One of the most remarkable aspects of reproductive biology is the simple fact that a healthy woman with a fully functional immune system can successfully carry a semiallogeneic pregnancy to full term without immune rejection. A recent study by Samstein and colleagues1 provides new insights into the mechanism of maternal–fetal tolerance and suggests that our previous notions have been naive. This study may also explain why prior approaches to preventing immune rejection of the fetus by the mother have failed and suggests an alternative approach to treatment.
Human reproduction is far from perfectly efficient; one in five pregnancies ends in miscarriage, with the vast majority of miscarriages occurring in the first trimester, commonly owing to sporadic chromosomal abnormalities. Recurrent miscarriage, which is defined as two or more miscarriages, occurs in up to 5% of couples attempting to conceive. In these cases, typically, the fetus is euploid and no cause for the miscarriage is found.
For decades, it was thought that one possible cause of recurrent miscarriage was the rejection of the fetus by the maternal immune system. The notion that an overzealous immune system recognizes the fetus as a foreign element led to the use of therapeutic interventions, such as the administration of glucocorticoids and intravenous immune globulin, with the goal of suppressing the maternal immune system. These therapies, however, proved largely disappointing; inhibiting the maternal immune system did not prevent miscarriage.
Regulatory T cells (Tregs) are a subset of inhibitory CD4+ helper T cells that function to curb the immune response to infection, inflammation, and autoimmunity (Fig. 1). Forkhead box P3 (FOXP3), a transcription factor expressed by Tregs, mediates this suppressor function. A deficiency in Tregs, whether it is congenital or acquired, results in a fatal systemic autoimmune response. There are two developmental pathways of Tregs: thymic (tTreg) and extrathymic or peripheral (pTreg). Recently, a FOXP3 enhancer element, conserved noncoding sequence 1 (CNS1), was shown to be essential for pTreg but not tTreg generation, suggesting different biologic functions of these two cell populations.2 Specifically, tTregs appear to suppress autoimmunity, whereas pTregs may restrain immune responses to foreign antigens, such as those from diet, commensal bacteria, and allergens.
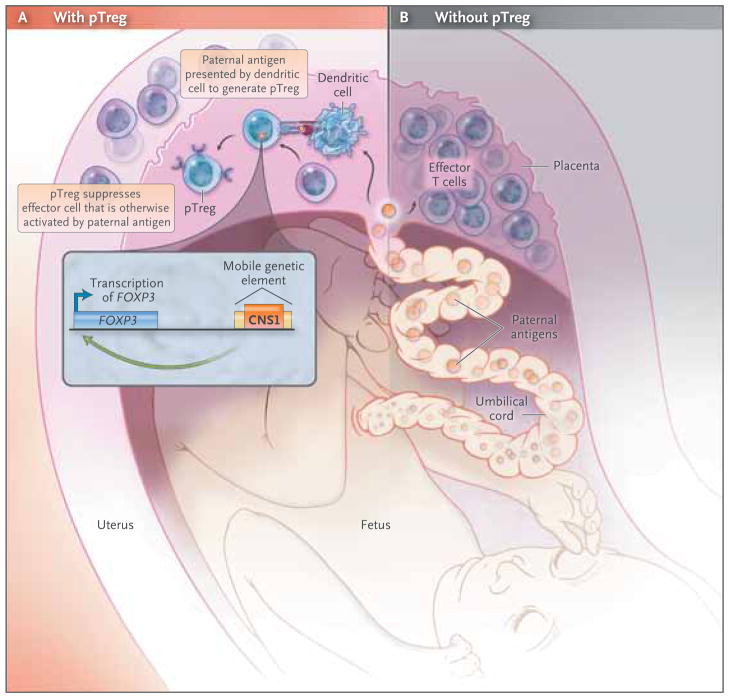
Figure 1. Regulatory T Cells and Spontaneous Abortion.
Fetal antigens, including paternal alloantigens, interface with the maternal immune system within the uteroplacental unit and proximal lymph nodes. A recent study by Samstein et al.1 suggests that insertion of a mobile genetic element containing the conserved noncoding sequence 1 (CNS1) near the FOXP3 gene at the time of the evolutionary radiation of placental mammals enabled the emergence of extrathymic or peripheral regulatory T cells (pTregs) (Panel A). These investigators found that paternal alloantigens trigger the expansion of pTregs that suppress effector T cells and mitigate maternal–fetal conflict induced by the maternal immune system. This suggests that when pTregs are absent, effector T cells respond to the paternal alloantigens, infiltrate the placenta, and cause inflammatory changes that ultimately result in spontaneous abortion (Panel B).
The evolutionary emergence of placental mammals provided survival advantages, such as allowing for more complex fetal development and protection. However, this came at the cost of prolonged exposure to paternal alloantigens and hence the need to mitigate maternal–fetal conflict by the maternal immune system. Samstein and colleagues hypothesized that pTregs emerged during the evolution of placental mammals to mitigate maternal–fetal conflict. In support of this hypothesis is their finding that CNS1, which is required for pTreg development, is present in all 14 placental mammals they tested and is absent in nonplacental mammals such as the wallaby, opossum, and platypus, as well as in nonmammals such as zebra fish. Because CNS1 is located within a retrotransposon (“jumping gene”) family that was active during the evolutionary radiation of placental mammals, Samstein and colleagues suggested that the retro-transposition of CNS1 into the Foxp3 locus allowed for the emergence of pTregs.
In a series of elegant experiments in mice, the authors showed that pregnancy-induced maternal pTregs specifically recognized paternal antigens. These pTregs suppressed maternal effector T cells and mitigated the maternal–fetal conflict caused by paternal alloantigens. In CNS1-deficient mice, the inability to induce pTregs in the mother resulted in the infiltration of activated T cells into the placenta and spontaneous abortions.
These findings indicate that maternal–fetal tolerance to paternal alloantigens is an active process in which pTregs specifically respond to paternal antigens to induce tolerance. Thus, therapies should aim not to suppress the maternal immune system but rather to enhance tolerance. These findings are consistent with an increase in the percentage of Tregs during pregnancy and with no such increase in women with recurrent pregnancy loss.3 These results also provide biologic plausibility for the seemingly paradoxical observation that, among women with a history of unexplained recurrent miscarriage, the administration of filgrastim (granulocyte colony-stimulating factor) in early pregnancy reduces the rate of miscarriage.4
We are now beginning to better understand the mechanism by which the fetus is protected from rejection by the maternal immune system. It will be important to see whether aberrant induction of pTregs is a cause of miscarriage in humans and, if so, how frequently it occurs. Although having an explanation for recurrent miscarriage often brings a sense of relief and closure for the patient, it would be enormously gratifying to see these results translated into therapies to help those who have recurrent miscarriage.
Footnotes
Disclosure forms provided by the author are available with the full text of this article at NEJM.org.
References
- 1.Samstein RM, Josefowicz SZ, Arvey A, et al. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflict. Cell. 2012;150:29–38. doi: 10.1016/j.cell.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng Y, Josefowicz SZ, Chaundhry A, Peng XP, Forbush K, Rudensky AY. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature. 2010;463:808–12. doi: 10.1038/nature08750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Somerset DA, Zheng Y, Kilby MD, Sansom DM, Drayson MT. Normal human pregnancy is associated with an elevation in the immune suppressive CD25+CD4+ regulatory T-cell subset. Immunology. 2004;112:38–43. doi: 10.1111/j.1365-2567.2004.01869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scarpellini F, Sbracia M. Use of granulocyte colony-stimulating factor for the treatment of unexplained recurrent miscarriage: a randomised controlled trial. Hum Reprod. 2009;24:2703–8. doi: 10.1093/humrep/dep240. [DOI] [PubMed] [Google Scholar]