonal dysfunction and demyelination.
Keywords: Adult, Brain Diseases; Magnetic Resonance Imaging; Magnetic Resonance Spectroscopy; Neurology; Pediatric; Tuberous Sclerosis
INTRODUCTION
Tuberous sclerosis complex (TSC), a neurocutaneous disorder affecting ~1:6000 live births, affects ~50,000 in the US (1). In 85% of cases, the disorder results from mutations in the TSC1 (9q34) or TSC2 (16p13·3) genes that code for hamartin and tuberin, respectively. The latter are more common and cause more severe pathology (2 disease in multiple organs). Clinical symptoms and pathological findings in TSC are primarily from mammalian target of rapamycin complex 1 (mTORC1) hyperactivity. TSC affects multiple organ systems prominently involving the brain, skin, kidneys and lungs (3, 4). Neurological disorders are probably the leading cause of morbidity and mortality (5) - seizures affect up to 90% of patients, often with early onset and medical intractability, and are associated with high rates of intellectual disability and autism (6–9). Other neuropsychiatric disorders complicate TSC: language delays, impaired social and emotional skills, aggressive behavior, attention-deficit, anxiety, and affective and motor disorders (9, 10).
TSC brain lesions include subependymal giant cell astrocytomas (SEGAs) and nodules that can occur throughout the brain [although predominate in subcortical nuclei (11)], cortical and subcortical tubers, cerebral cortex and cerebellum developmental malformations comprised of disorganized structures lacking six-layered lamination and containing dysplastic neurons, astrocytes and/or giant cells. The tubers’ number and size correlate in some studies with autism, intellectual disability and epilepsy severity (12, 13). In patients with refractory epilepsy and a single large tuber, its resection is associated with more than 90% reduction in seizures (14). The relation between MRI lesion burden and neurologic phenotype, however, is imperfect: TSC patients with many tubers can have normal IQ, while others with just a few may suffer severe intellectual disability (13). Moreover, some TSC patients with clinically symptomatic SEGAs have many cortical tubers but no seizures, and require no anti-epileptic medication. Finally, TSC animal models have been able to replicate hyper-excitable brains and seizures, but lack tubers (15–17).
Fluid-attenuated inversion recovery (FLAIR) MRI is the most sensitive sequence to identify the tubers, with a false-negative rate of ‹0.5% versus 21% for T2-weighted MRI (18). This is due to the long T2 weighting and cerebrospinal fluid (CSF) signal suppression that enhances the sensitivity to small subcortical and gyral core (enlarged gyri with central hyperintensity) tubers, although in neonates and infants, MRI signal intensity changes may be opposite to those of older children or adults (19). Despite FLAIR’s high sensitivity, only 90–95% of TSC patients exhibit brain tubers on MRI (18, 19). The remaining show none despite neuropsychiatric symptoms (18). In these patients, mTORC1 pathway hyperactivation--the underlying pathogenic mechanism of TSC--may interfere with cortical development and contribute to functional impairments by producing giant cells, dysplastic neurons and other MRI-occult microscopic changes (20, 21).
Aberrant neuronal and glial function in TSC may be monitored non-invasively through their proton-MR spectroscopy (1H-MRS)-observed markers: N-acetylaspartate (NAA) for neurons (22); myo-inositol (mI) for the astroglia (23, 24), creatine (Cr) and choline (Cho) that are more abundant in the latter (25). Our goal, therefore, was to test whether “MRI-negative” TSC patients exhibit: (a) increases in the astroglial metabolites: mI, Cr and Cho; and (b) NAA levels similar to controls’, since mTORC1 processes produce dysfunctional neurons but do not damage existing ones. Towards these ends and since TSC pathology is diffuse, we applied three-dimensional (3D) 1H-MRS to the brains of two MRI-negative TSC patients and their healthy matched controls.
MATERIALS AND METHODS
Human Subjects
Two female TSC patients, a 5- and 43-year old, and their age- and gender-matched controls were prospectively enrolled. The 5-year old patient did not have either TSC1 or 2 mutation although she met criteria for “clinically-definite” TSC (26) and was diagnosed with cardiac rhabdomyomas at birth. Seizures began at age 2 years and she developed medically refractory partial (simple and partial complex, secondary generalized) and symptomatic generalized (myoclonic, atypical absence, and tonic) seizures. Electroencephalogram showed generalized spike-wave and left occipital discharges. Her examination revealed five hypopigmented macules, global developmental delay and gait ataxia. Bilateral subdural strip studies revealed multifocal and diffuse seizure onsets that were inoperable. The 43-year old woman was diagnosed with a TSC2 gene mutation, facial angiofibromas and renal angiomyolipomas, but had no history of neurological symptoms. Both patients and controls had unremarkable brain MRI. The children’s parents and the adults gave written Institutional Review Board-approved informed consent.
MR Acquisition
Measurements on pediatric subjects were done in a 1.5 T MRI scanner with its standard transmit-receive circularly-polarized head-coil (AVANTO®, Siemens AG, Erlangen Germany). Axial T2-weighted turbo-spin echo (TSE; TE/TR=107/4770 ms) MRI were acquired at 220×220 mm2 field-of-view (FOV), 256×256 matrix, 5.0 mm slice thickness. Measurements on the adults were done in a 3 T MRI scanner (TRIO®, Siemens AG, Erlangen, Germany) with a transmit-receive circularly-polarized head-coil (TEM3000, MRinstruments, Minneapolis, MN). The TSE MRI were acquired at 256×256 mm2 FOV, 512×512 matrix, 3.7 mm slice thickness.
In the children, following our chemical-shift imaging (CSI) based autoshim procedure (27), and depending on brain size, a 9 – 10 cm anterior-posterior (AP) by 7 – 8 cm left-right (LR) by 4 cm inferior-superior (IS) =252 – 320 cm3 1H-MRS volume-of-interest (VOI) was centered on the corpus callosum, as shown in Fig. 1. It was excited with TE/TR=30/1360 ms PRESS and partitioned using Hadamard spectroscopic imaging into four axial slices, each encoded with 16×16 2D-CSI over a 16×16 cm2 (LR×AP) FOV (28). The VOI was defined in these slices’ planes with two 8 ms numerically optimized 180° pulses (6.3 kHz bandwidth) to yield 252 – 320 voxels, each1.0 cm3. The MRS signal was acquired for 512 ms at ±0.5 kHz bandwidth. At three averages the 1H-MRS took 35 minutes and the protocol was ¾ hour.
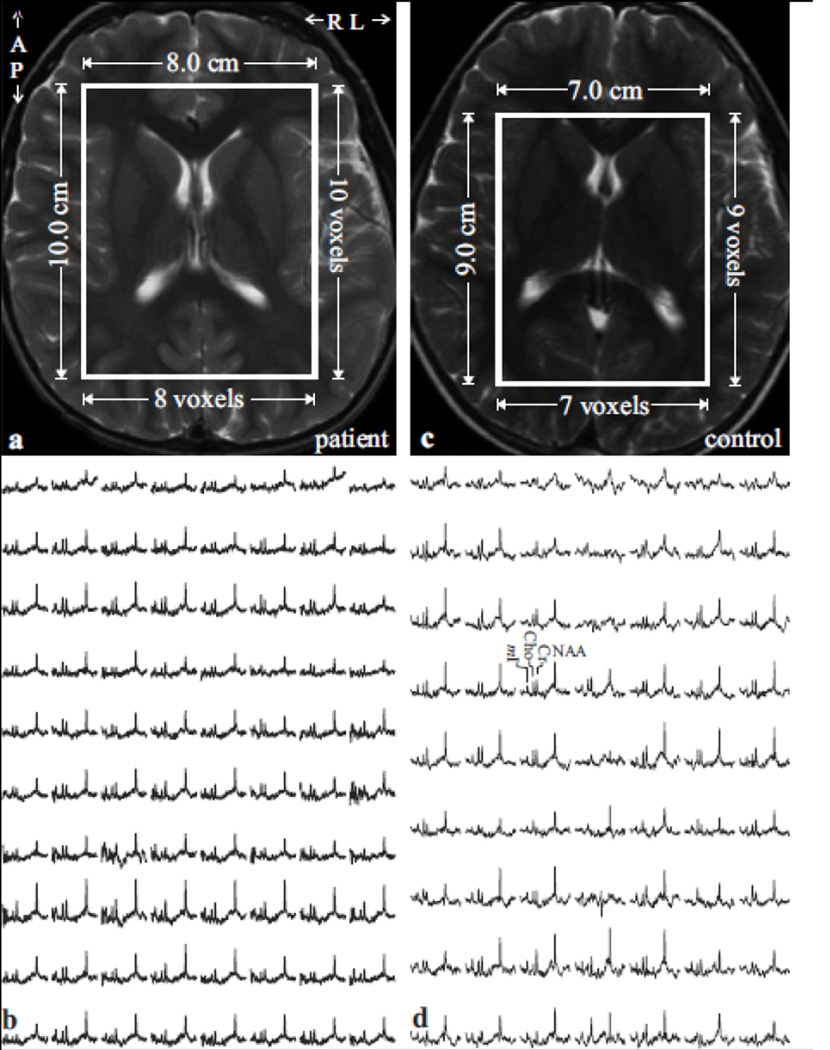
Fig. 1.
Top left, (a): Axial T2-weighted TSE MRI of a 5-year old female TSC patient brain shows the location and size of the VOI (solid white frame). Note the absence of tubers.
Bottom left, (b): Real part of the 8×10 (LR×AP) 1H spectra matrix from the VOI in a, on common chemical shift and intensity scales. Note the spectra resolution and SNR from these 1.0 cm3 voxels in ~35 minutes of 3D 1H-MRS acquisition at 1.5 T.
Top/Bottom right: (c, d): Same as (a, b), except for an age- and gender-matched control.
In the two adults, a 10×8×4.5 cm3=360 cm3 (AP×LR×IS) 1H-MRS VOI was used, as shown in Fig. 2 and excited with TE/TR=35/1800 ms PRESS. It was partitioned into six axial slices with three second-order Hadamard encoded slabs (28). These were encoded with 16×16 2D-CSI over a 16×16 cm2 (LR×AP) FOV and the VOI was defined in slices’ planes by two 11.2 ms optimized 180° pulses (4.5 kHz bandwidth) yielding 8×10×6=480 voxels, 0.75 cm3 each. The 1H-MRS signal was acquired for 256 ms at ±1 kHz bandwidth; at two averages, it took 31 minutes.
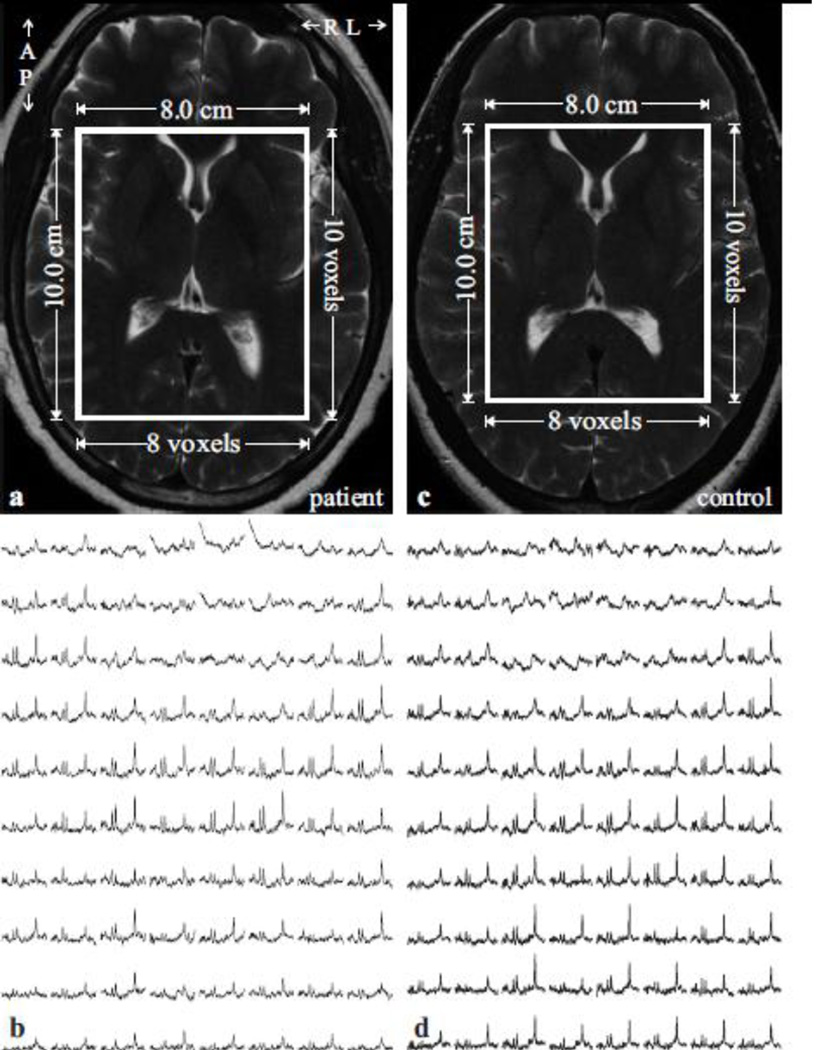
Fig. 2.
Top left, (a): Axial T2-weighted TSE MRI of a 43-year old female TSC patient brain shows the location and size of the VOI (solid white frame). Note the absence of tubers.
Bottom left, (b): Real part of the 8×10 (LR×AP) 1H spectra matrix from the VOI in a, on common chemical shift and intensity scales. Note the spectra resolution and SNR of the 0.75 cm3 voxels in ~30 minutes of 3D 1H-MRS acquisition at 3 T.
Top/Bottom right: (c, d): Same as (a, b), except for an age- and gender-matched control.
Metabolite Quantification
Processing was done with in-house software that removed the residual water signal in the time domain, zero-filled the data from 512 to 2048 points, voxel-shifted to align the CSI grid with the NAA VOI and Fourier transformed in the time, AP and LR and Hadamard reconstructed along the IS directions. The spectra were frequency-aligned and phased in reference to the NAA peak in each voxel (29); and the relative level of the ith=NAA, Cr, Cho or mI in each voxel estimated from its peak area, Si, using prior knowledge modeling software (30). These were scaled into absolute concentrations, Ci, relative to their signals from a 2 L reference sphere of Civitro=12.5, 10.0, 3.0 and 7.5 mM NAA, Cr, Cho and mI in water at physiological ionic strength:
| [1] |
where SR is the reference metabolites' signal and Tf the VOI tissue fraction, P180° and PR180° the radio-frequency power for a non-selective 1 ms 180° pulse on subject and reference and fi-s correct for different relaxation times in vivo (T1vivo, T2vivo) and in the reference (T1vitro, T2vitro):
| [2] |
We used the reported 3 T T1vivo values of 1360, 1300, 1145 and 1170 ms for NAA, Cr, Cho and mI (31) and T2vivo of 350, 174, 251 and 200 ms (32, 33). The 1.5 T metabolite T2vivo values used were 357, 216, 332 and 200 ms (34). Since the corresponding T1vivos are reported to not significantly differ from 3 T (34), the same values were used at both fields.
The global concentration of the i-th metabolite in the VOI of a subject, Ci, was obtained by summing all the spectra in the VOI. This strategy is appropriate for diffuse disorders, retains the individual (narrow) spectra linewidth and dramatically improves the signal-to-noise-ratio (SNR), i.e., the precision (35), by the square root of the number of voxels, ≈20 fold in this study (36):
| [3] |
where n is the number of voxels in the VOI and fi is given by Eq. [2] for the i-th metabolite.
Brain Volumetry
The TSE images were segmented with our FireVoxel package that also works well on pediatric brains, as shown in Fig. 3 (37). It first corrects the images for non-uniform intensities due to the coil’s RF inhomogeneity, using the common histogram devolution method of Sled et al. (38). Next, a WM signal intensity, IWM, is selected in a periventricular seed region. Following automatic detection of all pixels at or above 50% (but below 192.5% to exclude the CSF) of IWM, every slice’s tissue-mask is constructed in three steps: morphological erosion; recursive region growth retaining pixels connected to the seed; and morphological inflation to reverse the erosion effect. Pixels of intensity above 192.5% of IWM are classified as CSF, above 130% (but below 192.5%) IWM, classified as part of the GM mask; and under 130% (but over 50% to exclude air cavities) classified as WM, as shown in Figs. 3b, c and d. In-house software (MATLAB, The Mathworks Inc., Natick, MA), estimated each mask’s volumes in every j-th voxel in the k-th subject (VjkGM, VjkWM, VjkCSF). The overall VOI, GM and WM tissue fractions (Tf, GMf, WMf) were obtained by dividing Vjk(GM+WM), VjkGM, VjkWM by the VOI volume.
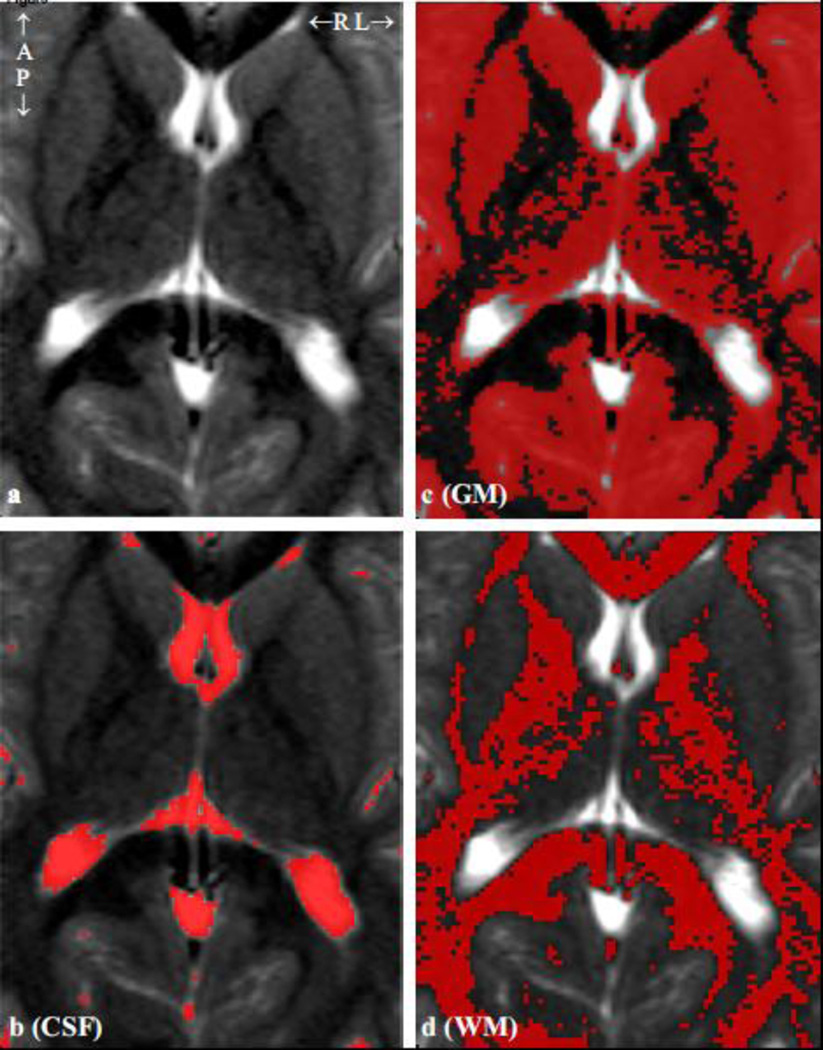
Fig. 3.
a: Axial T2-weighted TSE MRI of a healthy 6-year old brain showing the 7×9 cm2 VOI. b – d: same as a, overlaid with FireVoxel generated CSF, GM and WM masks used to obtain their VOI fractions for Eqs. [3] and [4]. Note the tissue segmentation accuracy.
Global GM and WM Concentrations
Since the CSF does not contribute to the 1H-MRS signal, the i-th metabolite amount in the j-th voxel in the k-th subject can be modeled as the sum of two compartments’ (GM, WM) amounts:
| [4] |
where CikGM, CikWM are the (unknown) global GM and WM metabolites’ concentrations and the fi-s are given by Eq. [2]. Since no significant GM and WM T1vivo differences are reported between 1.5 and 3 T (34), we used the values below Eq. [2]. For NAA, Cr, Cho and mI T2vivos at 1.5 T we used 317, 208, 300 and 130 ms for the GM; and 361, 215, 330 and 110 ms for WM (34, 39). At 3 T, the T2vivos used were 275, 157, 241 and 200 ms in GM; 400, 185, 258 and 200 ms in WM (32, 33). Although CikGM and CikWM cannot both be derived from Eq. [4], since the brain’s GM and WM spatial heterogeneity is on a scale much smaller than the voxels, each has different, independent VjkGM and VjkWM coefficients. The resulting over-determined system of equations in CikGM and CikWM was solved with linear regression. The inter-subject coefficient of variation (CV=standard deviation/mean) of this approach has been shown to be under 15% (40).
Statistical Analysis
Due to the small sample size, there was not enough statistical power to formally test patient to control values for statistical significance. Therefore, a sample of 18 previously-studied healthy adults was used to derive 90% prediction intervals (40). Each metabolite concentration’s (or tissue fraction’s) mean, and standard deviation, Si, in the VOI and in its GM and WM moieties for a sample of N controls can be used to derive a 90% prediction interval for their value in a randomly-selected individual from the same population as,
| [5] |
Consequently, if a new subject is observed to have a measured metric outside its prediction interval, either he or she is one of the 10% for whom this metric falls outside this interval, or, more likely, came from a population with a different distribution of values.
RESULTS
Our automatic shimming adjusted the whole-head water linewidth to 14 and 27 Hz at 1.5 and 3 T, yielding 12 and 22 Hz in the VOI without further adjustments. The VOI size and placement and 1H spectra for the patients and controls, are shown in Figs. 1 and 2. The spectral fit quality is reflected by ‹20% mean Cramer-Rao lower bounds for all metabolites in the 1.0 cm3 voxels’ and ‹5.5% in the averaged VOI spectra, shown in Fig. 4. The global VOI and its GM and WM metabolite concentrations are compiled in Table 1. The NAA, Cr, Cho and mI concentrations 90% prediction intervals for the adults were obtained from their published values: 7.7±0.5, 5.4±0.5, 1.3±0.1 and 4.8±0.6 mM in the VOI; 7.6±0.5, 4.8±0.4, 1.4±0.2 and 4.6±0.7 mM in its WM; and 8.4±0.7, 6.7±0.6, 1.2±0.2 and 5.4±0.7 mM in the GM, using Eq. [5] (40).
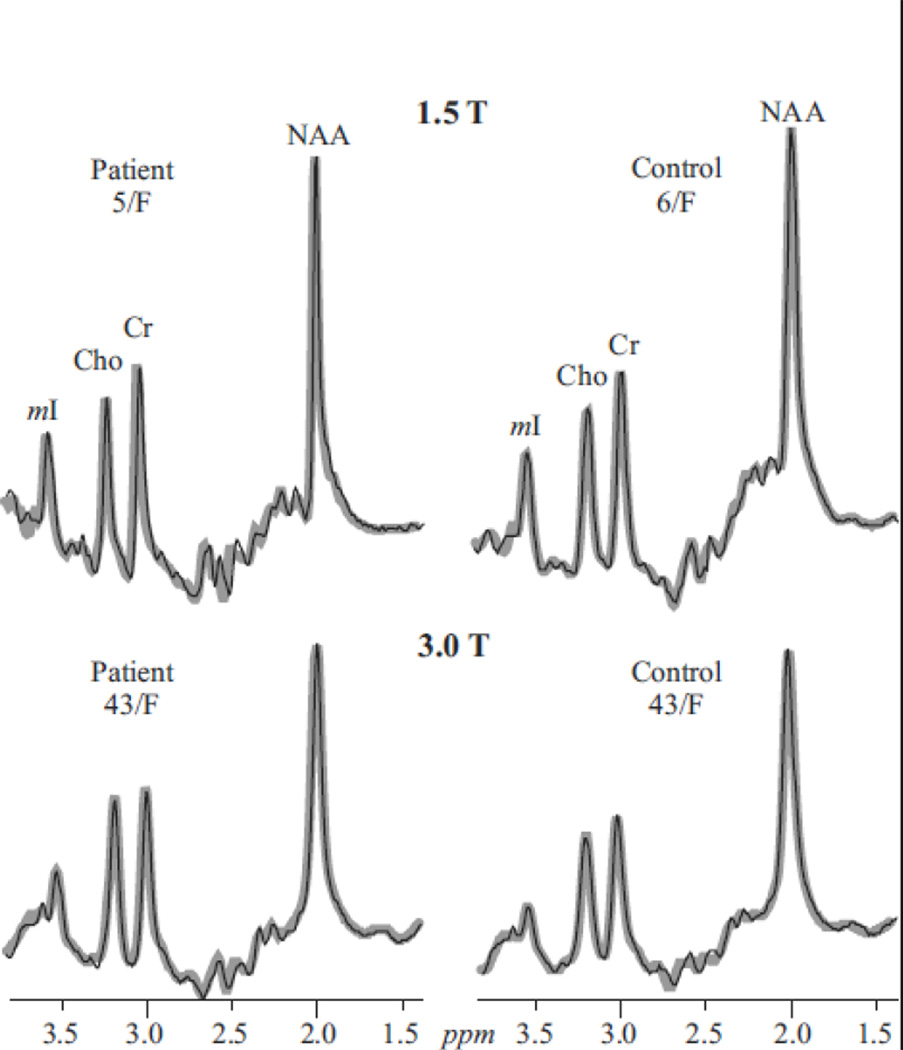
Fig. 4.
Real part of the aligned and globally-averaged 1H spectra from all VOI voxels (thin black lines) representing Eq. [3], for each subject, superimposed with their fitted model functions (thick gray lines), on common intensity (at each field) and chemical shift scales. Note the excellent SNRs and resolution (compared with the single voxels in Figs. 1 and 2) and observable Cho, Cr and mI increases in the adults (See Table 1).
TABLE 1.
Absolute metabolite concentrations and tissue fraction for each region for both TSC patients and their matched controls.
| Region | Metabolitea | aPediatric | aAdult | ||
|---|---|---|---|---|---|
| TSC | bControl | TSC | bControl | ||
| VOI | NAA | 9.7 | 9.8 | 7.4 | 7.8 [6.8, 8.6] |
| Cr | 6.2 | 63 | 6.1 | 5.8 [4.7, 6.2] | |
| Cho | 1.4 | 1.4 | 1.6 | 1.3 [1.1, 1.5] | |
| mI | 6.0 | 5.7 | 5.2 | 4.3 [3.8,5.8] | |
| cTf | 91 | 89 | 92 | 91 [90, 99] | |
| GM | NAA | 12.5 | 12.5 | 8.8 | 8.0 [7.2, 9.6] |
| Cr | 8.3 | 9.0 | 7.4 | 6.9 [5.7, 7.8] | |
| Cho | 1.6 | 1.6 | 1.4 | 1.3 [0.9, 1.5] | |
| mI | 7.9 | 7.3 | 6.2 | 4.7 [4.2, 6.5] | |
| cGMf | 41 | 44 | 38 | 42 [36, 43] | |
| WM | NAA | 8.5 | 8.4 | 6.3 | 7.7 [6.8, 8.5] |
| Cr | 5.0 | 4.6 | 4.9 | 4.8 [4.1,5.6] | |
| Cho | 1.5 | 1.4 | 1.6 | 1.4 [1.1, 1.6] | |
| mI | 5.0 | 4.6 | 4.4 | 4.3 [3.4, 5.7] | |
| cWMf | 51 | 45 | 53 | 49 [47, 57] | |
The mean tissue fractions from the 18-subject sample were: 91±2%, 39±2% and 52±3% in VOI, GM and WM, and the resulting prediction intervals are also given in Table 1. In the adult patient, we observed 25% and 16% higher Cho in the VOI and its WM, respectively, and 17% lower NAA concentration in WM compared with controls’ mean. All differences were outside their respective 90% prediction intervals (40). No metabolite was observed outside its 90% prediction interval for the adult control. For the children, no meaningful differences were observed between TSC patient and her control for any metabolite or tissue compartment, as shown in Table 1 and Fig. 4. Based on the reported similarity of pediatric spectra at this age to adults' (41, 42), we assume that because the differences between the patient and her control were relatively small (‹10% for any metabolite), the patient is within the 90% prediction interval of a representative healthy pediatric population.
Global VOI, GM and WM tissue fractions, Tf, GMf. WMf, are also shown in Table 1 for all subjects. For the adults, no tissue fraction was outside its relevant 90% prediction interval.
DISCUSSION
The low to modest correlation between brain tubers on clinical MRI and the neurologic phenotype of TSC strongly suggests that this modality does not define the full range of pathologic abnormalities. We therefore sought to use 1H-MRS in MRI-negative TSC patients. The presence of MRS abnormalities in the adult TSC brain but not in the child is paradoxical since the child has developmental delay and refractory epilepsy, whereas the adult is neurologically normal. Several explanations may account for this double dissociation. First, the child did not have either TSC1 or TSC2 mutations on genetic testing although she met criteria for clinically-definite TSC (26). The cause for TSC in patients without TSC1 or 2 mutations remains unknown, and may relate to either a third gene or regulatory DNA controlling TSC1 or 2 (43). Thus, the pathophysiology of her disorder, although clinically parallel to patients with TSC1/2 mutations, may result from another genetic disorder acting via a non-mTORC1 pathway. Second, the presence (or lack) of a TSC1/2 mutation may be functionally dissociated from neurological symptoms; approximately 15% of children and adolescents with TSC have no CNS complications (43).
The MRS abnormalities in the adult, despite being neurologically asymptomatic, suggest that longer disease duration and/or TSC2 gene mutation can cause: (i) axonal dysfunction, reflected by decreased WM NAA and elevated VOI and WM Cho suggesting demyelination that may lead to subsequent neuronal loss by Wallerian degeneration; and (ii) aberrant glial growth , reflected by elevated mI and Cr in the VOI and its GM (though not outside their 90% prediction intervals), possibly due to mTORC1 hyperactivity.
Several other neurologic processes could account for the dissociations between our pediatric and adult TSC subjects. Lack of abnormalities in the pediatric TSC brain may reflect ongoing development; e.g., myelination may prevent or offset the demyelinating effects of hamartomas. Indeed, previous studies of the diffusion characteristics showed focal microstructural abnormalities limited to hamartomas, i.e., no apparent diffusion coefficient differences in TSC patients versus controls in normal-appearing WM (44). In addition, cortical remodeling could also reverse or prevent changes to neurons and glia. Either mechanism, if substantiated, could have implications for therapy. Another possibility is that the underlying neuropathology in the child is in the 80–90% of the cortex outside the VOI (Fig. 1) missed in our study.
It is noteworthy that although not outside their 90% prediction intervals, GMf was lower and WMf was higher in both patients than their controls’ (cf. Table 1). Although a curiosity, given the small N, and that TSC pathology is known to affect the cortex, it is perhaps not surprising that possible microscopic lesions and/or atrophy (that leaves healthy neurons intact) is responsible for both GMf reductions. If deep GM nuclei within the VOI, e.g., caudate, thalamus, etc., shrink, the surrounding WM that would collapse inward to replace that lost volume may be responsible for the observed increased WMf.
Admittedly, this study is also subject to several limitations: First, only two patients were enrolled, each belonging to a different age group and each representing a different genotype. However, given the rarity of MRI-negative TSC cases nationwide (about 1:105), the Manhattan (2011 census population: 1.6 million (45)) area served by our epilepsy center would expect to see at most about sixteen. Since 10–20% of TSC patients do not experience seizures (43), they would not require an epilepsy center, the actual number of MRI-negative TSC patients available will usually underestimate the true prevalence; making two out of (at most) 16 a reasonable yield given our patient base (4). Second, each patient represented a subset not typically found in TSC. While these are rare conditions, their exploratory study is important for wider understanding of gene-function and/or its biomarker correlates. One patient without TSC1/2 mutation expresses a neurological phenotype while the other with the TSC2 mutation had no neurological phenotype; this “double dissociation” points to the possible conclusion that mutation by itself has little bearing on neurologic outcome. It is noteworthy that approximately 15% of TSC patients have no CNS complications (43). Our MRS findings seem to support this functional dissociation. For instance, the asymptomatic adult showed lower WM NAA and higher WM Cho, which suggest neuro-axonal abnormalities associated with mutation, but not symptoms. Meanwhile similar MRS profiles between the children suggest that perhaps a different cause is needed to explain the patient’s neurological symptoms. Either scenario, if substantiated, could have implications for treatment. Third, due to lipid contamination and signal “bleed” into surrounding voxels near/on lateral surfaces of the cerebral cortex, as well as poor shimming in those areas, our cortical coverage within the VOI was limited to cortex near the midline, e.g., anterior and posterior cingulate.
Future studies with larger samples are needed to define the role of different genotypic features, e.g.TSC1 versus TSC2 versus non-TSC1/2 specific mutations; and phenotypic features, e.g., disease duration and severity, age, and specific clinical features such as intellectual disability, autism, epilepsy and their correlation with MRS abnormalities. Further investigation of the relationship of MRS abnormalities to neurologic phenotype in TSC could potentially provide diagnostic and prognostic information regarding brain development and function. It may also provide data relevant to defining epileptogenic versus nonepileptogenic brain tissue that could influence the identification and localization of seizure foci. Lastly, MRS could reveal new diagnostic biomarkers of mTORC1 brain dysfunction, which may help identify patients most likely to respond to mTOR inhibitors and monitor their response to therapy.
ACKNOWLEDGEMENTS
This work was supported by National Institutes of Health grants NS050520 and EB01015. Assaf Tal also acknowledges the support of the Human Frontiers Science Project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Osborne JP, Fryer A, Webb D. Epidemiology of tuberous sclerosis. Ann N Y Acad Sci. 1991;615:125–127. doi: 10.1111/j.1749-6632.1991.tb37754.x. Epub 1991/01/01. [DOI] [PubMed] [Google Scholar]
- 2.Dabora SL, Jozwiak S, Franz DN, Roberts PS, Nieto A, Chung J, et al. Mutational analysis in a cohort of 224 tuberous sclerosis patients indicates increased severity of TSC2, compared with TSC1, disease in multiple organs. Am J Hum Genet. 2001;68(1):64–80. doi: 10.1086/316951. Epub 2000/12/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bourneville DM. Sclérose tubéreuse des circonvolutions cérébrales. Arch Neurol. 1880;1:81–91. [Google Scholar]
- 4.Roach ES, Sparagana SP. Diagnosis of tuberous sclerosis complex. J Child Neurol. 2004;19(9):643–649. doi: 10.1177/08830738040190090301. Epub 2004/11/26. [DOI] [PubMed] [Google Scholar]
- 5.Shepherd CW, Gomez MR. Mortality in the Mayo Clinic Tuberous Sclerosis Complex Study. Ann N Y Acad Sci. 1991;615:375–377. doi: 10.1111/j.1749-6632.1991.tb37786.x. Epub 1991/01/01. [DOI] [PubMed] [Google Scholar]
- 6.Jozwiak S, Schwartz RA, Janniger CK, Bielicka-Cymerman J. Usefulness of diagnostic criteria of tuberous sclerosis complex in pediatric patients. J Child Neurol. 2000;15(10):652–659. doi: 10.1177/088307380001501003. Epub 2000/11/04. [DOI] [PubMed] [Google Scholar]
- 7.Curatolo P, Bombardieri R, Verdecchia M, Seri S. Intractable seizures in tuberous sclerosis complex: from molecular pathogenesis to the rationale for treatment. J Child Neurol. 2005;20(4):318–325. doi: 10.1177/08830738050200040901. Epub 2005/06/01. [DOI] [PubMed] [Google Scholar]
- 8.Sparagana SP, Delgado MR, Batchelor LL, Roach ES. Seizure remission and antiepileptic drug discontinuation in children with tuberous sclerosis complex. Arch Neurol. 2003;60(9):1286–1289. doi: 10.1001/archneur.60.9.1286. Epub 2003/09/17. [DOI] [PubMed] [Google Scholar]
- 9.Curatolo P, Cusmai R, Cortesi F, Chiron C, Jambaque I, Dulac O. Neuropsychiatric aspects of tuberous sclerosis. Ann N Y Acad Sci. 1991;615:8–16. doi: 10.1111/j.1749-6632.1991.tb37743.x. Epub 1991/01/01. [DOI] [PubMed] [Google Scholar]
- 10.Muzykewicz DA, Newberry P, Danforth N, Halpern EF, Thiele EA. Psychiatric comorbid conditions in a clinic population of 241 patients with tuberous sclerosis complex. Epilepsy Behav. 2007;11(4):506–513. doi: 10.1016/j.yebeh.2007.07.010. Epub 2007/10/16. [DOI] [PubMed] [Google Scholar]
- 11.Ridler K, Suckling J, Higgins N, Bolton P, Bullmore E. Standardized whole brain mapping of tubers and subependymal nodules in tuberous sclerosis complex. J Child Neurol. 2004;19(9):658–665. doi: 10.1177/08830738040190090501. Epub 2004/11/26. [DOI] [PubMed] [Google Scholar]
- 12.O'Callaghan FJ, Harris T, Joinson C, Bolton P, Noakes M, Presdee D, et al. The relation of infantile spasms, tubers, and intelligence in tuberous sclerosis complex. Arch Dis Child. 2004;89(6):530–533. doi: 10.1136/adc.2003.026815. Epub 2004/05/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jansen FE, Vincken KL, Algra A, Anbeek P, Braams O, Nellist M, et al. Cognitive impairment in tuberous sclerosis complex is a multifactorial condition. Neurology. 2008;70(12):916–923. doi: 10.1212/01.wnl.0000280579.04974.c0. Epub 2007/11/23. [DOI] [PubMed] [Google Scholar]
- 14.Madhavan D, Schaffer S, Yankovsky A, Arzimanoglou A, Renaldo F, Zaroff CM, et al. Surgical outcome in tuberous sclerosis complex: a multicenter survey. Epilepsia. 2007;48(8):1625–1628. doi: 10.1111/j.1528-1167.2007.01112.x. Epub 2007/05/09. [DOI] [PubMed] [Google Scholar]
- 15.Wong M. The utility of tuberless models of tuberous sclerosis. Epilepsia. 2007;48(8):1629–1630. doi: 10.1111/j.1528-1167.2007.01178_1.x. author reply 32–4. Epub 2007/08/19. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Greenwood JS, Calcagnotto ME, Kirsch HE, Barbaro NM, Baraban SC. Neocortical hyperexcitability in a human case of tuberous sclerosis complex and mice lacking neuronal expression of TSC1. Ann Neurol. 2007;61(2):139–152. doi: 10.1002/ana.21058. Epub 2007/02/07. [DOI] [PubMed] [Google Scholar]
- 17.Meikle L, Talos DM, Onda H, Pollizzi K, Rotenberg A, Sahin M, et al. A mouse model of tuberous sclerosis: neuronal loss of Tsc1 causes dysplastic and ectopic neurons, reduced myelination, seizure activity, and limited survival. J Neurosci. 2007;27(21):5546–5558. doi: 10.1523/JNEUROSCI.5540-06.2007. Epub 2007/05/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Griffiths PD, Hoggard N. Distribution and conspicuity of intracranial abnormalities on MR imaging in adults with tuberous sclerosis complex: A comparison of sequences including ultrafast T2-weighted images. Epilepsia. 2009;50(12):2605–2610. doi: 10.1111/j.1528-1167.2009.02107.x. Epub 2009/06/06. [DOI] [PubMed] [Google Scholar]
- 19.Luat AF, Makki M, Chugani HT. Neuroimaging in tuberous sclerosis complex. Curr Opin Neurol. 2007;20(2):142–150. doi: 10.1097/WCO.0b013e3280895d93. Epub 2007/03/14. [DOI] [PubMed] [Google Scholar]
- 20.Marcotte L, Aronica E, Baybis M, Crino PB. Cytoarchitectural alterations are widespread in cerebral cortex in tuberous sclerosis complex. Acta Neuropathol. 2012;123(5):685–693. doi: 10.1007/s00401-012-0950-3. Epub 2012/02/14. [DOI] [PubMed] [Google Scholar]
- 21.Magri L, Cambiaghi M, Cominelli M, Alfaro-Cervello C, Cursi M, Pala M, et al. Sustained activation of mTOR pathway in embryonic neural stem cells leads to development of tuberous sclerosis complex-associated lesions. Cell Stem Cell. 2011;9(5):447–462. doi: 10.1016/j.stem.2011.09.008. Epub 2011/11/08. [DOI] [PubMed] [Google Scholar]
- 22.Benarroch EE. N-acetylaspartate and N-acetylaspartylglutamate: neurobiology and clinical significance. Neurology. 2008;70(16):1353–1357. doi: 10.1212/01.wnl.0000311267.63292.6c. Epub 2008/04/17. [DOI] [PubMed] [Google Scholar]
- 23.Soares DP, Law M. Magnetic resonance spectroscopy of the brain: review of metabolites and clinical applications. Clinical radiology. 2009;64(1):12–21. doi: 10.1016/j.crad.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 24.Mountford CE, Stanwell P, Lin A, Ramadan S, Ross B. Neurospectroscopy: the past, present and future. Chem Rev. 2010;110(5):3060–3086. doi: 10.1021/cr900250y. Epub 2010/04/15. [DOI] [PubMed] [Google Scholar]
- 25.Ross B, Bluml S. Magnetic resonance spectroscopy of the human brain. Anat Rec. 2001;265(2):54–84. doi: 10.1002/ar.1058. Epub 2001/04/27. [DOI] [PubMed] [Google Scholar]
- 26.Roach ES, Gomez MR, Northrup H. Tuberous sclerosis complex consensus conference: revised clinical diagnostic criteria. J Child Neurol. 1998;13(12):624–628. doi: 10.1177/088307389801301206. Epub 1999/01/09. [DOI] [PubMed] [Google Scholar]
- 27.Hu J, Javaid T, Arias-Mendoza F, Liu Z, McNamara R, Brown TR. A fast, reliable, automatic shimming procedure using 1H chemical-shift-imaging spectroscopy. J Magn Reson B. 1995;108(3):213–219. doi: 10.1006/jmrb.1995.1126. [DOI] [PubMed] [Google Scholar]
- 28.Goelman G, Liu S, Hess D, Gonen O. Optimizing the efficiency of high-field multivoxel spectroscopic imaging by multiplexing in space and time. Magn Reson Med. 2006;56(1):34–40. doi: 10.1002/mrm.20942. [DOI] [PubMed] [Google Scholar]
- 29.Gonen O, Murdoch JB, Stoyanova R, Goelman G. 3D multivoxel proton spectroscopy of human brain using a hybrid of 8th-order Hadamard encoding with 2D chemical shift imaging. Magn Reson Med. 1998;39(1):34–40. doi: 10.1002/mrm.1910390108. [DOI] [PubMed] [Google Scholar]
- 30.Soher BJ, Young K, Govindaraju V, Maudsley AA. Automated spectral analysis III: application to in vivo proton MR spectroscopy and spectroscopic imaging. Magn Reson Med. 1998;40(6):822–831. doi: 10.1002/mrm.1910400607. [DOI] [PubMed] [Google Scholar]
- 31.Ethofer T, Mader I, Seeger U, Helms G, Erb M, Grodd W, et al. Comparison of longitudinal metabolite relaxation times in different regions of the human brain at 1.5 and 3 Tesla. Magn Reson Med. 2003;50(6):1296–1301. doi: 10.1002/mrm.10640. [DOI] [PubMed] [Google Scholar]
- 32.Kirov II, Fleysher L, Fleysher R, Patil V, Liu S, Gonen O. Age dependence of regional proton metabolites T2 relaxation times in the human brain at 3 T. Magn Reson Med. 2008;60(4):790–795. doi: 10.1002/mrm.21715. Epub 2008/09/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Posse S, Otazo R, Caprihan A, Bustillo J, Chen H, Henry PG, et al. Proton echo-planar spectroscopic imaging of J-coupled resonances in human brain at 3 and 4 Tesla. Magn Reson Med. 2007;58(2):236–244. doi: 10.1002/mrm.21287. Epub 2007/07/05. [DOI] [PubMed] [Google Scholar]
- 34.Traber F, Block W, Lamerichs R, Gieseke J, Schild HH. 1H metabolite relaxation times at 3.0 tesla: Measurements of T1 and T2 values in normal brain and determination of regional differences in transverse relaxation. J Magn Reson Imaging. 2004;19(5):537–545. doi: 10.1002/jmri.20053. [DOI] [PubMed] [Google Scholar]
- 35.Kreis R, Slotboom J, Hofmann L, Boesch C. Integrated data acquisition and processing to determine metabolite contents, relaxation times, and macromolecule baseline in single examinations of individual subjects. Magn Reson Med. 2005;54(4):761–768. doi: 10.1002/mrm.20673. Epub 2005/09/15. [DOI] [PubMed] [Google Scholar]
- 36.Kirov II, George IC, Jayawickrama N, Babb JS, Perry NN, Gonen O. Longitudinal interand intra-individual human brain metabolic quantification over 3 years with proton MR spectroscopy at 3 T. Magn Reson Med. 2012;67(1):27–33. doi: 10.1002/mrm.23001. Epub 2011/06/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mikheev A, Nevsky G, Govindan S, Grossman R, Rusinek H. Fully automatic segmentation of the brain from T1-weighted MRI using Bridge Burner algorithm. J Magn Reson Imaging. 2008;27(6):1235–1241. doi: 10.1002/jmri.21372. Epub 2008/05/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17(1):87–97. doi: 10.1109/42.668698. Epub 1998/06/09. [DOI] [PubMed] [Google Scholar]
- 39.Frahm J, Bruhn H, Gyngell ML, Merboldt KD, Hanicke W, Sauter R. Localized proton NMR spectroscopy in different regions of the human brain in vivo. Relaxation times and concentrations of cerebral metabolites. Magn Reson Med. 1989;11(1):47–63. doi: 10.1002/mrm.1910110105. Epub 1989/07/01. [DOI] [PubMed] [Google Scholar]
- 40.Tal A, Kirov II, Grossman RI, Gonen O. The role of gray and white matter segmentation in quantitative proton MR spectroscopic imaging. NMR Biomed. 2012 doi: 10.1002/nbm.2812. Epub 2012/06/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bluml S, Wisnowski JL, Nelson MD, Jr, Paquette L, Gilles FH, Kinney HC, et al. Metabolic Maturation of the Human Brain From Birth Through Adolescence: Insights From In Vivo Magnetic Resonance Spectroscopy. Cereb Cortex. doi: 10.1093/cercor/bhs283. Epub 2012/09/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kreis R, Ernst T, Ross BD. Development of the human brain: in vivo quantification of metabolite and water content with proton magnetic resonance spectroscopy. Magn Reson Med. 1993;30(4):424–437. doi: 10.1002/mrm.1910300405. [DOI] [PubMed] [Google Scholar]
- 43.Curatolo P, Bombardieri R, Jozwiak S. Tuberous sclerosis. Lancet. 2008;372(9639):657–668. doi: 10.1016/S0140-6736(08)61279-9. Epub 2008/08/30. [DOI] [PubMed] [Google Scholar]
- 44.Firat AK, Karakas HM, Erdem G, Yakinci C, Bicak U. Diffusion weighted MR findings of brain involvement in tuberous sclerosis. Diagnostic and interventional radiology. 2006;12(2):57–60. Epub 2006/06/06. [PubMed] [Google Scholar]
- 45.New York County QuickFacts from the U.S. Census Bureau. United States Census Bureau. 2011 Available from: http://quickfacts.census.gov/qfd/states/36/36061.html.