Abstract
Planar lipid bilayer is an electrophysiological technique that enables study of functional activities of ion channels, porins, and other pore-forming molecular complexes. The main purpose of this method is to monitor ion channels’ behavior at the single molecule level in the artificial membranes. Here, I describe the details of this technique that will underline formation of the lipid bilayers and incorporation and activation of the ion channel protein.
Keywords: Planar lipid bilayer, Black lipid membranes, Ion channel, Ionic current, Conductance
1 Introduction
Electrophysiological approaches are designed to evaluate physical properties and characteristics of ion channels. Planar lipid bilayer is one of the unique electrophysiological techniques that is intended to study specific channel properties of the purified complexes in a well-controlled artificial environment (1). The effectiveness of this method reveals the possibility to study and characterize ion channel behavior at the single molecule level. Furthermore, this technique is very useful for investigation of the direct effects of chemicals and enzymes on the channel while excluding possible indirect effects, which might be caused by the presence of regulatory proteins of the native membranes.
Planar lipid bilayer experiments enable monitoring the transport rates of ions across membranes through incorporated ion channels. The experiments are performed in the chamber with cis- and trans-compartments that are connected through a small aperture with a diameter ranging from 50 to 250 μm. A lipid solution is applied to the aperture with subsequent formation of a planar bilayer membrane on the hole. This is followed by reconstitution of ion channel proteins. Ion channels can be inserted into bilayer lipid membrane (BLM) directly from a micellar solution or fused with liposomes. After the channel is incorporated into the bilayer, the ionic current can be induced by applying a driving force. This force represents an electrochemical potential that has two components—electrical, which is called membrane potential (Vm or Δψ) derived from charges or voltages applied across the membrane, and chemical component that derives from a chemical gradient with asymmetric ionic solutions. The ionic current through typical ion channel is measured in pico-amperes (pA), and potential or voltages are measured in millivolts (mV). Conductance of ions at a single-channel level is usually measured in a range of 10–1,000 pico-siemens (pS).
Some ion channels such as ligand-gated channels would also require the presence of specific molecules-activators (ligands) in order to induce channel openings. In case of many mammalian channels this regulation can be quite complex and would require simultaneous presence of a number of molecular components and/or various physical factors (temperature, pressure) in order to stimulate channel openings. The advantage of planar lipid bilayer is that it increases likelihood to precisely identify all the chemical and physical factors that are required for, or supporting, the channel activity. The other benefits of this method include a possibility to alternate lipid composition or chemical compounds regulating channel activity that can be applied to either side of the membrane.
Like any technique, planar lipid bilayer also has its disadvantages. Among the disadvantages of lipid bilayers are large capacitances due to the large sizes of the aperture, comparing it to the diameters used in excised patches. The larger capacitances cause for a slow voltage response time. Another disadvantage of this technique, relative to the native membrane patch-clamp, is the generation of high-amplitude noise due to the large area of bilayers in traditional systems. In order to reduce the peak-to-peak amplitude of the noise in the system, intensive filtering may be applied. However low-pass filtering of the single-channel signal produces a significant loss of time resolution and at some point will make fast-gating events undetectable. This issue has been addressed by recently developed alternative BLM systems that allow performing low-noise and higher bandwidth recordings (2, 3).
Overall, the complexity of planar lipid bilayer technique at first glance seems to arise from the intricacy of the steps in formation of the artificial membranes that require thorough knowledge of physical chemistry of BLM. Nevertheless, the theoretical insights that describe physicochemical properties of lipid bilayer as well as methodological details are well established (for more references see refs. 1, 4–6), along with the practical side of the method (including advanced technology for the electrophysiological setup, voltage-clamp recordings, and availability of high quality and purity of synthetic lipids). All these factors aid in the formation of artificial lipid bilayer suitable for channel study and make it a straightforward and reproducible method. The main challenge of this technique is rather hindered in the capricious nature of the membrane proteins, which ion channels represent, and therefore the success of the experiment often heavily relies on the protein part, which includes protein isolation, purification, folding, and incorporation. The reason for this is that each protein is unique and ways to handling it may vary greatly. Here, as an example, I describe planar lipid bilayer method (Fig. 1) of incorporation and activation of the cold and menthol receptor—TRPM8, which we have successfully applied in our studies (7, 8). The method is comprised of (1) formation of the artificial membranes, (2) incorporation of the ion channel, and (3) activation of the channel protein by its agonists and other molecules that are involved in the channel activity.
Fig. 1.
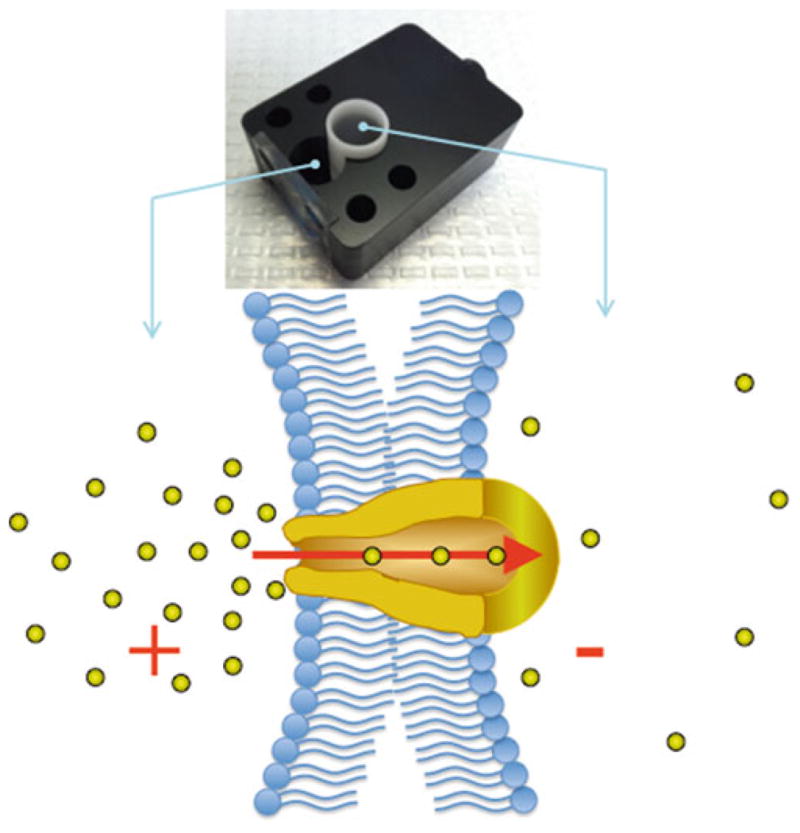
Planar lipid bilayer chamber with inserted cuvette, lipid bilayers are painted on the aperture in the middle of the cuvette. Silver-silver chloride electrodes are connected to each compartment (cis and trans ), those allow to obtain the ionic current that is amplified using a voltage-clamp amplifier. Cups and chambers are filled with equal volumes in the cis- and trans -sides, which results in a balanced solution height, thus minimizing mechanical gradients across the bilayer membrane. Below is a graphical representation for formation of planar lipid bilayers and incorporated ion channel that conducts ions across the membrane
2 Materials
2.1 Solutions and Reagents
-
Lipids include synthetic 1-palmitoyl-2-oleoyl-glycero-3-phosphocoline (POPC) and 1-palmitoyl-2-oleoyl-glycero-3-phos-phoethanolamine (POPE, Avanti Polar Lipids, Birmingham, AL). Lipids are stored in septum glass vials and screw caps with Teflon discs inserts that are from Thermo Scientific Pierce (Rockford, IL).
Short- and long-chain phosphoinositides are from Cayman Chemicals (Ann Arbor, MI).
Organic solvent to dissolve the lipids—n-decane (Sigma-Aldrich, St. Louis, MO).
Experimental bathing solution: 150 mM KCl, 0.2 mM MgCl2, 20 mM Hepes, pH 7.4. All salts are ultrapure (>99%) (Sigma-Aldrich, St. Louis, MO). To increase the purity of the solutions it is important to filter all the buffers with 0.22 μm filters.
Planar lipid bilayer chamber and cuvette are made of Delrin (acetyl resin); we use cuvette with an aperture of ~150 μm in diameter (Warner Instruments, Hamden, CT).
The TRPM8 protein is purified from the human embryonic kidney cells (HEK-293), stably expressing the channel as previously described (7), for updates in the purification procedure (see Note 1).
NCB buffer: 500 mM NaCl, 50 mM NaH2PO4, 20 mM Hepes, 2 mM Na-orthovanadate, 10% glycerol, pH 7.5.
Cell homogenization buffer: NCB buffer with addition of 1 mM of protease inhibitor PMSF, 5 mM β-mercaptoethanol.
Protein isolation buffer: NCB buffer with addition of one tablet of protease inhibitor cocktail, 1 mM PMSF, 20 μg/mL DNase, 20 μg/mL RNase, 0.1% Nonidet P40 (Roche, Indianapolis, IN), and 0.5% dodecyl-maltoside (DDM) (CalBiochem, Darmstadt, Germany).
Phosphate buffer saline (PBS).
2.2 Equipment and Materials
Electrophysiological setup: Axopatch 200B amplifier; CV-203BU headstage; Baseplate; Electrode holder; Series Resistance Dither Box; Patch-1U Model Cell (Molecular Devices, Sunnyvale, CA).
DD1440A Digidata 1440A data acquisition system and pClamp-10 Electrophysiology software (Molecular Devices, Sunnyvale, CA).
8-Pole Bessel filter—950 TAF (Frequency Devices, Ottawa, IL).
The entire setup is fixed in the Faraday cage to block sound and electrical noise (AutoMate Scientific, Inc, Berkeley, CA).
Bilayer chamber and cuvette (Warner Instruments, Hamden, CT).
For temperature experiments we use bilayer chamber made of a thermally conductive plastic; the pyroelectric heating/cooling stage connected to temperature controller CL-100 (Warner Instruments, Hamden, CT).
Stereo microscope (Olympus, Center Valley, PA).
Bath sonicator (Branson, Hatfield, PA).
Glass capillary tubes (Fisher Scientific).
Bunsen burner (Fisher Scientific).
Molecular sieves (Sigma).
Speed-Vac (VWR).
Nitrogen gas tank (GTS Welco, Inc., Newark, NJ).
3 Methods
3.1 Preparing Working Equipment for Planar Lipid Bilayer: Chambers, Cuvettes, and Glass Capillaries
Preparation of the working equipment such as bilayer chambers and cuvettes is the very first and important step and should be carried before each experiment. To optimize the rate for a successful BLM experiment and eliminate false positive results due to possible contaminants, the bilayer chamber and cuvette have to be cleaned thoroughly.
Wash with dishwashing detergent in warm water; rinse well several times with warm running water to remove detergent; rinse three times with milli-Q water.
Wash with organic solvents—first with ethanol and then with methanol; dry with nitrogen gas. It is useful to press on top of cuvette when filled with solvent, the intense solvent flow through the aperture helps to remove residual lipids and other content from the aperture. When switching from one target-protein to another, or in case of difficulties to remove contaminants, all washing steps can be done along with bath sonication (see Note 2).
Delrin cuvettes can also be cleaned with chloroform, when necessary to remove highly hydrophobic content from the aperture. Note that not all materials are suitable for a chloroform use; be sure that your cuvette will not be damaged during this cleaning procedure (see Note 3).
Prepare a set of air-bubble glass capillaries. Holding to the end of capillary, flame the other end, simultaneously pooling the edge with forceps until the thin prolonged glass tube is formed. Remove the forceps and continue to flame the thin tube in order to make the bubble-like structure.
3.2 Preparation of the Lipids
Open the ampoules with lipids in chloroform solution and transfer the content into new and prerinsed with organic solvent glass vials, add the molecular sieves (4 Å) and cover with Teflon-side screw cap. For storage conditions see Note 4.
Prepare the mixture of POPC/POPE in 3:1 ratio and dry the lipids by lyophilization in Speed-Vac at least for 2 h to completely remove the chloroform content. Next, resuspend the lipidic mixture in an organic solvent such as n-decane to a final concentration of 25–30 mM. Mix the lipids under the stream of nitrogen gas.
3.3 Formation of Planar Lipid Bilayer
Fill the chamber in the following order: first add 1 mL of bathing solution to the trans-compartment (cuvette); press slightly on the top of cuvette until a tiny droplet will appear in the area of aperture (see Note 5); add 1 mL of bathing solution to the cis-compartment.
Immerse a pair of matched Ag-AgCl electrodes in the cis- and trans-compartments. Adjust the background-leak current near 0 values, this can be done by changing the voltage in a range of ±1 mV and adjusting manually the offset appropriately so that at positive voltage baseline would go upwards and at negative it would shift downwards (see Note 6).
Dip the air-bubble glass capillary in the lipid/decane solution and carefully apply the lipids to the aperture in the cuvette by “painting.” After several gentle movements of the air-bubble in the area of aperture, the film will form. We prefer not to pre-apply the lipidic solution beforehand; instead, we distribute the lipids by painting with the air-bubble glass capillary when the aqueous solvents are present (see Note 7).
Monitor the thinning of the lipid film by either observing it in the microscope—planar lipid bilayers reflect no light and appear black after formation, or alternatively estimate the capacitance of the membrane—as lipid bilayers will form the capacitance will gradually increase, indicating that the film is thinning. The capacitance of the bilayer can reach 100–200 pF, depending on the size of aperture used (see Note 8).
3.4 Incorporation and Activation of Ion Channel
After the bilayers are formed, add 0.2 μL of the TRPM8 micellar solution (0.002 μg/mL) to the cis-compartment with gentle stirring. Normally it will take some time for the channel to incorporate in the membrane. Fusion of the proteoliposomes or micelles can be observed by fusion spike appearance. After the incorporation stop the stirring.
To activate TRPM8 in planar lipid bilayer, add its agonist menthol (500 μM) to the cis- and/or trans-compartment. TRPM8 inserts into lipid bilayer without preferences and may be oriented either way. To induce TRPM8 channel openings, apart from the ligand, it also requires the presence of its gating factor phophoinositol 4,5-biphosphate (PIP2) (Fig. 2). The (2.5 μM) can be added to short acyl-chain dioctanoyl diC8PIP2 either side of the membrane, but it activates the channel from the intracellular side (see Note 9). Channel current recordings can be then obtained with different voltages. Orientation of TRPM8 can be determined according to the outward rectification and/or by an intracellular block with polylysine (polyK can be added at the end of the experiment).
Outward currents of TRPM8 exhibit mean slope conductance values of ~72 pS, and Po of ~0.89 at 100 mV, and inward currents may be observed in two conductance states with main conductance level of ~42 pS and Po of ~0.4 (at −100 mV) and rarely detected burst openings of a subconductance state with mean conductance of ~30 pS (Po ≤ 0.001), which would step to the fully open magnitude (72 pS) of the channels. In case if inconsistency in channel behavior is observed see Note 10.
Unitary currents are recorded with an integrating patch clamp amplifier. The trans-solution (voltage command side) is connected to the preamplifier headstage input, and the cis-solution is held at virtual ground via a pair of matched Ag-AgCl electrodes. Currents through the voltage-clamped bilayers (background conductance <3 pS) are filtered at the amplifier output (low pass, −3 dB at 10 kHz, 8-pole Bessel response). Data are secondarily filtered at 100 Hz and digitized at 1 kHz.
Single-channel conductance events, all points’ histograms, open probabilities, and other parameters are identified and analyzed using the appropriate software.
Fig. 2.
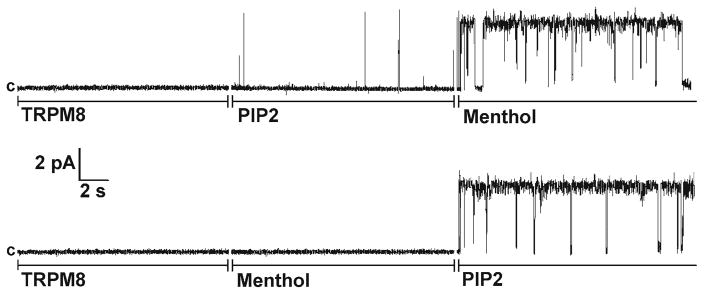
Activation of TRPM8 channels in planar lipid bilayers by menthol and PIP2. Representative single-channel current recordings of TRPM8 channels incorporated in planar lipid bilayers formed from POPC/POPE (3:1) in n-decane, between in 20 mM Hepes symmetric bathing solutions of 150 mM KCl, 0.2 mM MgCl2 buffer, pH 7.4 at 22°C. 0.2–0.5 μL of 0.2 μg/mL TRPM8 protein was incorporated in POPC/POPE micelles, which were added to the cis-compartment. Clamping potential was +60 mV. Upper and lower traces consist of three segments with subsequent additions of components as indicated in the figure: 2 μM of diC8 PIP2 and 500 μM of menthol were added to both compartments (the figure is reproduced from ref. 8 with permission)
3.5 Temperature Studies
Planar lipid bilayer is a convenient system to study temperature sensitivity of the cold receptor TRPM8.
For temperature studies, position a Delrin cuvette in the bilayer chamber made of a thermally conductive plastic.
The chamber is fitted on a conductive stage containing a pyroelectric heater/cooler.
Turn on the pump to circulate deionized water through the heating/cooling stage: the water is pumped into the system to remove the generated heat.
Set the temperature of interest on the temperature controller connected to the pyroelectric heating/cooling stage. The temperature of the bath is monitored constantly with a thermoelectric device in the cis-side, i.e., the groundside of the cuvette. Although there will be a temperature gradient build between the bath solution and conductive stage, the temperature within the bath could be reliably controlled within ±0.5°C.
Footnotes
HEK-293 cells stably expressing Myc-tagged TRPM8 are grown to ~80% confluence, washed and collected with PBS. Cells are harvested and resuspended in cell homogenization buffer. Then the cells are lysed by freeze-thawing method and centrifuged at low speed to remove cell debris and DNA. The supernatant is further centrifuged at 40,000 × g for 2.5 h., and the pellet is resuspended in protein isolation buffer. The suspension is incubated overnight at 4°C on a shaker with gentle agitation and then centrifuged for 1 h at 40,000 × g. The supernatant is collected and incubated with the beads, where the TRPM8 protein is purified by immunoprecipitation with anti-Myc-IgG conjugated to A/G protein magnetic beads (Pierce, Thermo Scientific), following the procedure provided by the manufacturer. All steps of purification are performed at 4°C. For the planar lipid bilayer experiments the protein is eluted with Myc-peptide (50 μg/mL).
Place chamber and cuvette into a beaker and perform all the washing steps (warm water with detergent; rinse; organic media) while using bath sonicator at low intensity, do not exceed 5 minutes for each washing step. Longer and more frequent sonication may affect the quality of the chamber.
Polystyrene cuvettes have poor resistance to organic solvents, which can lead to degradation of the aperture; therefore these cannot be cleaned with strong organic solvents. We also do not recommend using chloroform-cleaning step for the bilayer chamber, although it is made of Delrin, but the observation window is made of a different plastic polymer that can be entirely destroyed with chloroform.
Stock lipids/chloroform solutions are degassed with nitrogen stream and stored in the presence of molecular sieves 4 Å (Sigma) at −20°C. The molecular sieves are used for dehydration and absorb molecules with 4 Å effective diameter (H2O capacity ~20% by weight). Vials with lipids should be tightened with a Teflon-insert cap and covered with parafilm.
This step is important and helps to remove air from the aperture and create nice contact between the cis- and trans-compartments. Air entrapped in the aperture may cause a problematic formation of the bilayers.
At certain experimental conditions, such as with gradient solutions that introduce different ionic strengths across the membrane and cause formation of junction potentials, it is preferable to connect the electrodes with bath solutions using agar bridges. The agar bridges are made of glass capillaries and filled with 3 M KCl-agar solution.
Although it slightly increases the time for forming the bilayer, overall avoiding pre-application of lipids helps to minimize introduction of bulky amounts of lipids on the aperture. This in turn is useful in formation of good/thin bilayer with reduced torus of amorphous lipid that surrounds the membrane.
Planar lipid bilayer represents a capacitor that is capable of storing charge in electric field. The bilayer capacity is directly proportional to its surface area and dielectric constant (ε), and is inversely proportional to the thickness of the bilayer (d): Capacitance (C) of the bilayer formed across circular aperture can be calculated using the following formula C=ε·πr2/d (where r is the radius of the bilayer). Note that r is the radius of the actual bilayer, not the aperture, and the value of r generally reflects the quality of the bilayer. A phospholipid membrane has a capacitance in the range of 1 μF/cm2. A steady-state charge on a capacitor is expressed as q=CV, where q is the charge on the capacitor (in Coulombs) and V is the potential between the capacitor plates (in Volts). The time-dependent derivative for the charge can be obtained from the following expression: I=dq/dt=C·dV/dt. This expression is useful to estimate the capacitance of the bilayer, when the current measured under the conditions of the linear voltage ramp (constant dV/dt) is directly proportional to the bilayer capacitance.
It may take several (5–10) minutes for diC8PIP2 to incorporate in the membrane. Stirring the bath solution is helpful and can accelerate the incorporation of the phosphoinositide. Alternatively, PIP2 can be incorporated in the bilayers in a form of long dipalmitoyl chain—diC16PIP2 by adding it to the mixture of phospholipids beforehand at a final concentration of 1%.
In case if the channel behaves irregularly, do not rush to discard the solution and restart the experiment, sometimes the channel’s final folding may occur after incorporation in the lipid bilayer. In other words, channel may at first be misfolded; however it is possible that the protein will finally obtain the proper conformation state in the bilayer. This can be evidenced by stabilization of the channel currents that would change from irregular activity to nice strait openings and closures. Likewise, the channel may continue to misbehave. This would mean that most likely the protein purification encountered some problems and the protein is unstable. Alter the incorporation method and insert the channel with the liposomes. If this would not improve the result, the most logical would be to repeat the purification procedure. Note that presence of Mg2+ is important for the stability of the TRPM8 protein. We found that Mg2+ is important in supporting the native supramolecular complex of TRPM8 with polyhydroxybutyrate/polyphosphate (PHB/polyP). Affecting the molecular integrity of this supra-molecular complex of the channel has an immense effect on its activity (8).
References
- 1.Miller C. Ion channel reconstitution. Plenum; New York: 1986. [Google Scholar]
- 2.White RJ, Ervin EN, Yang T, Chen X, Daniel S, Cremer PS, White HS. Single ion-channel recordings using glass nanopore membranes. J Am Chem Soc. 2007;129:11766–11775. doi: 10.1021/ja073174q. [DOI] [PubMed] [Google Scholar]
- 3.Zhang B, Galusha J, Shiozawa PG, Wang G, Bergren AJ, Jones RM, White RJ, Ervin EN, Cauley CC, White HS. Bench-top method for fabricating glass-sealed nanodisk electrodes, glass nanopore electrodes, and glass nanopore membranes of controlled size. Anal Chem. 2007;79:4778–4787. doi: 10.1021/ac070609j. [DOI] [PubMed] [Google Scholar]
- 4.White SH. The physical nature of planar lipid bilayer membranes. In: Miller C, editor. Ion channel reconstitution. Plenum; New York: 1986. pp. 3–35. [Google Scholar]
- 5.Montal M, Mueller P. Formation of bimolecular membranes from lipid monolayers and a study of their electrical properties. Proc Natl Acad Sci U S A. 1972;69:3561–3566. doi: 10.1073/pnas.69.12.3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams AJ. An introduction to the methods available for ion channel reconstitution. In: Ogden D, editor. Microelectrode techniques: the plymouth workshop handbook. 2. Company of Biologists; Cambridge: 1994. pp. 79–99. [Google Scholar]
- 7.Zakharian E, Cao C, Rohacs T. Gating of transient receptor potential melastatin 8 (TRPM8) channels activated by cold and chemical agonists in planar lipid bilayers. J Neurosci. 2010;30:12526–125348. doi: 10.1523/JNEUROSCI.3189-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zakharian E, Thyagarajan B, French RJ, Pavlov E, Rohacs T. Inorganic polyphosphate modulates TRPM8 channels. PLoS One. 2009;4:e5404. doi: 10.1371/journal.pone.0005404. [DOI] [PMC free article] [PubMed] [Google Scholar]


