Abstract
Inhibitors of the mammalian target of rapamycin (mTOR), sirolimus and everolimus, reduce the incidence of acute rejection following kidney transplantation but their impact on clinical outcomes beyond two years after transplantation is unknown. We examined risks of mortality and allograft loss in a prospective observational study of 993 prevalent kidney transplant recipients who enrolled a median of 72 months after transplantation. During a median follow-up of 37 months, 87 patients died and 102 suffered allograft loss. In the overall population, use of mTOR inhibitors at enrollment was not associated with altered risk of allograft loss, and their association with increased mortality was of borderline significance. However, history of malignancy was the strongest predictor of both mortality and therapy with an mTOR inhibitor. Among patients without a history of malignancy, use of mTOR inhibitors was associated with significantly increased risk of mortality in propensity score-adjusted (hazard ratio [HR] 2.6; 95%CI, 1.2, 5.5; P = 0.01), multivariable-adjusted (HR 3.2; 95%CI, 1.5, 6.5; P = 0.002) and one-to-one propensity score-matched analyses (HR 5.6; 95% CI 1.2, 25.7; P = 0.03). Additional studies are needed to examine the long-term safety of mTOR inhibitors in kidney transplantation, especially among recipients without a history of malignancy.
Keywords: mTOR inhibitors, kidney transplantation, mortality, allograft loss
Introduction
Despite great strides in short-term preservation of renal allografts following the introduction of calcineurin inhibitors, there has been less progress in extending graft survival beyond 5 years (1). This lack of progress in long-term graft survival can be partially attributed to chronic calcineurin inhibitor nephrotoxicity, a concern which prompted the search for alternative immunosuppressive strategies in kidney transplantation (2). Inhibitors of the mammalian target of rapamycin (mTOR), sirolimus and everolimus, have emerged as a novel class of immunosuppressive agents with the promise of reduced nephrotoxicity compared with calcineurin inhibitors (3, 4).
Sirolimus and everolimus are macrolide antibiotics derived from Streptomyces hygroscopicus (5, 6). These agents engage the intracellular immunophilin FK binding protein 12, and the receptor-ligand complex binds mTOR, which is a highly conserved serine/threonine kinase involved in the control of cell growth and metabolism. In rat models, effective immunosuppressive doses of mTOR inhibitors do not induce kidney injury (3). In addition, the antiproliferative effects of sirolimus and everolimus are associated with reduced incidence of malignancies in kidney transplant populations (7, 8). In contrast to these potentially beneficial effects, mTOR inhibitors have been associated with impaired wound healing, and increased risk of dyslipidemia and proteinuria (9–12).
Several randomized controlled trials tested the efficacy and safety of using mTOR inhibitors in the management of kidney transplant recipients. A meta-analysis of 8 trials that compared mTOR inhibitors versus calcineurin inhibitors as part of the primary immunosuppressive regimen demonstrated lower serum creatinine and higher estimated glomerular filtration rate (eGFR) among users of mTOR inhibitors, but no differences in rates of acute rejection, allograft loss, or mortality during a maximum of 2 years of follow-up (13). In contrast, the SYMPHONY study found higher rates of biopsy-proven rejection and lower eGFR in the sirolimus arm, and no differences in hard clinical outcomes during the first year post-transplant (14). Beyond these discrepant results for renal function during the early post-transplant period, an important limitation of the published literature on mTOR inhibitors in kidney transplantation is the exclusive focus on the early transplant period. Data on clinical outcomes beyond 2 years following kidney transplantation are sparse (13). We investigated the impact of treatment with mTOR inhibitors on long-term clinical outcomes in a prospective observational study of kidney transplant recipients who had undergone transplantation a median of 6 years earlier and were followed longitudinally for 3 additional years.
Materials and Methods
Study Population
The study population consisted of kidney transplant recipients followed by the Department of Transplantation and Surgery at Semmelweis University in Budapest, Hungary. The center performs approximately 150 kidney transplants annually, and provides post-transplant care to the majority of recipients with minimal loss to follow up. Kidney transplant recipients followed at the center as of December 31, 2006 (n=1,214) were considered for inclusion in a prospective observational study (the Malnutrition-Inflammation in Transplant – Hungary (MINIT-HU Study) aimed at evaluating risk factors for adverse clinical outcomes that occur years after successful transplantation (15–19). Exclusion criteria were current hospitalization or an episode of acute rejection within the previous 4 weeks, transplantation within the preceding 3 months, or an active infection at the time of enrollment. Sixteen patients (1%) met exclusion criteria and 205 (17%) refused to participate, leaving 993 who enrolled in the cohort. During the three years of prospective observation, there was 100% retention of participants in the cohort. The study was approved by the Institutional Review Board of the Semmelweis University and written informed consent was obtained from all patients prior to enrollment.
Baseline visits for all participants occurred between February and August 2007, during which the following data were collected: age, gender, body mass index (BMI), blood pressure (BP), past medical history, medications, primary etiology of end stage renal disease (ESRD), and previous time spent on dialysis. The modified Charlson Comorbidity Index, which is associated with outcomes in transplant populations (20), was calculated as a summary measure of comorbidity. Transplant-specific data included duration post-transplant at enrollment, donor type, number of HLA mismatches, titer of panel reactive antibodies at the time of transplantation, cold ischemia time, current immunosuppressive medications, and history of acute rejection or delayed graft function, defined as the need for hemodialysis at any point within the first week post-transplant. Standard maintenance immunosuppressive regimens at enrollment included prednisone plus cyclosporine A or tacrolimus, and mycophenolate-mofetil, azathioprine, or sirolimus, but deviations from this regimen were permitted for individual patients at the discretion of the primary transplant physician. The local practice at the time at the Semmelweis transplant center was to convert kidney transplant recipients to mTOR inhibitors without performing an allograft biopsy if there was concern for calcineurin inhibitor toxicity or if they had a history of malignancy. Laboratory data measured at the baseline visit in fasting blood specimens included basic chemistries, lipid panels, albumin, calcium, phosphate, intact parathyroid hormone (PTH), and complete blood counts. Baseline 25-hydroxyvitamin D was measured in frozen samples after recruitment was complete. Dipstick proteinuria was not available at baseline but was collected annually during the follow up period. Annual estimated GFR was calculated using the equation derived from the Modification of Diet in Renal Disease Study (21).
Exposure and Outcomes
The primary exposure was use of an mTOR inhibitor, either sirolimus or everolimus, which was ascertained at enrollment and annually during follow-up. Data on immunosuppressive medications that were used prior to enrollment and the duration of use of current medications were not available. The primary outcomes were all-cause mortality and allograft loss, defined as the need to resume dialysis. Outcomes were ascertained from the hospital’s electronic medical records and validated with national death registries. Follow-up of study participants was 100% complete and continued until they died, returned to dialysis, or the three-year observation period ended.
Statistical Analysis
We compared baseline characteristics among users versus non-users of mTOR inhibitors using two-sample t-tests, Wilcoxon rank-sum test, or χ2 test as appropriate. Since follow-up of individual patients was discontinued when they died or resumed dialysis, each outcome event precluded the occurrence of the other. To address this issue of competing risks, we used separate competing risk analyses of the specific outcomes (22). We used cumulative incidence plots to present the univariate association between use of mTOR inhibitors at enrollment and outcomes, and multivariable competing risk regression to adjust for confounding factors.
Given important differences in clinical characteristics among users versus non-users of mTOR inhibitors, the relatively low rates of outcome events and mTOR inhibitor use, and the large number of potential confounders, we used propensity scores to parsimoniously adjust for confounding factors and to address potential confounding by indication (23). We calculated the propensity score of receiving an mTOR inhibitor at enrollment after fitting a multivariable logistic regression model with mTOR inhibitor use as the dependent variable. Data that were evident to the clinical transplant team in real time and thus could affect the choice of immunosuppressive agents were used to calculate the propensity score and included age, sex, BP, BMI, smoking, diabetes, history of non-cutaneous malignancy (any prior history of non-cutaneous malignancy ascertained at the time of enrollment), Charlson comorbidity index, etiology of renal failure, duration of dialysis prior to transplant, duration post-transplant at enrollment, titer of panel reactive antibodies, number of HLA mismatches, donor type, cold ischemia time, history of rejection, history of delayed graft function, eGFR, serum albumin, calcium, phosphate, PTH and 25-hydroxyvitmain D, hemoglobin, and white blood cell and platelet counts.
We expected a history of non-cutaneous malignancy to be a risk factor for mortality and among the strongest predictors of receiving an mTOR inhibitor years after transplantation. Therefore, we performed pre-specified stratified analyses according to malignancy status at enrollment. To adjust for confounding factors in the stratified analyses, we recalculated a new propensity score exclusively in the subgroup without a history of malignancy. To assess the robustness of our results, we also adjusted for individual covariates instead of the propensity score, performed a one-to-one propensity score-matched analysis of mortality (matching propensity scores ± 0.05 of treated and untreated patients), and analyzed longitudinal use of mTOR inhibitors during follow-up as a time-varying factor adjusted for propensity scores and the individual covariates. Since the study recruited patients several years after transplantation, bias could have been introduced by excluding other patients who died or lost their allografts before they could have enrolled. To address this potential bias, we performed a left-truncated Cox regression analysis. In exploratory analyses, we also adjusted for baseline lipid values and dipstick proteinuria during follow-up. Finally, to determine whether the results were specific to mTOR inhibitors, we tested the impact on outcomes of treatment with tacrolimus, cyclosporine, mycophenolate mofetil and corticosteroids at enrollment after substituting use versus non-use of these agents for mTOR inhibitors in the multivariable analyses. Analyses were performed using Intercooled Stata 11 (College Station, TX) and SAS 9.2 (SAS Institute, Cary, NC, USA). P values < 0.05 were considered statistically significant.
Results
Baseline characteristics
The median duration post-transplant when participants enrolled was 72 months (interquartile range, 39, 113 months). At enrollment, 101 (10%) patients were treated with mTOR inhibitors, 79 with sirolimus and 22 with everolimus. Among the 101 users of mTOR inhibitors, 37 received combination therapy with calcineurin inhibitors and 64 received no calcineurin inhibitors. Baseline clinical and transplant characteristics of the study population are presented in Table 1 according to treatment status with mTOR inhibitors. Greater than 95% of patients who were on an mTOR inhibitor at enrollment remained on one of these agents during the longitudinal follow-up period, while only 2.5% of previously untreated patients initiated an mTOR inhibitor during follow-up.
Table 1.
Comparison of baseline characteristics among users and non-users of mTOR inhibitors.
| + mTOR inhibitor n=101 |
−mTOR inhibitor n=892 |
P | |
|---|---|---|---|
| Post-transplant duration, months | |||
| Median (interquartile range) | 54 (36, 99) | 74 (40, 115) | 0.21 |
| Mean ± standard deviation | 76 ± 54 | 80 ± 52 | 0.43 |
|
| |||
| Age, years | 53.8 ± 10.6 | 50.6 ± 13.0 | 0.02 |
|
| |||
| Male (%) | 57 | 53 | 0.41 |
|
| |||
| Etiology of primary kidney disease (%) | <0.001 | ||
|
| |||
| Glomerulonephritis | 20 | 23 | |
|
| |||
| Tubulointerstitial nephritis | 5 | 14 | |
|
| |||
| Polycystic Kidney Disease | 19 | 18 | |
|
| |||
| Diabetes | 15 | 3 | |
|
| |||
| Hypertension | 9 | 6 | |
|
| |||
| Other/Unknown | 33 | 35 | |
|
| |||
| Dialysis duration, months | 18 (9, 36) | 20 (9, 38) | 0.61 |
|
| |||
| Deceased donor transplant (%) | 93 | 94 | 0.86 |
|
| |||
| HLA-A mismatches (%) | 0.10 | ||
| 0 | 7 | 13 | |
| 1 | 64 | 65 | |
| 2 | 29 | 22 | |
|
| |||
| HLA-B mismatches (%) | 0.02 | ||
| 0 | 8 | 14 | |
| 1 | 59 | 64 | |
| 2 | 33 | 22 | |
|
| |||
| HLA-DR mismatches (%) | <0.001 | ||
| 0 | 31 | 28 | |
| 1 | 53 | 65 | |
| 2 | 16 | 7 | |
|
| |||
| Delayed graft function (%) | 25 | 26 | 0.89 |
|
| |||
| History of acute rejection (%) | 33 | 34 | 0.85 |
|
| |||
| Body mass index, kg/m2 | 26 ± 5 | 27 ± 5 | 0.05 |
|
| |||
| Systolic blood pressure, mmHg | 143 ± 20 | 142 ± 19 | 0.42 |
|
| |||
| Diastolic blood pressure, mmHg | 82 ± 11 | 84 ± 12 | 0.32 |
|
| |||
| Current smoker (%) | 17 | 19 | 0.62 |
|
| |||
| Charlson comorbidity index | 4 (2, 5) | 2 (2, 3) | <0.001 |
|
| |||
| History of malignancy (%) | 33 | 2 | <0.001 |
|
| |||
| Creatinine, mg/dl | 1.8± 1.2 | 1.6 ± 0.9 | 0.04 |
|
| |||
| eGFR, ml/min/1.73m2 | 47 ± 23 | 51 ± 21 | 0.05 |
|
| |||
| Cholesterol, mg/dl | 243 ± 60 | 208 ± 47 | <0.001 |
|
| |||
| Low-density lipoprotein, mg/dl | 138 ± 44 | 120 ± 34 | <0.001 |
|
| |||
| High-density lipoprotein, mg/dl | 56 ± 22 | 50 ± 16 | 0.002 |
|
| |||
| Triglycerides, mg/dl | 225 ± 134 | 180 ± 130 | 0.001 |
|
| |||
| Albumin, g/dl | 3.8 ± 0.4 | 4.0 ± 0.4 | <0.001 |
|
| |||
| Calcium, mg/dl | 9.4 ± 0.6 | 9.5 ± 0.6 | 0.24 |
|
| |||
| Phosphate, mg/dl | 3.3 ± 0.8 | 3.3 ± 0.9 | 0.55 |
|
| |||
| 25-hydroxyvitamin D, ng/ml | 7.9 (5.4, 11.7) | 10.0 (5.9, 14.9) | 0.01 |
|
| |||
| Parathyroid hormone, pg/ml | 76 (48, 116) | 70 (47, 102) | 0.13 |
|
| |||
| Hemoglobin, g/dl | 13.4 ± 1.9 | 13.5 ± 1.7 | 0.79 |
|
| |||
| White blood cells (cells × 103) | 7.4 ± 2.2 | 7.9 ± 2.3 | 0.04 |
Results are reported as mean ± standard deviation, median (interquartile range) or proportions.
Factors Associated with Use of an mTOR Inhibitor at Enrollment
Given the significant differences in clinical characteristics among users versus non-users of mTOR inhibitors, we calculated a propensity score of likelihood of mTOR inhibitor use at enrollment. The most powerful independent clinical predictors of use of mTOR inhibitors, derived from the multivariable model used to calculate the propensity score, were history of malignancy followed, in descending order of strength of association, by diabetes, lower serum albumin, BMI, and eGFR (Table 2).
Table 2. Independent predictors of use of mTOR inhibitors at enrollment.
Odds ratios and 95% confidence intervals (CI) of use versus non-use of mTOR inhibitors were derived from the multivariable propensity score model. Individual predictors are listed in descending order of strength of association with use of mTOR inhibitors.
| Clinical factor | Odds ratio | 95% CI | P |
|---|---|---|---|
| Non-cutaneous malignancy versus no malignancy | 41.5 | 16.1, 106.9 | <0.001 |
| Diabetes | 9.8 | 4.0, 24.1 | <0.001 |
| Serum albumin (per 1 g/dl increase) | 0.31 | 0.16, 0.60 | <0.001 |
| Body mass index (per 1 kg/m2 increase) | 0.92 | 0.87, 0.98 | 0.01 |
| Estimated GFR (per 10 ml/min/1.73m2 increase) | 0.84 | 0.72, 0.99 | 0.03 |
In addition to the factors listed in the Table, the propensity score model included age, sex, BP, transplant vintage, previous dialysis vintage, current smoking, current diabetes, white blood cell count, hemoglobin, platelet count, calcium, phosphate, PTH, panel reactive antibodies and cold ischemic time at the time of transplantation, donor type, HLA mismatches, history of delayed graft function, and history of acute rejection.
Outcomes
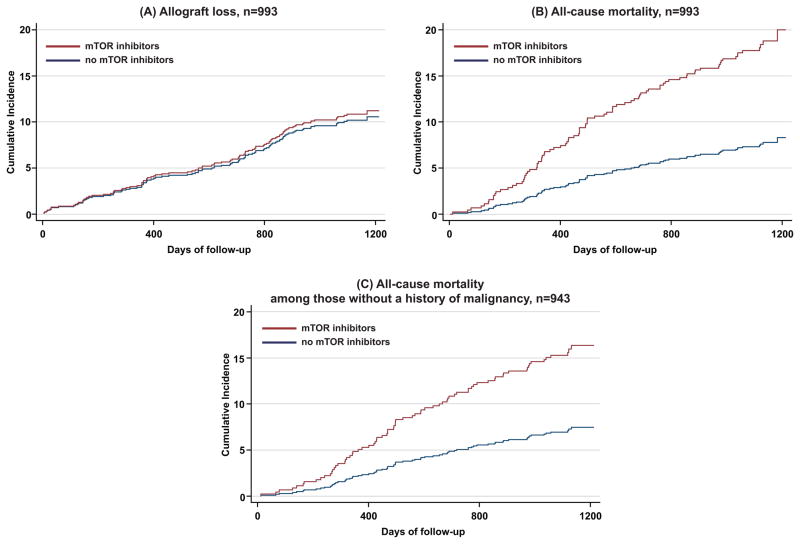
During a median follow-up of 37 months (interquartile range 35–39), 87 patients died and 102 suffered allograft loss. Although there was no significant difference in allograft loss according to treatment with mTOR inhibitors (Figure 1A), patients receiving mTOR inhibitors at baseline had a significantly increased risk of death in the overall cohort (Figure 1B) and in the subgroup without a history of malignancy (Figure 1C; P < 0.001 for each).
Figure 1. Cumulative incidence plots of allograft loss (A) and all-cause mortality (B) in the overall population, and all-cause mortality among those without a history of malignancy (C) according to baseline use versus non-use of mTOR inhibitors.
The mortality plots considered allograft loss as a competing risk, and the allograft loss considered death as a competing risk. Study participants who remained event-free were censored at the end of the 3-year observation period. No participants were prematurely lost to follow-up.
In multivariable models of the overall population that adjusted for the propensity score (likelihood) of receiving mTOR inhibitors, use of mTOR inhibitors was no longer independently associated with increased risk of mortality, while the lack of association between mTOR inhibitors and allograft loss persisted (Table 3). In contrast, in models that adjusted individually for covariates (instead of the propensity score), use of mTOR inhibitors was associated with increased mortality (P = 0.02). This was attenuated with further adjustment for lipids (Table 3). In the multivariable-adjusted analyses, reduced renal function at baseline was the strongest predictor of allograft loss (hazard ratio [HR] 1.8 per 10 ml/min/1.73m2 decrease in eGFR, 95%CI 1.5, 2.1; P < 0.001), but it was not independently associated with mortality. There was no difference in the slope of eGFR loss over time between the mTOR inhibitor treatment groups (data not shown).
Table 3. Crude and multivariable-adjusted risks of all-cause mortality and allograft loss comparing users versus non-users of mTOR inhibitors in the overall population (n = 993) and in the subgroup without a history of malignancy (n = 943).
Hazard ratios (HR) for death were derived from competing risks regression models that considered allograft loss as a competing risk. Hazard ratios for allograft loss were derived from similar models that considered death as a competing risk.
| All-cause Mortality | Allograft Loss | |||
|---|---|---|---|---|
| Hazard Ratio (95%CI) | P | Hazard Ratio (95%CI) | P | |
| Overall population, n=993 | ||||
| Crude | 2.6 (1.6, 4.3) | <0.001 | 1.1 (0.6, 2.0) | 0.84 |
| + Propensity Score | 1.8 (0.8, 4.0) | 0.14 | 0.7 (0.3, 1.6) | 0.40 |
| Full model* | 2.3 (1.1, 4.5) | 0.02 | 0.7 (0.4, 1.4) | 0.32 |
| Full model + LDL, triglycerides | 1.9 (0.9, 4.1) | 0.08 | 0.8 (0.4, 1.5) | 0.42 |
| Patients without a history of malignancy (n = 943) | ||||
| Crude | 2.3 (1.2, 4.3) | 0.01 | 1.0 (0.5, 2.1) | 0.95 |
| + Propensity Score | 2.6 (1.2, 5.5) | 0.01 | 0.6 (0.2, 1.5) | 0.27 |
| Full model* | 3.2 (1.5, 6.5) | 0.002 | 0.7 (0.3, 1.7) | 0.41 |
| Full model + LDL, triglycerides | 2.9 (1.4, 6.1) | 0.004 | 0.7 (0.3, 1.9) | 0.53 |
Adjusted for age, sex, history of malignancy, eGFR, systolic blood pressure, BMI, albumin, calcium, phosphate, PTH, 25-hydroxyvitamin D, Charlson comorbidity index, etiology of renal failure, transplant vintage at enrollment, smoking, diabetes, donor type and HLA mismatches.
Stratified Analyses
History of malignancy was not associated with allograft loss (HR 1.0, 95% CI 0.4, 2.5; P = 0.9), but was strongly associated with greater risk of mortality (HR 3.8, 95% CI 2.1, 7.0; P < 0.001). Thus, a history of malignancy markedly increased risk of death and it was also the single strongest predictor of treatment with mTOR inhibitors. Therefore, we performed additional pre-specified analyses that stratified by history of malignancy. In the stratum of 50 patients with a history of malignancy, the unadjusted HR for death comparing users versus non-users of mTOR inhibitors was 0.8 (95%CI 0.3, 2.5; P = 0.7). The limited number of patients who received mTOR inhibitors (n = 33) and the few deaths (n = 13) in this stratum precluded multivariable analyses.
In the subgroup of patients without a history of malignancy (n = 943), use of mTOR inhibitors was independently associated with increased risk of death in unadjusted and propensity score-adjusted analyses, and in an analysis that adjusted for covariates individually (Table 3). Further adjusting the latter model for lipids did not substantially alter the results, nor did adjustment for follow up dipstick proteinuria (data not shown). The results were also qualitatively unchanged in the malignancy-free subgroup when we analyzed longitudinal use of mTOR inhibitors during follow-up as a time-varying factor in univariate (HR 2.1, 95%CI 1.2, 3.9; P = 0.01), propensity score-adjusted (HR 2.2, 95%CI 1.1, 4.5; P = 0.03), and fully adjusted models (HR 2.0, 95%CI 1.1, 3.8; P = 0.03).
Sensitivity Analyses
To further address potential confounding by indication, we compared mortality in a subcohort of 78 pairs of users and non-users of mTOR inhibitors who were one-to-one matched on their likelihood of being treated. The mean propensity scores in the two groups were identical at 0.21, and Table 4 confirms that the one-to-one matching attenuated differences in baseline characteristics between the groups compared to the overall unmatched cohort (Table 1). In the matched subcohort (n=156), use of mTOR inhibitors was associated with an identical point estimate for mortality as in the overall cohort (Table 3) but the result did not reach statistical significance (HR 2.3; 95%CI 0.9, 6.2; P = 0.08). However, among participants without a history of malignancy in the matched subcohort (n=128), use of mTOR inhibitors was associated with significantly increased risk of mortality versus non-use (HR 5.6; 1.2, 25.7; P = 0.03).
Table 4.
Comparison of baseline characteristics in one-to-one matched pairs of users and non-users of mTOR inhibitors.
| + mTOR inhibitor n=78 |
−mTOR inhibitor n=78 |
P | |
|---|---|---|---|
| Post-transplant duration, months | 74 ± 56 | 73 ± 51 | 0.88 |
| < 50 months, n (%) | 33 (42) | 33 (42) | |
| 50–99 months, n (%) | 26 (33) | 25 (32) | |
| > 99 months, n (%) | 19 (25) | 20 (26) | |
|
| |||
| Age, years | 53.3 ± 9.6 | 53.8 ± 11.1 | 0.78 |
|
| |||
| Diabetes, n (%) | 13 (17) | 10 (13) | 0.50 |
|
| |||
| Deceased donor transplant, n (%) | 75 (96) | 76 (97) | 0.65 |
|
| |||
| History of acute rejection, n (%) | 28 (36) | 26 (33) | 0.74 |
|
| |||
| HLA A mismatches, n (%) | 0.58 | ||
| 0 | 6 (8) | 4 (5) | |
| 1 | 46 (59) | 52 (67) | |
| 2 | 26 (33) | 22 (28) | |
|
| |||
| HLA B mismatches, n (%) | 1.0 | ||
| 0 | 8 (10) | 8 (10) | |
| 1 | 48 (62) | 48 (62) | |
| 2 | 22 (28) | 22 (28) | |
|
| |||
| HLA DR mismatches, n (%) | 0.09 | ||
| 0 | 24 (31) | 18 (23) | |
| 1 | 44 (56) | 56 (72) | |
| 2 | 10 (13) | 4 (5) | |
|
| |||
| eGFR, ml/min/1.73m2 | 47 ± 21 | 45 ± 19 | 0.57 |
|
| |||
| Current malignancy, n (%) | 13 (17) | 15 (19) | 0.68 |
|
| |||
| Charlson comorbidity index | 3 (2, 5) | 3 (2, 5) | 0.82 |
In the left truncated analyses of the overall population, use of mTOR inhibitors was associated with significantly increased risk of mortality in crude (HR 2.8; 95%CI 1.7, 4.8; P < 0.001) and multivariable-adjusted analyses (HR 2.2; 95%CI 1.2, 4.1; P = 0.02). The corresponding results were similar in participants without a history of malignancy (crude: HR 2.6; 95%CI 1.3, 5.0; P = 0.005; adjusted: HR 2.8; 95%CI 1.4, 5.8; P = 0.005).
Other Immunosuppressive Agents
To examine the specificity of the findings for mTOR inhibitors, we repeated the multivariable-adjusted analyses of death according to baseline treatment versus non-treatment with other immunosuppressive agents. In the overall population, treatment with tacrolimus (HR 0.8; 95%CI 0.5, 1.4; P = 0.44), cyclosporine (HR 0.8; 95%CI 0.5, 1.3; P = 0.32), mycophenolate mofetil (HR 0.8; 95%CI 0.4, 1.4; P = 0.41), and corticosteroids (HR 0.9; 95%CI 0.5, 1.6; P = 0.64) were not associated with altered risk of mortality. The results were qualitatively unchanged in patients without a history of malignancy (data not shown). Co-treatment with a calcineurin inhibitor did not modify the relationship between mTOR inhibitors and mortality.
Discussion
In this prospective observational study of prevalent kidney transplant recipients who had undergone transplantation approximately 6 years earlier, patients without a history of malignancy who were receiving mTOR inhibitors had a significantly increased risk of death. The results are indirectly supported by an unpublished trial of stable liver transplant recipients that was terminated prematurely because of increased mortality in the arm that was converted from a maintenance calcineurin inhibitor-based regimen to a sirolimus-based regimen (24). Although this is the first study to our knowledge to identify increased risk of mortality in association with mTOR inhibitors in kidney transplantation, the results should be considered hypothesis-generating, in need of validation, and, based on the study’s limitations, should not lead to a change in clinical practice.
Several scenarios lead clinicians to treat kidney transplant recipients with mTOR inhibitors, and these emerged in our study. Sirolimus and everolimus exert antiproliferative effects and are associated with decreased incidence of malignancy in kidney transplant populations (7, 8). In addition, mTOR inhibitors prolonged survival in randomized trials of metastatic neuroendocrine tumors (25, 26). Based on these properties, mTOR inhibitors are often used in transplant recipients with malignancy, which we confirmed was the leading predictor of therapy in the current study. Diabetes was the second most powerful predictor of mTOR inhibitor use, which was also expected because calcineurin inhibitors increase insulin resistance (27, 28), and mTOR inhibitors may be beneficial in diabetes by blocking diabetogenic cellular signaling pathways (29). Finally, patients with a lower eGFR were also more likely to receive an mTOR inhibitor, which reflects the usual practice at the transplant center of Semmelweis University to switch cyclosporine or tacrolimus to an mTOR inhibitor when there is concern for calcineurin inhibitor-induced nephrotoxicity.
A history of malignancy, diabetes, and reduced eGFR were the primary determinants of mTOR inhibitor therapy, and these are all risk factors for adverse outcomes in kidney transplantation (30, 31). Therefore, it could be argued that the link between mTOR inhibitors and mortality was driven by those patients who had a higher baseline risk of mortality and were preferentially treated with mTOR inhibitors on the basis of these same factors. The propensity score is particularly useful in such situations when confounding by indication is a concern (23). The finding that mTOR inhibitors were associated with mortality in the malignancy-free group in propensity score-adjusted analyses that modeled mTOR inhibitor use as a fixed or time-varying factor and in a one-to-one propensity score-matched analysis argues against confounding by indication. The divergence of results for allograft loss versus mortality strengthens this view. Reduced renal function at baseline was more strongly predictive of allograft loss than mortality, and use of mTOR inhibitors was not associated with allograft loss or differences in slope of eGFR. It is therefore unlikely that the slightly lower eGFR among users of mTOR inhibitors could explain their 2-fold increased risk of mortality.
As an observational, hypothesis-generating study, this report was unable to define potential mechanisms to explain increased mortality among malignancy-free users of mTOR inhibitors. Cardiovascular disease is a leading cause of death in kidney transplant recipients (8), and sirolimus and everolimus exert dueling protective and detrimental effects on the cardiovascular system. For example, mTOR inhibitors prevent proliferation of vascular smooth muscle cells that contribute to atherosclerosis and restenosis of coronary artery stents, suggesting they may slow progression of arterial disease (32). Conversely, mTOR inhibitors are associated with hyperlipidemia, which can promote atherosclerosis (28). In a systematic review, treatment with an mTOR inhibitor was associated with higher cholesterol levels or increased use of lipid lowering agents in all but one of 17 trials (10). Indeed, receiving an mTOR inhibitor was associated with higher levels of LDL and triglycerides in our population. Although it is possible that dyslipidemia associated with use of mTOR inhibitors contributed to increased mortality, it is interesting that adjusting for lipid levels did not markedly attenuate the effect. This suggests additional possible mechanisms, but further studies are needed to longitudinally assess the impact of repeated lipid measurements on outcomes in patients receiving mTOR inhibitors.
Our study has limitations that require emphasis. First, we studied a homogenous population of Caucasian kidney transplant recipients from Eastern Europe with a low prevalence of diabetes, and thus, the results may not generalize to other transplant populations. Second, because the low overall rate of mTOR inhibitor use required us to analyze sirolimus and everolimus as a group, we could not investigate the agents individually. Likewise, although we were able to carry out detailed analyses of patients without malignancy, the small number with malignancy may have precluded us from detecting a survival benefit of mTOR inhibitors in this subgroup. Third, the lack of data on the duration of use of specific immunosuppressive regimens prior to enrollment and their associated impact on cumulative cardiovascular burden may have been incompletely captured in the baseline variables we studied. Another limitation is the lack of data on the specific cause of death, which could have shed light on possible mechanisms underlying our findings. Similarly, we did not have complete data on proteinuria or dyslipidemia, which are independently associated with mortality and are known side effects of mTOR inhibitors (33). However, since proteinuria and dyslipidemia are consequences of mTOR inhibitor therapy and not indications for their use, they would lie along a causal pathway rather than representing true confounders, and thus, adjustment for them in the primary analysis would have been inappropriate.
Although our study is limited by its relatively small size and observational design, it is unlikely that a randomized trial will be performed with an adequate sample size and duration of follow-up capable of detecting the long-terms differences in mortality that our study suggests. In such situations when data is needed but resources are limited to conduct lengthy and costly clinical trials, observational studies are an attractive option to fill the void and direct further investigation. The literature abounds with examples of observational studies detecting important outcomes data missed by randomized trials due to insufficient power or length of follow-up (34, 35). Furthermore, direct comparisons of randomized trials and carefully performed observational studies that evaluated identical interventions often demonstrated similar results (36, 37). The current hypothesis-generating study should stimulate larger studies of the long-term safety and efficacy of mTOR inhibitors in transplantation, both as de-novo and conversion therapy, and especially in patients without a history of malignancy.
Acknowledgments
This study was supported by grants R01DK076116 and R01DK081374 from the National Institutes of Health, grants from the National Research Fund (OTKA) (F-68841; HUMAN-MB08-A-81231), ETT (206/09), the Hungarian Kidney Foundation, Hungarian Society of Hypertension, Hungarian Society of Nephrology, the Foundation for Prevention in Medicine, the János Bolyai Research Scholarship of the Hungarian Academy of Sciences, and the Hungarian Eötvös Scholarship (MÖB/66-2/2010). The authors thank the patients and the staff in the Department of Transplantation and Surgery, Semmelweis University Budapest.
Abbreviations
- mTOR
mammalian target of rapamycin
- eGFR
estimated glomerular filtration rate
- ESRD
end stage renal disease
- BP
blood pressure
- PTH
parathyroid hormone
- HLA
human leukocyte antigen
Footnotes
Disclosures
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Meier-Kriesche HU, Schold JD, Kaplan B. Long-term renal allograft survival: have we made significant progress or is it time to rethink our analytic and therapeutic strategies? Am J Transplant. 2004;4:1289–1295. doi: 10.1111/j.1600-6143.2004.00515.x. [DOI] [PubMed] [Google Scholar]
- 2.Nankivell BJ, Borrows RJ, Fung CL, O’Connell PJ, Allen RD, Chapman JR. The natural history of chronic allograft nephropathy. N Engl J Med. 2003;349:2326–2333. doi: 10.1056/NEJMoa020009. [DOI] [PubMed] [Google Scholar]
- 3.DiJoseph JF, Mihatsch MJ, Sehgal SN. Renal effects of rapamycin in the spontaneously hypertensive rat. Transpl Int. 1994;7:83–88. doi: 10.1007/BF00336467. [DOI] [PubMed] [Google Scholar]
- 4.Morales JM, Wramner L, Kreis H, Durand D, Campistol JM, Andres A, et al. Sirolimus does not exhibit nephrotoxicity compared to cyclosporine in renal transplant recipients. Am J Transplant. 2002;2:436–442. doi: 10.1034/j.1600-6143.2002.20507.x. [DOI] [PubMed] [Google Scholar]
- 5.Schuler W, Sedrani R, Cottens S, Haberlin B, Schulz M, Schuurman HJ, et al. SDZ RAD, a new rapamycin derivative: pharmacological properties in vitro and in vivo. Transplantation. 1997;64:36–42. doi: 10.1097/00007890-199707150-00008. [DOI] [PubMed] [Google Scholar]
- 6.Sehgal SN. Rapamune (RAPA, rapamycin, sirolimus): mechanism of action immunosuppressive effect results from blockade of signal transduction and inhibition of cell cycle progression. Clin Biochem. 1998;31:335–340. doi: 10.1016/s0009-9120(98)00045-9. [DOI] [PubMed] [Google Scholar]
- 7.Kauffman HM, Cherikh WS, Cheng Y, Hanto DW, Kahan BD. Maintenance immunosuppression with target-of-rapamycin inhibitors is associated with a reduced incidence of de novo malignancies. Transplantation. 2005;80:883–889. doi: 10.1097/01.tp.0000184006.43152.8d. [DOI] [PubMed] [Google Scholar]
- 8.Rostaing L, Kamar N. mTOR inhibitor/proliferation signal inhibitors: entering or leaving the field? J Nephrol. 2010;23:133–142. [PubMed] [Google Scholar]
- 9.Dean PG, Lund WJ, Larson TS, Prieto M, Nyberg SL, Ishitani MB, et al. Wound-healing complications after kidney transplantation: a prospective, randomized comparison of sirolimus and tacrolimus. Transplantation. 2004;77:1555–1561. doi: 10.1097/01.tp.0000123082.31092.53. [DOI] [PubMed] [Google Scholar]
- 10.Kasiske BL, de Mattos A, Flechner SM, Gallon L, Meier-Kriesche HU, Weir MR, et al. Mammalian target of rapamycin inhibitor dyslipidemia in kidney transplant recipients. Am J Transplant. 2008;8:1384–1392. doi: 10.1111/j.1600-6143.2008.02272.x. [DOI] [PubMed] [Google Scholar]
- 11.Letavernier E, Legendre C. mToR inhibitors-induced proteinuria: mechanisms, significance, and management. Transplant Rev (Orlando) 2008;22:125–130. doi: 10.1016/j.trre.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Letavernier E, Pe’raldi MN, Pariente A, Morelon E, Legendre C. Proteinuria following a switch from calcineurin inhibitors to sirolimus. Transplantation. 2005;80:1198–1203. doi: 10.1097/01.tp.0000185200.17589.74. [DOI] [PubMed] [Google Scholar]
- 13.Webster AC, Lee VW, Chapman JR, Craig JC. Target of rapamycin inhibitors (sirolimus and everolimus) for primary immunosuppression of kidney transplant recipients: a systematic review and meta-analysis of randomized trials. Transplantation. 2006;81:1234–1248. doi: 10.1097/01.tp.0000219703.39149.85. [DOI] [PubMed] [Google Scholar]
- 14.Ekberg H, Tedesco-Silva H, Demirbas A, Vitko S, Nashan B, Gurkan A, et al. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med. 2007;357:2562–2575. doi: 10.1056/NEJMoa067411. [DOI] [PubMed] [Google Scholar]
- 15.Kovesdy CP, Czira ME, Rudas A, Ujszaszi A, Rosivall L, Novak M, et al. Body mass index, waist circumference and mortality in kidney transplant recipients. Am J Transplant. 2010;10:2644–2651. doi: 10.1111/j.1600-6143.2010.03330.x. [DOI] [PubMed] [Google Scholar]
- 16.Kovesdy CP, Molnar MZ, Czira ME, Rudas A, Ujszaszi A, Rosivall L, et al. Associations between serum leptin level and bone turnover in kidney transplant recipients. Clin J Am Soc Nephrol. 2010;5:2297–2304. doi: 10.2215/CJN.03520410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Molnar MZ, Czira ME, Rudas A, Ujszaszi A, Haromszeki B, Kosa JP, et al. Association between the malnutrition-inflammation score and post-transplant anaemia. Nephrol Dial Transplant. 2011;26:2000–2006. doi: 10.1093/ndt/gfq690. [DOI] [PubMed] [Google Scholar]
- 18.Molnar MZ, Czira ME, Rudas A, Ujszaszi A, Lindner A, Fornadi K, et al. Association of the malnutrition-inflammation score with clinical outcomes in kidney transplant recipients. Am J Kidney Dis. 2011;58:101–108. doi: 10.1053/j.ajkd.2010.11.027. [DOI] [PubMed] [Google Scholar]
- 19.Molnar MZ, Keszei A, Czira ME, Rudas A, Ujszaszi A, Haromszeki B, et al. Evaluation of the malnutrition-inflammation score in kidney transplant recipients. Am J Kidney Dis. 2010;56:102–111. doi: 10.1053/j.ajkd.2010.02.350. [DOI] [PubMed] [Google Scholar]
- 20.Jassal SV, Schaubel DE, Fenton SS. Baseline comorbidity in kidney transplant recipients: a comparison of comorbidity indices. Am J Kidney Dis. 2005;46:136–142. doi: 10.1053/j.ajkd.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 21.K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42:S1–201. [PubMed] [Google Scholar]
- 22.Fine J, Gray R. A proportional hazards model for the subdistribution of competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 23.Rosenbaum P, Rubin D. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 24. [Accessed on July 10, 2011];Information for Healthcare Professionals: Sirolimus (marketed as Rapamune): FDA ALERT. 2009 Jun 11; http://wwwfdagov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm165015htm.
- 25.Vignot S, Faivre S, Aguirre D, Raymond E. mTOR-targeted therapy of cancer with rapamycin derivatives. Ann Oncol. 2005;16:525–537. doi: 10.1093/annonc/mdi113. [DOI] [PubMed] [Google Scholar]
- 26.Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514–523. doi: 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Araki M, Flechner SM, Ismail HR, Flechner LM, Zhou L, Derweesh IH, et al. Posttransplant diabetes mellitus in kidney transplant recipients receiving calcineurin or mTOR inhibitor drugs. Transplantation. 2006;81:335–341. doi: 10.1097/01.tp.0000195770.31960.18. [DOI] [PubMed] [Google Scholar]
- 28.Halloran PF. Immunosuppressive drugs for kidney transplantation. N Engl J Med. 2004;351:2715–2729. doi: 10.1056/NEJMra033540. [DOI] [PubMed] [Google Scholar]
- 29.Rui L. A link between protein translation and body weight. J Clin Invest. 2007;117:310–313. doi: 10.1172/JCI31289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Locatelli F, Pozzoni P, Del Vecchio L. Renal replacement therapy in patients with diabetes and end-stage renal disease. J Am Soc Nephrol. 2004;15 (Suppl 1):S25–29. doi: 10.1097/01.asn.0000093239.32602.04. [DOI] [PubMed] [Google Scholar]
- 31.Ojo AO, Hanson JA, Wolfe RA, Leichtman AB, Agodoa LY, Port FK. Long-term survival in renal transplant recipients with graft function. Kidney Int. 2000;57:307–313. doi: 10.1046/j.1523-1755.2000.00816.x. [DOI] [PubMed] [Google Scholar]
- 32.Morice MC, Serruys PW, Sousa JE, Fajadet J, Ban Hayashi E, Perin M, et al. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med. 2002;346:1773–1780. doi: 10.1056/NEJMoa012843. [DOI] [PubMed] [Google Scholar]
- 33.Grimm RH, Jr, Svendsen KH, Kasiske B, Keane WF, Wahi MM. Proteinuria is a risk factor for mortality over 10 years of follow-up. MRFIT Research Group. Multiple Risk Factor Intervention Trial Kidney. Int Suppl. 1997;63:S10–14. [PubMed] [Google Scholar]
- 34.Bergkvist L, Adami HO, Persson I, Hoover R, Schairer C. The risk of breast cancer after estrogen and estrogen-progestin replacement. N Engl J Med. 1989;321:293–297. doi: 10.1056/NEJM198908033210505. [DOI] [PubMed] [Google Scholar]
- 35.Friis-Moller N, Sabin CA, Weber R, d’Arminio Monforte A, El-Sadr WM, Reiss P, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349:1993–2003. doi: 10.1056/NEJMoa030218. [DOI] [PubMed] [Google Scholar]
- 36.Benson K, Hartz AJ. A comparison of observational studies and randomized, controlled trials. N Engl J Med. 2000;342:1878–1886. doi: 10.1056/NEJM200006223422506. [DOI] [PubMed] [Google Scholar]
- 37.Concato J, Shah N, Horwitz RI. Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med. 2000;342:1887–1892. doi: 10.1056/NEJM200006223422507. [DOI] [PMC free article] [PubMed] [Google Scholar]