Abstract
Purpose
Excessive scarring leading to failure of the filtering bleb continues to be a major problem after glaucoma filtration surgery. This study examines the antifibrotic effects of the anti-S1P monoclonal antibody LT1009 (Sonepcizumab) in prolonging bleb survival in a rabbit model of glaucoma filtering surgery.
Methods
The frequency of LT1009 dosage was determined initially using an enzyme-linked immunosorbent assay assay measuring LT1009 eye tissue retention in 6 New Zealand White rabbits. A further 21 New Zealand White rabbits underwent glaucoma filtering surgery. Bleb tissues were observed and compared clinically and histologically. The duration of bleb elevation was compared among LT1009, balanced saline solution (BSS) negative control, and mitomycin-C (MMC)-positive control.
Results
The mean duration of bleb survival was 28.5 ± 8.5 days for rabbits receiving injections of LT1009, 21.0 ± 5.6 days for those receiving injections of BSS, and 33.8 ± 5.6 days for rabbits receiving MMC. Analysis of variance with post hoc testing suggests a statistically significant trend of improvement in bleb duration for LT1009 when compared with BSS controls. Non-painful, upper eyelid edema was noted after 5 injections of LT1009, which resolved over a 10-day period. MMC eyes developed avascular conjunctivas with areas of thinning and sparse cellularity, whereas the conjunctiva of LT1009 and BSS eyes remained relatively normal.
Conclusions
The monoclonal antibody LT1009 demonstrated a longer duration of bleb elevation than BSS control without adverse conjunctival effects associated with MMC. However, after multiple doses LT1009 use was associated with short-term upper eyelid edema.
Keywords: glaucoma, wound healing, anti-S1P monoclonal antibody, rabbit model
When medication and/or laser have proven ineffective for patients with glaucoma, glaucoma filtering surgery (GFS) is the most common treatment used to control intraocular pressure (IOP). This procedure involves the creation of a new drainage channel to filter aqueous humor from the anterior chamber to the outer surface of the sclera, resulting in the formation of a subconjunctival bleb and a decrease in IOP. However, the filter can fail if scar tissue forms between the conjunctiva/Tenon capsule and the sclera at the surgical site. This scarring, caused by increased fibroblast proliferation and activation, can occur months or years after surgery resulting in poor postoperative control of IOP.1,2
In current clinical practice, the antimetabolites 5-fluorouracil and mitomycin-C (MMC) are used in conjunction with topical steroids to help reduce or prevent scarring of the filtering bleb. MMC is a potent antitumor drug derived from the bacterium Streptomyces caespitosus that inhibits fibroblast proliferation and alters conjunctival vascular epithelium.1–3 However, these agents are nonspecific and increase the risk of severe side effects by producing a thin, avascular bleb, which can be prone to late bleb leakage, infections (blebitis and endophthalmitis), hypotony, and vision loss.1,3–6 Therefore, more targeted therapeutic agents that might achieve a similar reduction in bleb scarring with fewer complications are being investigated.
One alternative method for limiting tissue fibrosis is through the regulation of sphingosine-1-phosphate (S1P), a lysophospholipid signal molecule that regulates a wide range of physiological processes such as cell proliferation, migration, survival, and differentiation.7 S1P, which is synthesized from ceramide and sphingosine by the actions of sphingosine kinase , acts as an extracellular messenger by activating a set of 5 G-protein-coupled receptors.8–10 It is a key component in the regulation of the immune, cardio-vascular, and central nervous systems.7,10–12 In addition, the dysregulation and subsequent overproduction of S1P has been implicated in the processes of angiogenesis and tumor progression.7,9–12
S1P signaling has been recently associated with the regulation of ocular tissue fibrosis.8 In the eye, the presence of S1P promotes proliferation, myofibroblast transformation, collagen production, and profibrotic protein expression.8 LT1009 (Sonepcizumab) is a humanized anti-S1P monoclonal antibody that can bind to S1P, thereby limiting the amount of free S1P molecules in tissues and reducing fibrotic activity.9 Currently, the antibody is undergoing phase I clinical trials for the treatment of cancer and age-related macular degeneration.9
In this study, we evaluated the effect of local application of LT1009 on ocular scarring after GFS in the rabbit. The rabbit model is well established as a standard animal model for the investigation of wound healing and scarring after GFS.13–16 Our goals were to (I) establish the duration of LT1009 antibody in external ocular tissues after a single subconjunctival injection; (II) evaluate bleb survival of an eye treated with LT1009 compared with negative balanced saline solution (BSS) and positive MMC controls; and (III) describe the side effect profile of the antibody after GFS. Our evaluations were based on longevity of bleb elevation, together with both clinical and histologic examinations of ocular tissues.
METHODS
Study Design
All animal experiments performed adhered to the Association for Research in Vision and Ophthalmology Statement for the use of animals in Ophthalmic and Vision Research and were approved by the University of Florida’s Institutional Animal Care and Use Committee.
The study was divided into 2 parts. In the first part, 5 New Zealand White (NZW) rabbits were given a single injection of 0.1 mL LT1009 at maximum solubility (3 mg in 0.1 mL) beneath the superior conjunctiva. LT1009 was provided courtesy of LPath Inc. Conjunctiva, Tenon capsule, and scleral tissues were harvested at the following time points after injection: 30 minutes, 1 hour, 2 hours, 4 hours, 8 hours, 1 day, 2 days, 3 days, 4 days, and 7 days. The tissue samples were homogenized in ×1 phosphate buffered saline and the levels of LT1009 monoclonal antibody were measured using a custom quantitative enzyme-linked immunosorbent assay (ELISA) sandwich capture array prescribed by LPath Inc.
Based on analysis of the ELISA data from the first 5 rabbits, an additional NZW rabbit was injected in each eye with LT1009 to confirm tissue LT1009 concentrations at the most critical time period between days 3 and 4. By comparing the data to a standard curve, we were able to determine that the optimum treatment interval for LT1009 application to maintain recordable tissue levels would be once every 3 days.
In the second part of the study, 21 NZW rabbits were randomized to 3 equal-sized treatment groups. All 21 rabbits underwent glaucoma filtering operation in their left eyes with 1 surgeon (Mark B. Sherwood) performing all the procedures; the right eyes were not operated on and served as a control.
At the end of the surgery, the rabbits in group 1 were given an injection of the LT1009 monoclonal antibody at a dose of 3 mg/0.1 mL under the superior conjunctiva, adjacent to the bleb. On the basis of the results from part 1, additional postoperative injections of 0.1 mL LT1009 were administered once every 3 days, to a maximum of 8 total injections. In some animals, treatment was halted early after 6 total injections due to significant upper eyelid edema.
Rabbits in group 2 received a partial-thickness Weck sponge (Alcon Surgical, Fort Worth, TX) application of 0.4 mg/mL of MMC for 3 minutes at the time of surgery and were not given any postoperative injections.
The rabbits in group 3 were given a 0.1 mL BSS injection under the superior conjunctiva at the end of surgery. Additional postoperative injections of 0.1 mL BSS were given every 3 days, to mimic the LT1009 dose schedule.
Glaucoma Filtering Operation
The rabbits were anesthetized with an intramuscular injection of a combination of Ketamine (“Ketaject”, Phoenix, MO) (50 mg/kg) and Xylazine (“Xyla-ject”, Phoenix, MO) (10 mg/kg). A topical anesthetic, 0.1% Proparacaine eye drop (Bausch & Lomb, Tampa, FL), was also administered. The surgical technique for the glaucoma filtration operations was similar to the procedures described in previous publications.13 In brief, an eyelid speculum retracted the eyelids. A partial thickness, corneal traction suture made in the superior cornea rotated the eye inferiorly. In the superior lateral quadrant of the eye at the limbus, a measured standard sized 5 mm fornix-based conjunctival flap was created. Blunt dissection helped undermine the conjunctiva and Tenon capsule. At this point for the group 2 rabbits, a 0.4 mg/mL MMC soaked 5×5 mm partial thickness. Weck cell was placed locally between the conjunctiva/Tenon capsule and the sclera for 3 minutes. After the 3 minutes, the sponge was removed and the area was washed with 30 mL of saline. Next, in all rabbits, a clear corneal paracentesis tract was fashioned in the superonasal quadrant using a #75 Beaver blade (Becton Dickinson & Co., Franklin Lakes, NJ). A viscoelastic material (Healon 10 mg/mL, Pharmacia & Upjohn) was injected to maintain the anterior chamber.
A 25-gauge needle was then used to create a beveled, full-thickness tract through the sclera into the anterior chamber approximately 1 mm posterior to the limbus. This was followed by insertion of a 22-gauge, IV cannula (Insyte Becton Dickinson Vascular Access, Sandy, UT) along this tract into the anterior chamber. The cannula needle was pulled out, and the cannula was positioned beyond the pupillary margin to prevent the tube from being occluded by iris. The cannula was trimmed at its scleral end so it would not protrude more than approximately 1 mm from the point of insertion. A 10-0 nylon suture (Ethicon Inc., Somerville, NJ) was used to anchor the tube to the sclera.
A running suture of 8-0 absorbable suture material (Vicryl, Ethicon Inc., Somerville, NJ) was used to close the conjunctiva/Tenon capsule at the limbus in a watertight fashion. At the end of the procedure, saline was injected into the anterior chamber through the paracentesis tract to elevate the bleb and a Seidel test was performed to ensure that there was no bleb leak. An ointment composed of Neomycin and Dexamethasone was applied topically immediately after the surgery.
The rabbits in the LT1009 and BSS treatment groups (groups 1 and 3) were given their assigned subconjunctival injections of either 0.1 mL LT1009 monoclonal antibody or 0.1 mL BSS respectively, adjacent to the blebs immediately after the surgery in a masked fashion.
Postoperative Injections and Clinical Evaluations
After the operation, the eyes were examined every 3 days by an examiner masked to the treatment group. The examiner looked for the presence of bleb elevation and for any complications that may have resulted from the surgeries or various treatments, including conjunctival injection, edema, anterior chamber shallowing, tube malpositioning, hyphema or subconjunctival hemorrhage, bleb leaks, and corneal or lens opacification. For each rabbit, bleb failure was declared after the bleb appeared flat in 2 consecutive masked clinical examinations. The first of the 2 dates was recorded as the endpoint. After the clinical examination, rabbits in the LT1009 and BSS treatment groups (groups 1 and 3) were also given injections of 0.1 mL LT1009 monoclonal antibody or 0.1 mL BSS, respectively, every 3 days after surgery by a second, unmasked observer.
The rabbits were anesthetized with Isoflurane during their postoperative examinations, and a topical 0.1% proparacaine anesthetic eye drop was also administered for those that required a postoperative injection. A speculum was used to retract the eyelids. Nontoothed Bishop-Harmon forceps were used to tent the conjunctiva, and 0.1 mL injections were given near the bleb using a 30-gauge needle on a 1 mL syringe.
Histology
Eyes were obtained from 1 rabbit in each treatment group on postoperative day 12 so bleb tissues could be examined while all blebs remained elevated and at an identical postoperative timepoint. Eyes were obtained from all other rabbits after the bleb was observed to be flat in 2 consecutive clinical evaluations (bleb failure).
The tissues were fixed for 24 hours in a 10% neutral buffered bormalin solution. The globes were placed in tissue cassettes, and then processed through graded ethanol and xylene, using a Sakura Tissue-Tek VIP 5 tissue processor (Torrance, CA). The tissues were infiltrated with paraffin (Richard-Allan Scientific, Kalamazoo, MI) and embedded on a Tissue-Tek III embedding center. Sagittal serial sections of the globes were taken. Once deparaffinized, the sections were stained using either standard Harris hematoxylin and eosin or Masson trichrome. The slides were examined under a light microscope and representative sections were photographed using a Canon EOS T1i digital camera attached to an Olympus Vanox microscope.
Statistical Analysis
Analysis of variance (ANOVA) testing was used to determine any significant difference in bleb survival duration among the LT1009, MMC, and BSS treatment groups. Two post hoc tests—Tukey Honestly Significantly Different test and Fisher Least Significant Difference (LSD) test—were then used to further compare pairs of relevant groups.
RESULTS
ELISA for LT1009
Figure 1 summarizes the concentration of the monoclonal antibody LT1009 detected by the ELISA assay at 25-fold and 100-fold dilutions of the original homogenate based on the first 5 rabbits. The repeat testing at days 3 and 4 in the additional rabbit confirmed these results. In both assays, the concentration of LT1009 in the tissues approaches 0, 4 days after injection.
FIGURE 1.
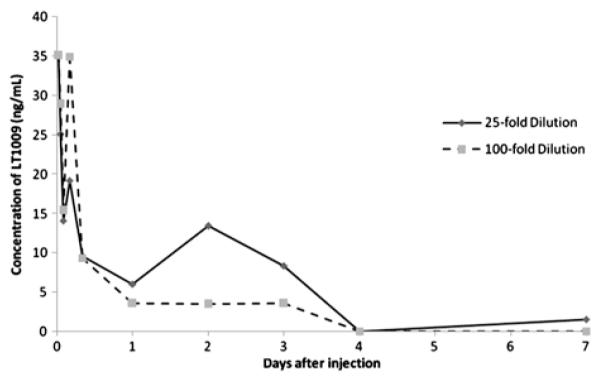
Enzyme-linked immunosorbent assay analysis of LT1009 levels in eye tissue homogenates (consisting of conjunctiva, Tenon capsule, and sclera) after initial injection.
Bleb Survival
In the second part of the study, the average time to bleb failure for rabbits receiving BSS injections was 21.0 ± 5.6 days. For rabbits receiving injections of LT1009, the average time to bleb failure was 28.5 ± 8.5 days. Lastly, for rabbits which had MMC applied topically at the time of surgery, the average time to bleb failure was 33.8 ± 5.6 days. The number of days to bleb failure for each treatment group is represented in a Kaplan-Meier survival plot in Figure 2 and summarized in Figure 3. Clinical bleb images taken on postoperative day 12 show similar vascularity between eyes treated with LT1009 and BSS (Figs. 4A, C, respectively). In contrast, MMC-treated eyes developed characteristic bleb avascularity (Fig. 4B).
FIGURE 2.
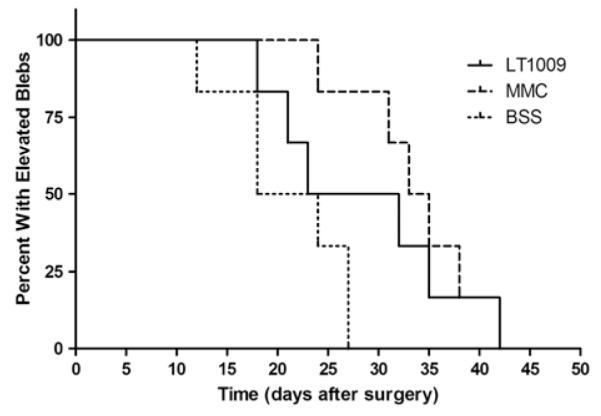
Kaplan-Meier bleb survival plot of eyes treated with LT1009 (solid line), mitomycin-C (MMC) (dashed line), and balanced saline solution (BSS) (dotted line). For each rabbit, bleb failure was declared after the bleb appeared flat in 2 consecutive masked clinical examinations. The first of the 2 dates was recorded as the endpoint.
FIGURE 3.
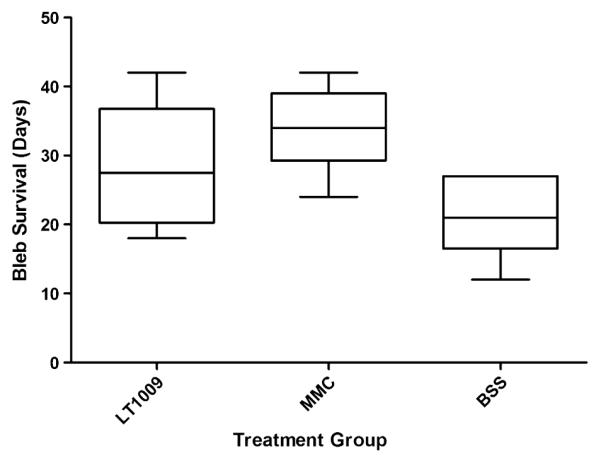
Box plot of bleb survival for each treatment group. First and third quartiles are delineated by the boxes. Whiskers represent maxima and minima.
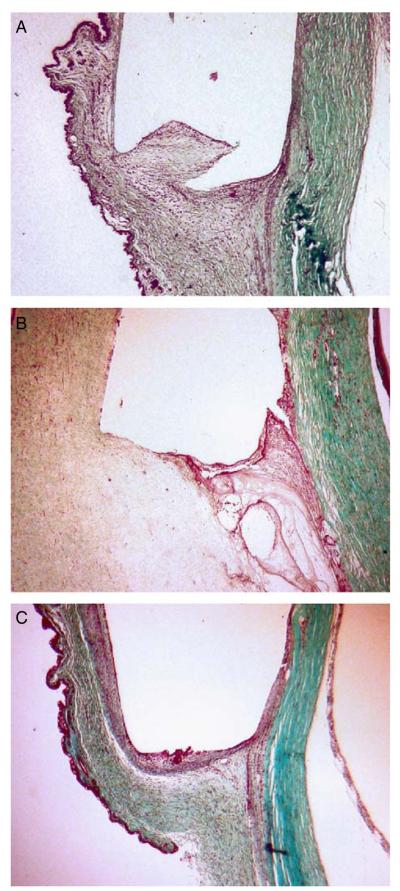
FIGURE 4.

Bleb appearance on postoperative day 12 (A) LT1009, (B) mitomycin-C (MMC), (C) balanced saline solution (BSS). Note the relative avascularity of MMC bleb when compared with LT1009 and BSS.
Statistical analysis of the bleb survival data using ANOVA revealed a significant difference among the 3 treatment groups (P = 0.0268) at a 95% confidence interval (CI). A Tukey Honestly Significantly Different test showed a significant increase in bleb survival for the MMC treatment group over the BSS group at a 95% CI. This result was confirmed using Fisher LSD test at a 95% CI (P = 0.008). In addition, LSD analysis suggested that the LT1009 offered a significant improvement in bleb survival over BSS at a 90% CI (P = 0.096). There was no statistically significant difference between the MMC and LT1009 treatment groups using either test (P>0.1).
Rabbits receiving MMC treatment developed avascular, but otherwise unremarkable blebs. Within the LT1009 treatment group, rabbits receiving multiple (>5) injections developed significant eyelid edema that improved after 2 to 3 days (Fig. 5) and completely resolved after 10 days (Fig. 6). After consultation with the veterinarians of animal care services at the University of Florida, the number of additional LT1009 and BSS injections after surgery was limited to a maximum of 5 over the course of 12 days postoperative. In addition, a once-daily oral non-steroidal antiinflammatory drug (Meloxicam) was added for those with lid swelling.
FIGURE 5.
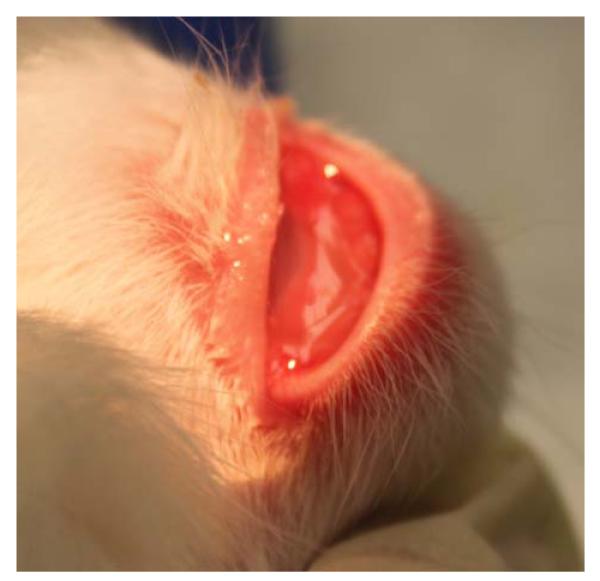
Significant upper eyelid edema observed on postoperative day 15 in a rabbit in the LT1009 treatment group (group 1), 3 days after injection number 5.
FIGURE 6.
Decrease in upper eyelid swelling of the same rabbit 6 days postinjection number 5 on postoperative day (POD) 18 (A), then near-complete resolution of upper eyelid swelling 13 days postinjection on POD 25 (B).
Histology
Histologic examination of the eyes taken 12 days after surgery showed differences in the implant site among the 3 treatment groups. Rabbits that received BSS injections consistently formed fibrotic capsules at the end of the implant cannula (Fig. 7C) whereas those receiving MMC (Fig. 7B) or LT1009 (Fig. 7A) did not. Specimens from the BSS treatment group were also observed to have a greater amount of cellularity than samples from the MMC and LT1009 treatment groups. In the 12-day histology specimen from the LT1009 treatment group, there was a diffuse inflammatory response within the conjunctiva. In addition, nearby lymph vessels were observed to be filled with lymphocytes.
FIGURE 7.
Implant site 12 days postoperative (Masson Trichrome, ×20). A, Representative section of LT1009 treatment group implant site after 3 injections—note lack of capsule formation. B, Representative section of mitomycin-C treatment group implant site—note lack of capsule formation. C, Representative section of balanced saline solution treatment group implant site after 3 injections—note formation of a capsule around the implant site.
Eyes from rabbits in the MMC treatment group obtained after bleb failure showed a relatively avascular conjunctiva with irregularities in cell density and overall thickness which extended beyond the implant site (Fig. 8B). The conjunctiva in rabbits in the LT1009 and BSS groups showed localized thinning at the immediate implant site, but was otherwise unremarkable (Figs. 8A, C). A scattered presence of inflammatory cells could be seen in the bulbar conjunctiva of some samples as in Figures 8A, B. However, this was not unique to any treatment group.
FIGURE 8.
Conjunctiva near implant site 12 days postoperative (hemotoxylin and eosin, ×100). A, Representative section of an LT1009 treatment group rabbit after 3 injections—note healthy appearance of conjunctival epithelium. B, Representative section of a mitomycin-C treatment group rabbit—note thinning of conjunctival epithelium with sparse goblet cells. C, Representative section of a balanced saline solution treatment group rabbit after 3 injections—note healthy appearance of conjunctival epithelium.
Multiple injections of LT1009 did not seem to affect the inflammatory response in the eye tissues. However, a fibrotic capsule began to form at the end of the implant cannula in rabbits receiving the maximum (6) treatments (Fig. 9).
FIGURE 9.

A, Representative section of LT1009 treatment group implant site after 6 total injections, obtained 37 days postoperative after bleb failure on day 32 [hemotoxylin and eosin (H&E), ×20]—note the capsule formation, but no substantial inflammatory response. B, Close up (H&E, ×100).
DISCUSSION
Although it is effective at preventing scarring of the filtering bleb, MMC is also well known to be associated with a range of long-term adverse effects.1,3–6 In this study, we investigated in a rabbit model an alternative method of prolonging bleb survival by lowering the amount of free S1P signaling molecules in tissues at the bleb using the anti-S1P antibody LT1009. Currently, LT1009 has been shown to be effective in depleting unbound S1P from tissues and is undergoing phase I clinical trials for the treatment of cancer and age-related macular degeneration.9
The first part of our investigation was to determine the optimum application interval for LT1009, based on antibody retention by the eye tissues. ELISA assay analysis showed that the level of LT1009 rapidly diminished after day 3. Based on these data, we elected to give injections of LT1009 at the end of surgery and then every third day.
The second part of our study was to evaluate the effect of LT1009 on bleb survival. The overall trend was encouraging when compared with BSS controls, with a 36% increase in the average time to bleb failure in rabbits receiving LT1009 injections. ANOVA analysis with post hoc testing suggests a marginally significant difference between the LT1009 and BSS treatment groups despite the relatively small sample size. Although the improvement in bleb duration in the LT1009 group compared with BSS was not as great as with MMC, the histology showed less conjunctival thinning and avascularity. Of interest, comparison to a previous study17 examining bleb survival duration using 5-fluorouracil in the same model at this center suggests greater efficacy for the LT1009. Histologic examination showed that both the LT1009 and MMC groups had less fibrotic activity than the BSS controls.
In clinical examinations, rabbits receiving LT1009 did not develop the bleb avascularity (a well-described effect of MMC1,3–6) that was seen in the MMC treatment group rabbits. The long-term histopathologic effects of MMC on human eye tissues have been well documented and are characterized by irregular conjunctival epithelium of varying thickness, paucity of conjunctival vessels, stromal thickening and fibrosis, and low-grade inflammatory infiltrate.2,18 Even in the relatively short (up to 42 d postoperative) span of this study, histologic examination of the MMC group showed conjunctival thinning with a noticeable reduction in conjunctival vasculature that extended past the immediate implant site. In comparison, the LT1009 and BSS groups had relatively healthy conjunctival morphology.
One notable finding we observed in the LT1009 treatment group was the development of an upper eyelid edema after 5 injections in all of the first 3 treated rabbits. Clinically, the eyelid edema did not seem painful to touch or affect feeding and other activities of the rabbit. With discontinuation of injections, the eyelid swelling improved after a few days, and resolved completely by 10 days.
Previous experiments showing prolongation of bleb survival in this same rabbit model with antifibrotic agents have correlated with increased efficacy of bleb survival in humans.17 Both clinical and histologic evidence suggest that the anti-S1P antibody LT1009 may be useful in preventing fibrosis compared with negative BSS controls. In addition, we did not observe any of the conjunctival thinning or avascularity in the LT1009 group. In future larger studies, it may be important to determine the optimal dose and delivery method for LT1009 to achieve a good antiscarring response while minimizing the side effect of lid edema.
ACKNOWLEDGMENTS
The authors thank LPath Inc. for providing the LT1009 monoclonal antibody and France Lapierre-Holme (of LPath Inc.) for technical assistance.
This study was funded by a grant from LPath Inc.
The study was funded in part by an unrestricted grant from RPB to the UF Department of Ophthalmology.
Footnotes
Disclosure: In addition, coauthor G.S. is an employee of LPath Inc. and therefore has a financial interest in the subject being studied. The other authors declare no conflict of interest.
REFERENCES
- 1.Muckley ED, Lehrer RA. Late-onset blebitis/endophthalmitis: incidence and outcomes with mitomycin C. Optom Vis Sci. 2004;81:499–504. doi: 10.1097/00006324-200407000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Francis BA, Du LT, Najafi K, et al. Histopathologic features of conjunctival filtering blebs. Arch Ophthalmol. 2005;123:166–170. doi: 10.1001/archopht.123.2.166. [DOI] [PubMed] [Google Scholar]
- 3.Bindlish R, Condon GP, Schlosser JD, et al. Efficacy and safety of mitomycin-C in primary trabeculectomy: five year follow-up. Ophthalmology. 2002;109:1336–1341. doi: 10.1016/s0161-6420(02)01069-2. [DOI] [PubMed] [Google Scholar]
- 4.Anand N, Arora S, Clowes M. Mitomycin C augmented glaucoma surgery: evolution of filtering bleb avascularity, transconjunctival oozing, and leaks. Br J Ophthalmol. 2006;90:175–180. doi: 10.1136/bjo.2005.077800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beckers HJ, Kinders KC, Webers CA. Five-year results of trabeculectomy with mitomycin C. Graefes Arch Clin Exp Opthalmol. 2003;241:106–110. doi: 10.1007/s00417-002-0621-5. [DOI] [PubMed] [Google Scholar]
- 6.DeBry PW, Perkins TW, Heatley G, et al. Incidence of late-onset bleb-related complications following trabeculectomy with mitomycin. Arch Ophthalmol. 2002;120:297–300. doi: 10.1001/archopht.120.3.297. [DOI] [PubMed] [Google Scholar]
- 7.Skoura A, Sanchez T, Claffey K, et al. Essential role of sphingosine 1-phosphate receptor 2 in pathological angiogenesis of the mouse retina. J Clin Invest. 2007;117:2506–2516. doi: 10.1172/JCI31123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swaney JS, Moreno KM, Gentile AM, et al. Sphingosine-1-phosphate (S1P) is a novel fibrotic mediator in the eye. Exp Eye Res. 2008;87:367–375. doi: 10.1016/j.exer.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 9.O’Brien N, Jones ST, Williams DG, et al. Production and characterization of monoclonal anti-sphingosine-1-phosphate antibodies. J Lipid Res. 2009;50:2245–2257. doi: 10.1194/jlr.M900048-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wymann MP, Schneiter R. Lipid signaling in disease. Nat Rev Mol Cell Biol. 2008;9:162–176. doi: 10.1038/nrm2335. [DOI] [PubMed] [Google Scholar]
- 11.Gardell SE, Dubin AE, Chun J. Emerging medicinal roles for lysophospholipid signaling. Trends Mol Med. 2006;12:65–75. doi: 10.1016/j.molmed.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Murph M, Mills GB. Targeting the lipids LPA and S1P and their signaling pathways to inhibit tumour progression. Expert Rev Mol Med. 2007;15:1–18. doi: 10.1017/S1462399407000476. [DOI] [PubMed] [Google Scholar]
- 13.Cordeiro MF, Constable PH, Alexander RA, et al. Effect of varying the mitomycin-C treatment area in glaucoma filtration surgery in the rabbit. Invest Ophthalmol Vis Sci. 1997;38:1639–1646. [PubMed] [Google Scholar]
- 14.Sherwood MB, Esson DW, Neelakantan A, et al. A new model of glaucoma filtering surgery in the rat. J Glaucoma. 2004;13:407–412. doi: 10.1097/01.ijg.0000131482.86547.5a. [DOI] [PubMed] [Google Scholar]
- 15.Doyle JW, Sherwood MB, Khaw PT, et al. Intraoperative 5-fluorouracil for filtration surgery in the rabbit. Invest Ophthalmol Vis Sci. 1993;34:3313–3319. [PubMed] [Google Scholar]
- 16.Connon CJ, Meek KM. Organization of corneal collagen fibrils during the healing of trephined wounds in rabbits. Wound Repair Regen. 2003;11:71–78. doi: 10.1046/j.1524-475x.2003.11111.x. [DOI] [PubMed] [Google Scholar]
- 17.Khaw PT, Doyle JW, Sherwood MB, et al. Effects of intraoperative 5-fluorouracil or mitomycin C on glaucoma filtration surgery in the rabbit. Ophthalmology. 1993;100:367–372. doi: 10.1016/s0161-6420(93)31640-4. [DOI] [PubMed] [Google Scholar]
- 18.Liang SY, Lee GA, Whitehead K. Histopathology of a functioning mitomycin-C trabeculectomy. Clin Experiment Ophthalmol. 2009;37:316–319. doi: 10.1111/j.1442-9071.2009.02023.x. [DOI] [PubMed] [Google Scholar]