Abstract
Purpose
The purpose of this study was to formulate and characterize experimental antibacterial fluoride-releasing sealants and compare them with commercial sealants for fluoride release, recharge, adhesion, and microleakage.
Methods
Two experimental sealants (Exp-1, Exp-2) containing a synthesized antibacterial fluoride-releasing monomer and fluoride-releasing filler were formulated. Exp-2 also contained NovaMin nanoparticles. Commercial sealants Clinpro (CL) FluoroShield (FS), and SeLECT Defense (E34) were also included. Fluoride release from disk samples in deionized water was measured daily using an ion-selective electrode for 14 days, and after recharging with Neutra-Foam (2.0% sodium fluoride), fluoride was measured for 5 days. Microtensile bonding strengths (MTBS) to enamel were tested after 24-hour storage in water at 37°C or thermocycling 5-55°C for 1,000 cycles. A microleakage test was conducted on extracted teeth using a dye-penetration method. The data were analyzed using analysis of variance with the Tukey’s honestly significant difference test and Kruskal-Wallis test.
Results
Exp-1 and Exp-2 had significantly higher fluoride release and recharge capabilities than CL and FL (P<.05). All tested sealants had similar MTBS before and after thermocycling. Exp-2 and Exp-1 had significantly lower microleakage scores (P<.05) than other groups.
Conclusion
The experimental sealants had higher fluoride release and recharge capabilities and similar or better retention than commercial sealants.
Keywords: ANTIBACTERIAL, FLUORIDE RELEASE, SEALANTS, MICROLEAKAGE, MICROTENSILE BOND STRENGTH
Dental caries is still a prevalent disease in children. Forty-two percent of 2- to 11-year-olds have dental caries in their primary teeth, and 59% of 12- to 19-year-olds have had dental caries in their permanent teeth.1 Sealants, which are fluid resins with or without fillers, have been widely used for nearly 40 years to prevent caries, mainly in pits and fissures of occlusal surfaces of premolars and molars.2,3 Use of pit-and-fissure sealants is an effective way to prevent caries in children and adults, even in early noncavitated (incipient) lesions, according to a recently published set of American Dental Association evidence-based clinical recommendations.4 Fissure sealing has been shown to inhibit not only the formation of occlusal caries, but also to impede the progression of existing carious lesions.5-8 A recently reported in vitro study showed that active management of incipient lesions with sealant application reduced lesion size.9
To increase the caries-preventing effect, fluoride-releasing sealants were introduced in the 1970s.10 Current commercial fluoride-releasing sealants (eg, FluoroShield from Caulk/Dentsply, Clinpro from 3M ESPE, and HelioSeal F from Vivadent) contain either a soluble fluoride salt such as sodium fluoride (NaF) or a fluoride-releasing glass filler or both. It is well documented in the in vitro studies that these materials can release fluoride and inhibit demineralization of the adjacent tooth structure.10-13
According to a National Institute of Dental and Craniofacial Research study,14 the method of adding soluble fluoride salt in sealants has been questioned, because the dissolution of a soluble salt might weaken the sealant and reduce its usefulness as a preventive agent. Numerous clinical studies have shown that the retention rate of fluoride-releasing sealants is approximately the same15 or lower16,17 than that of a nonfluoride sealant. The development frequency of carious lesions associated with fluoride-releasing sealants is comparable to that of nonfluoride sealants.10,18,19 Therefore, current sealant materials need to be further improved to achieve a better caries-inhibiting effect. In recent years, we have developed several F-exchange monomers containing ternary zirconium fluoride chelates.20,21 The composites containing these fluoride-exchange monomers have higher fluoride-releasing and recharging capacities than current commercial dental composites.22,23
Since a microbial effect on food residue is reported as the major cause of secondary caries, the antibacterial properties of fissure sealant materials may contribute to the reduction of microleakage and caries.24 Polymers with antimicrobial activities have drawn great interest in the field of biomedical materials and medical implants25 and have played an important role in reducing infection. The most common biocide moieties are quaternary ammonium, pyridinium, phosphonium, and sulfonium salts. The mechanism of action for these quaternary compounds may be the direct cationic binding to cell wall components, which leads to disruption of the cell wall membrane, leakage of critical cell contents, and cell death.26,27
One of the most extensively studied antibacterial dental monomers is methacryloyloxydodecyl pyrimidinium bromide (MDPB), synthesized by Imazato et al.28,29 MDPB-containing dental materials (eg, Protect Bond, Kuraray, Tokyo, Japan) have shown bactericidal activity against oral streptococci.30,31 Several reports, however, have also shown that Protect Bond and other commercial F-releasing bonding agents cannot prevent secondary caries.32-34 The antimicrobial monomer methacryloxylethyl cetyl dimethyl ammonium chloride added into the commercial sealant Helioseal F was reported to suppress Streptococcus mutans formation with an influence on microleakage at 1%.35 Surface antimicrobial properties of Clinpro have been reported,36 and such effects can be attributed to quaternary amine counter ions of the fluoride salt, not fluoride itself.
Our research group has recently synthesized several new antibacterial monomers.37 One of them (N-benzyl-11-[methacryloyloxy]-N, N-dimethylundecan-1-aminium fluoride [AM-2]) has shown higher antibacterial activity against S mutans than MDPB but lower cytotoxicity than MDPB. The experimental composite containing 3% of this new antibacterial monomer could significantly reduce S mutans biofilms compared to controls.38,39 The experimental antibacterial fluoride-releasing bonding agents containing AM-2 and a new synthesized hydrolytically stable adhesive monomer have also shown higher bonding strengths and hydrolytical stability than Protect Bond and other commercial bonding agents.40
The purpose of this study was to investigate the adhesion (bonding strength to enamel), fluoride release and recharge, and microleakage of several experimental and commercial antibacterial fluoride-releasing sealants.
Methods
Sealant materials
Two experimental sealants (Exp-1, Exp-2) were formulated using the synthesized antibacterial fluoride-releasing monomer,41 synthesized hydrolytically stable adhesive monomer,39 other dental monomers, and fluoride-releasing glass filler (Caulk/Dentsply). Exp-1 had the same monomers as Exp-2, but Exp-1 contained 15% NovaMin bioactive glass nanoparticles replacing the equal amount of fluoride-releasing filler. The NovaMin nanoparticles were prepared by suspending the NovaMin particles (GlaxoSmithKline, London, UK, 5 μm) in isopropanol under vibration for 2 minutes, decanting the suspension solution containing nanoparticles, evaporating the solvent from the suspension, and drying the nanoparticles under vacuum. The NovaMin nanoparticles had a 50% mean particle size of 45 nm, as determined by a Nanotrac 250 particle size analyzer (Microtrac, Montgomeryville, Pa., USA). Commercial fluoride-releasing sealants FluroShield (FS; Caulk/Dentsply, Milford, Del., USA), fluoride-releasing antimicrobial sealants Clinpro (CP; 3M-ESPE, St. Paul, Minn., USA), and an antibacterial nonfluoride-releasing sealant SeLECT Defense (E34; Element 34 Technology, Lubbock, Texas, USA) were also included. The compositions of the sealants are listed in Table 1.
Table 1.
COMPOSITIONS OF THE SEALANTS USED IN THIS STUDY
| Material | Manufacturer | Composition* |
|---|---|---|
| Clinpro | 3M ESPE, St. Paul, Minn., USA |
TEGDMA, BisGMA, tetrabuty lammoniumtetrafluoroborate, silane-treated silica |
| SeLECT Defense |
Element 34 Technologies, Lubbock, Texas, USA |
Organic selenium compound |
| FluoroShield | Dentsply Caulk, Milford, Del., USA |
Polyurethanes, BisGMA, sodium fluoride |
| Exp-1 | LSU School of Dentistry, New Orleans, La., USA |
Synthesized antibacterial fluoride releasing monomer (16.13%), BisGMA (6.45%), UEDMA (12.90%), HDDMA (12.90%), HEMA (12.90%), synthesized adhesive monomer (3.22%), silanized fluoride-releasing filler (Caulk/Dentsply, 35%), photo- initiators (0.5%) |
| Exp-2 | LSU School of Dentistry, New Orleans, La., USA |
Same monomers and photoini- tiators as in Exp-1, silanized fluoride-releasing filler (Caulk/ Dentsply, 20%), NovaMin bioactive glass particles (GlaxoSmithKline, 15%) |
BisGMA=2,2-bis[4-(2-hydroxy-3-methacroyloxypropoxy)phenyl]-propane; UEDMA=urethane dimethacrylate; HDDMA=1,6-hexanediol dimethacrylate; HEMA=2-hydroxyethyl methacrylate.
Fluoride release and recharge test
The disk specimens (5.0 mm diameter, 1.2 mm thickness, n=5) were prepared, light cured for 40 seconds using an Optilux 501 dental curing light (Kerr, Orange, Calif., USA output >600 mW/cm2) and immersed in 2.0 mL deionized water at 37°C. The fluoride concentration of the solution (1.8 mL immersion solution plus 0.2 mL concentrated Total Ion Strength Adjusting Buffer (TISAB III) was measured daily using an ion-selective electrode (model no. 96-09, Thermo Scientific Orion, Waltham Ma., USA) and 720 pH/ISE meter (Thermo Scientific Orion) for 14 days with daily replenishment of the solution. After 2 more days of fluoride release (serving as the baseline), the specimens were recharged with Neutra-Foam (2.0% NaF; Oral-B, Procter & Gamble, Cincinnati, Ohio, USA) for 1 minute and rinsed with running deionized water for 30 seconds. Fluoride release from recharged samples was measured daily for 4 days, and the recharge process was repeated 3 times. Data collected on the fourth day served as the new baseline and were not included in the cumulative fluoride release after recharge.
Microtensile bond strength (MTBS) test
Twenty extracted molar teeth were divided into 5 groups (n=4) and ground with 600-grit silicon carbide papers to expose the flat enamel of the occlusal surfaces. The specimens were etched with 37% phosphoric acid gel for 15 seconds, rinsed, and dried. The sealants were applied and light cured for 20 seconds. Filtek Supreme Plus composite (3M ESPE) was placed on a cured sealant, cured for 40 seconds, and built up incrementally to 4 to 5 mm. The samples were stored in deionized water for 24 hours at 37°C, and sectioned into bar specimens (1 × 1 mm cross-section). Each “restored” tooth generated 8 to 10 bar specimens, and each bar specimen was observed under a microscope to ensure no defect existed on the enamel-sealant interface. For each group, 15 specimens were tested on a microtensile tester (Bisco, Schaumburg, Ill., USA) for MTBS. The remaining 15 specimens were thermocycled at 5°C to 55°C for 1,000 cycles and tested for MTBS.
Microleakage test
Five groups of extracted teeth (6 maxillary molars and 4 mandibular molars per group) were randomly sorted, cleaned, and stored in Hanks balanced salt solution. A 1.0 mm fissure was made in each sample to ensure a uniform fissure depth for all samples, followed by etching with 37% phosphoric acid gel for 30 seconds, cleaning, and drying. Sealants were applied according to the manufacturer’s instructions. The samples were light cured for 40 seconds and checked with an explorer. The unsealed surface of the sample was coated with a nail polish and thermocycled at 5°C to 55°C for 2,000 cycles. The specimens were stained in 2% basic fuchsine for 24 hours, rinsed, air dried, and embedded in epoxy-resin. Each specimen was sectioned mesiodistally into 6 slabs (1.2 mm-thick over 5-7 surfaces), observed, and photographed under a microscope at 40× magnification. The microleakage degrees were scored on a scale of 0 to 4 (0 = no penetration; 1 = 1/4 penetration; 2 = 1/2 penetration; 3 = 3/4 penetration; 4 = penetration into the bottom of the fissure).
Statistical analysis
Fluoride release and bonding strength data were analyzed using analysis of variance and Tukey’s honestly significant differences test (THSDT). A paired t test was used to compare the mean MTBS under different storage conditions. The microleakage score data were analyzed using the Kruskal-Wallis test with post-hoc analysis performed using THSDT on the ranks of the scores and a Bonferroni adjusted significance level employed to maintain a 5% experiment-wise error rate.
Results
The fluoride release and recharge profiles of the tested sealants are shown in Figures 1 and 2, respectively. Experimental sealants (Exp-1 and Exp-2) had higher sustained fluoride release rates, while the commercial sealants (CL and FS) had much lower fluoride release rates after the first 3 days (Figure 1). The cumulative fluoride release and recharge data are shown in Table 2. Exp-1 and Exp-2 had significantly higher fluoride release and recharge rates than commercial sealants CL and FS (P<.005). Exp 2 also had cumulative higher fluoride release rates 14 days and 3 days after recharge than Exp-1 (P<.05), but Exp-1 should have a higher net F-recharge rate (see Discussion section). There seemed to be a slight increase in peak values 1 day after recharge (or over 3 days) with increasing cycles, but there was no significant difference in the values between each cycle. We also measured the fluoride release from E34, but no fluoride was detected.
Figure 1.
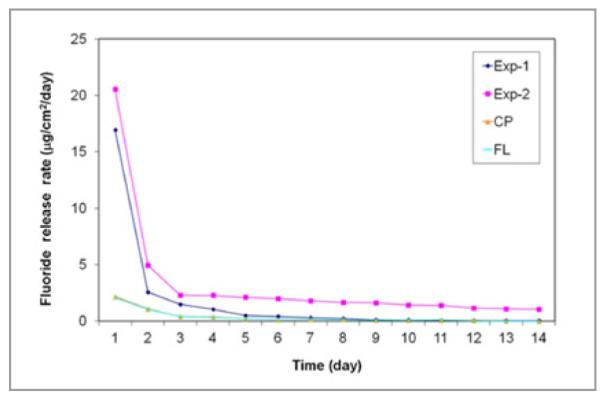
Fluoride release profiles of the sealants in 14 days.
Figure 2.
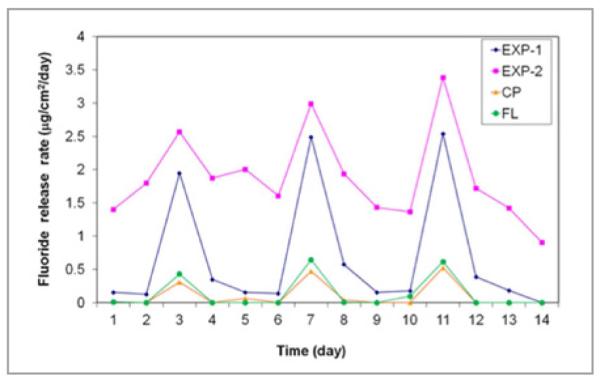
Fluoride recharge profiles of the sealants
Table 2.
CUMULATIVE FLUORIDE RELEASE IN 14 DAYS AND 3 DAYS AFTER RECHARGE (MEAN±SD)*
| Materials | Cumulative fluoride release in 14 days (μg/cm2) |
Cumulative fluoride release 3 days after recharge (μg/cm2) |
|---|---|---|
| Exp-1 | 23.90 ± 2.85B | 2.93 ± 0.61b |
| Exp-2 | 45.34 ± 4.35A | 6.44 ± 0.72a |
| Clinpro | 4.98 ± 0.97C | 0.46 ± 0.05c |
| FluoroShield | 4.79 ± 1.10C | 0.56 ± 0.13c |
Groups with the same superscript latter have no significant difference (P<.05).
MTBS of the sealants before and after 1,000 thermocycling treatments are shown in Table 3. FS and Exp-2 had higher MTBS than E34 (P<.05) and similar bonding strength to Exp-1 and CL (P>.05). There was no significant decrease of MTBS after thermal cycling. A typical micrograph and the microleakage score of the 4 groups’ samples are shown in Figure 3. The Kruskal-Wallis test revealed a significant difference (chisquare = 21.0, df = 4, P<.001) between microleakage scores for different sealants. The mean scores and standard deviations are shown in Figure 4. Exp-2 had significantly lower scores than all the commercial sealants (P<.005), but it was not significantly different from Exp-1 (P = .28). Exp-1 had a significantly lower score than FS (P>.04), but it was not significantly different from CL (P<.38) and E34 (P>.09).
Table 3.
MICROTENSILE BONDING STRENGTH (MTBS; MEAN±SD)*
| Materials | MTBS, 24 hs (MPa) |
MTBS, 1,000 thermocycles (MPa) |
|---|---|---|
| FluoroShield | 24.13 ± 4.76A | 25.62 ± 4.32a |
| Exp-2 | 24.60 ± 3.83A | 23.79 ± 4.90a |
| Exp-1 | 22.71 ± 3.39A,B | 21.99 ± 3.75a |
| Clinpro | 20.24 ± 4.81A,B | 22.53 ± 5.61a |
| E34 | 19.29 ± 4.73B | 22.97 ± 5.20a |
Groups with the same superscript latter have no significant difference (P>.05).
Figure 3.
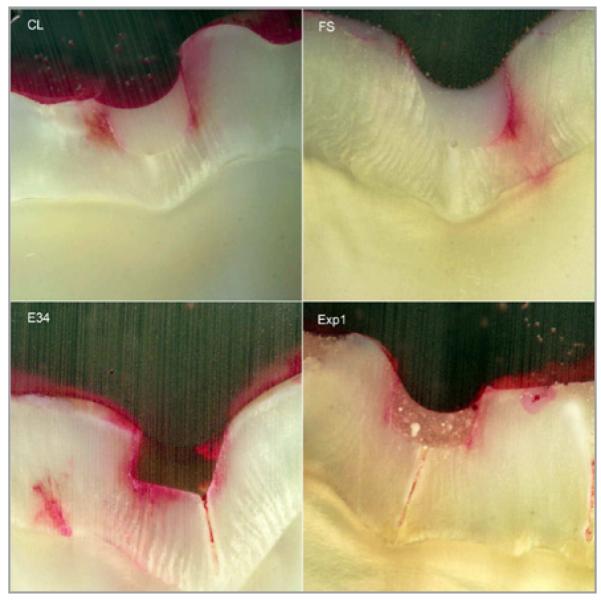
Microleakage scores of typical micrographs of sealants for groups CL (score=2), FS (score=3), E34 (score=4), and Exp1 (score=2) under 40×magnification.
Figure 4.
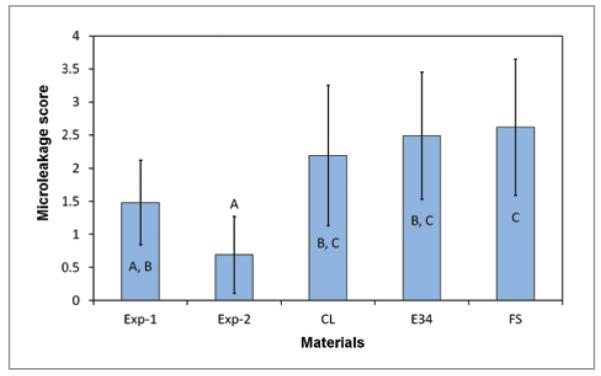
Microleakage scores of different sealants. Groups with different letters have significant differences (P<.05).
Discussion
Pit and fissure sealants are widely used to treat early stage occlusal caries. The application method for sealants is technique-sensitive, however, because saliva contamination may lead to a poor seal and poor mechanical retention of the sealant. The partially retained sealant can trap fermentable food debris underneath it and make it difficult to clean, which may facilitate the formation of secondary caries. The fluoride ions in most fluoride-releasing sealants do not show any antimicrobial property.41
The fluoride release from all fluoride-releasing sealants is usually high in the first 2 days (“burst effect”) and then tapers off to a much lower level (as shown in Figure 1).11,12 Another obvious drawback of the sealant containing NaF is that the salt is not miscible with the monomers, and porosity forms after the dissolution of the salt, which increases the water sorption and decreases durability. To increase the solubility of the fluoride salt in dental monomers, a large neutral organic salt—such as tetrabutylammonium fluoride—has been used during the synthesis of the fluoride-releasing monomer.22 The tetrabutylammonium ion, however, can form a tight ion-pair with fluoride, and such ion-pairs can leach out of the material. This may lead to higher water sorption and solubility and a decline in mechanical properties over time.22 A similar compound is also used in Clinpro sealant.
The experimental sealants in this study contain a new antibacterial fluoride-release monomer, which consists of a fluoride-exchange dimethacrylate monomer containing ternary zirconium fluoride chelate and an antibacterial monomer, N-benzyl-11-(methacryloyloxy)-N, N-dimethylundecan-1-aminium fluoride (AM-2).38,39,42 The fluoride-exchange monomer not only promotes the transport of the fluoride ion from the fluoride-releasing filler to the surface of the material (increasing fluoride release), but also enhances the uptake (recharge) of fluoride from high concentration fluoride sources, such as topical fluoride agents or fluoride-containing toothpaste. Therefore, the experimental sealants have shown significantly higher fluoride-releasing and recharging capabilities than the commercial sealants (Figures 1-2, Table 2). Since we have previously reported the antimicrobial effect of an AM-2-containing composite,38,39 an antimicrobial efficacy test was not included in the current study. It is anticipated that the combination of the fluoride-exchange monomer and antibacterial monomer will enhance the caries-inhibitive effect of the sealant and reduce secondary caries.
Our previous pilot study of experimental sealants containing bioactive glass fillers showed higher release of calcium, phosphate, and fluoride than a commercial fluoride-releasing sealant (FluroShield).43 Calcium (Ca) and phosphate (P) released from the pit and fissure sealant are able to promote the remineralization occurring beneath the sealant. There is a concern, however, about the possible reduced retention rate of such bioactive sealants as well as fluoride-releasing sealants.16,17 Our previous study of NovaMin-containing fluoride-releasing bonding agents has shown that adding bioactive glass particles in bonding agents can increase Ca and P release but will decrease the bond strength.44 To improve the adhesion and retention of the bioactive antibacterial fluoride-releasing sealant, 2 new approaches have been applied in this study:
Nanoparticles (45 nm) instead of coarse particles (5 μm) of NovaMin bioactive glass have been used. This could significantly reduce the precipitation of the particles and phase separation of the sealant. It could also allow the sealant to infiltrate into the microstructure of the etched pit and fissure.
Exp-1 and Exp-2 contain 5% newly synthesized adhesive monomer with a phosphonic acid moiety, which may provide additional chemical bonding to enamel, similar to dental adhesives. Through careful formulation, the viscosities of the experimental sealants are kept at the same or lower levels compared to those of commercial sealants to ensure their flowable property. Therefore, Exp-1 and Exp-2 have similar MTBS to FS, E34, and CL. The bonding strength after thermal cycling showed no significant difference among the sealants. This indicates that addition of antimicrobial fluoride release monomer in experimental groups does not decrease the desired mechanical properties. In Figure 3, however, the section of experimental sealant shows visible white granules, which may be the filler particle aggregation. All the commercial sealants are very homogenous, and E34 shows more transparency than FS and CL.
An unexpected result was that Exp-2 sealant (containing NovaMin nanoparticles) seemed to have higher fluoride release and recharge rates than Exp-1. This result was further confirmed by repeated experiments. A possible explanation is that the adhesive monomer (containing phosphonic acid) in Exp-2 reacted with NovaMin nanoparticles (through an acid-base reaction) and released calcium ions. These calcium ions reacted with the ternary zirconium fluoride chelate in the fluoride-exchange monomer, which led to the release of zirconium fluoride complex (perhaps as [ZrFn(OH)5-n]−, which was converted to zirconium dioxide, water, and fluoride ions in aqueous solution).
Although the formation of calcium fluoride cannot be excluded, the strong chelating ability of the monomer might suppress the formation of calcium fluoride. If this is true, the fluoride recharge capability of the Exp-2 should be lower than Exp-1 (since the chelated calcium has lower fluoride exchange ability than the zirconium counterpart). In fact, if we look carefully at the recharge profile (Figure 2), we can see that Exp-2 actually had a smaller net increase (smaller peak) of fluoride concentration than Exp-1. The higher cumulative fluoride release for Exp-2 was mainly caused by its higher baseline (continually released fluoride). In other words, Exp-1 indeed had a higher fluoride recharge capability than Exp-2. Further study may be warranted to fully understand the functions of the NovaMin nanoparticles.
Conclusions
Based on this study’s results, the following conclusions can be made:
The new experimental antibacterial fluoride-releasing sealants had significantly higher fluoride release and recharge capabilities than commercial sealants.
The experimental and commercial sealants had similar bonding strengths to enamel.
The experimental sealants showed very little to no microleakage and significantly less than the commercial sealants. Therefore, they are expected to have excellent retention.
Future studies are needed to evaluate the antimicrobial and biofilm-inhibitory properties of the experimental sealants, and clinical trials will need to be conducted to determine in vivo retention and anti-caries properties of these sealants.
Acknowledgments
This work was supported by NIH/NIDCR grant no. 5R01DE019203. The authors wish to thank 3M ESPE, St. Paul, Minn., Caulk/Dentsply, Milford, Del., E34 Technologies, Lubbock, Texas, Esstech, Essington, Pa., and GlaxoSmithKline, London, UK, for their donation of materials.
References
- 1. [Accessed January 16, 2013];Dental caries (tooth decay) in children (age 2 to 11) Available at: “ http://www.nidcr.nih.gov/DataStatistics/FindData ByTopic/DentalCaries/DentalCariesChildren2to11”.
- 2.Mejáre I, Lingström P, Petersson LG, et al. Caries-preventive effect of fissure sealants: A systematic review. Acta Odontol Scand. 2003;61:321–30. doi: 10.1080/00016350310007581. [DOI] [PubMed] [Google Scholar]
- 3.Hiiri A, Ahovuo-Saloranta A, Nordblad A, Mäkelä M. Pit and fissure sealants versus fluoride varnishes for preventing dental decay in children and adolescents (Review) Cochrane Database Syst Rev. 2010;3:CD003067. doi: 10.1002/14651858.CD003067.pub3. [DOI] [PubMed] [Google Scholar]
- 4.Beauchamp J, Caufield PW, Crall JJ, et al. Evidence-based clinical recommendations for the use of pit-and-fissure sealants: A report of the American Dental Association Council on Scientific Affairs. J Am Dent Assoc. 2008;139:257–68. doi: 10.14219/jada.archive.2008.0155. [DOI] [PubMed] [Google Scholar]
- 5.Oong EM, Griffin SO, Kohn WG, Gooch BF, Caufield PW. The effect of dental sealants on bacteria levels in caries lesions: A review of the evidence. J Am Dent Assoc. 2008;139:71–8. doi: 10.14219/jada.archive.2008.0156. [DOI] [PubMed] [Google Scholar]
- 6.Going RE, Loesche WJ, Grainger DA, Syed SA. The viability of microorganisms in carious lesions five years after covering with a fissure sealant. J Am Dent Assoc. 1978;97:455–62. doi: 10.14219/jada.archive.1978.0327. [DOI] [PubMed] [Google Scholar]
- 7.Mertz-Fairhurst E, Curtis JW, Jr, Ergle JW, Rueggeberg FA, Adair SM. Ultraconservative and cariostatic sealed restorations: Results at year 10. J Am Dent Assoc. 1998;129:55–66. doi: 10.14219/jada.archive.1998.0022. [DOI] [PubMed] [Google Scholar]
- 8.Azarpazhooh A, Main PA. pit and fissure sealants in the prevention of dental caries in children and adolescents: A systematic review. J Can Den Assoc. 2008;74:171–7. [PubMed] [Google Scholar]
- 9.Trairatvorakul C, Kladkaew S, Songsiripradabboon S. Active management of incipient caries and choice of materials. J Dent Res. 2008;87:228–32. doi: 10.1177/154405910808700301. [DOI] [PubMed] [Google Scholar]
- 10.Swartz ML, Phillips RW, Norman RD, Elliason S, Rhodes BF, Clark HE. Addition of fluoride to pit and fissure sealants: A feasibility study. J Dent Res. 1976;55:757–71. doi: 10.1177/00220345760550050901. [DOI] [PubMed] [Google Scholar]
- 11.Cooley RL, McCourt JW, Huddleston AM, Casmedes HP. Evaluation of a fluoride-containing sealant by SEM, microleakage, and fluoride release. Pediatr Dent. 1990;12:38–42. [PubMed] [Google Scholar]
- 12.Rock WP, Foulkes EE, Perry H, Smith AJ. A comparative study of fluoride-releasing composite resin and glass ionomer materials used as fissure sealants. J Dent. 1996;24:275–80. doi: 10.1016/0300-5712(95)00061-5. [DOI] [PubMed] [Google Scholar]
- 13.Dionysopoulos P, Kotsanos N, Pataridou A. fluoride release and uptake by four new fluoride releasing restorative materials. J Oral Rehabil. 2003;30:866–72. doi: 10.1046/j.1365-2842.2003.00993.x. [DOI] [PubMed] [Google Scholar]
- 14.National Institute of Dental Research Fluoride releasing sealants. J Am Dent Assoc. 1985;110:90. doi: 10.14219/jada.archive.1985.0304. [DOI] [PubMed] [Google Scholar]
- 15.Jensen OE, Billing RJ, Featherstone JD. Clinical evaluation of FluroShield pit and fissure sealant. Clinic Prevent Dent. 1990;12:24–7. [PubMed] [Google Scholar]
- 16.Yildiz E, Dorter C, Efes B, Koray F. A comparative study of two fissure sealants: A 2-year clinical follow-up. J Oral Rehabil. 2004;31:979–84. doi: 10.1111/j.1365-2842.2004.01334.x. [DOI] [PubMed] [Google Scholar]
- 17.Koch MJ, Garcia-Godoy F, Mayer T, Staehle HJ. Clinical evaluation of Helioseal F fissure sealant. Clin Oral Investig. 1997;1:199–202. doi: 10.1007/s007840050034. [DOI] [PubMed] [Google Scholar]
- 18.Lygidakis NA, Oulis KI. A comparison of FluroShield with Delton fissure sealant: Four-year results. Pediatr Dent. 1999;21:429–31. [PubMed] [Google Scholar]
- 19.William B, Laxton L, Holt RD, Winter GB. Fissure sealants: A 4-year clinic trial comparing an experimental glass polyalkenoate cement with a bisglycidyl methacrylate resin used as fissure sealants. Br Dent J. 1996;180:104–8. doi: 10.1038/sj.bdj.4808989. [DOI] [PubMed] [Google Scholar]
- 20.Xu X, Ling L, Ding X, Burgess JO. Synthesis and characterization of a novel fluoride-releasing dimethacrylate monomer and its dental composite. J Polym Sci A Polym Chem. 2004;42:985–98. [Google Scholar]
- 21.Xu X, Ding X, Ling L, Burgess JO. Synthesis and characterization of novel fluoride-releasing monomers 2: Dimethacrylates containing bis(aminodiacetic acid) and their ternary zirconium-fluoride complexes. J Polym Sci A Polym Chem. 2005;43:3153–66. [Google Scholar]
- 22.Xu X, Ling L, Wang R, Burgess JO. Formulation and characterization of a novel fluoride-releasing dental composite. Dent Mater. 2006;22:1014–23. doi: 10.1016/j.dental.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 23.Ling L, Xu X, Choi GY, Billodeaux D, Guo G, Diwan RM. Novel F-releasing composite with improved mechanical properties. J Dent Res. 2009;88:83–8. doi: 10.1177/0022034508328254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maltz M, de Oliveira EF, Fontanella V, Bianchi R. A clinical, microbiologic, and radiographic study of deep caries lesions after incomplete caries removal. Quintessence Int. 2002;33:151–9. [PubMed] [Google Scholar]
- 25.Kenawy E-R, Worley SD, Broughton R. The chemistry and applications of antimicrobial polymers: A state-of-the-art review. Biomacromolecules. 2007;8:1359–84. doi: 10.1021/bm061150q. [DOI] [PubMed] [Google Scholar]
- 26.Bynum AM, Donly KJ. Enamel de/remineralization on teeth adjacent to fluoride releasing materials without dentifrice exposure. J Dent Child. 1999;66:89–92. [PubMed] [Google Scholar]
- 27.Svanberg M. Class II amalgam restorations, glass ionomer tunnel restorations, and caries development on adjacent tooth surfaces: A 3-year clinical study. Caries Res. 1992;26:315–8. doi: 10.1159/000261459. [DOI] [PubMed] [Google Scholar]
- 28.Imazato S, Torii M, Tsuchitani Y, McCabe JF, Russell RR. Incorporation of bacterial inhibitor into resin composite. J Dent Res. 1994;73:1437–43. doi: 10.1177/00220345940730080701. [DOI] [PubMed] [Google Scholar]
- 29.Imazato S, Kinomoto Y, Tarumi H, Torii M, Russell RR, McCabe JF. Incorporation of antibacterial monomer MDPB into dentin primer. J Dent Res. 1997;76:768–72. doi: 10.1177/00220345970760030901. [DOI] [PubMed] [Google Scholar]
- 30.Imazato S, Imai T, Russell RR, Torii M, Ebisu S. Antibacterial activity of cured dental resin incorporating the antibacterial monomer MDPB and an adhesion-promoting monomer. J Biomed Mater Res. 1998;39:511–5. doi: 10.1002/(sici)1097-4636(19980315)39:4<511::aid-jbm1>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 31.Imazato S. Antibacterial properties of resin composites and dentin bonding systems. Dent Mater. 2003;19:449–57. doi: 10.1016/s0109-5641(02)00102-1. [DOI] [PubMed] [Google Scholar]
- 32.Lobo MM, Goncalves RB, Pimenta LA, Bedran-Russo AK, Pereira PN. In vitro evaluation of caries inhibition promoted by self-etching adhesive systems containing antibacterial agents. J Biomed Mater Res B Appl Biomater. 2005;75:122–7. doi: 10.1002/jbm.b.30312. [DOI] [PubMed] [Google Scholar]
- 33.Feuerstein O, Matalon S, Slutzky H, Weiss EI. Antibacterial properties of self-etching dental adhesive systems. J Am Dent Assoc. 2007;138:349–54. doi: 10.14219/jada.archive.2007.0167. [DOI] [PubMed] [Google Scholar]
- 34.de Carvalho FG, Puppin-Rontani RM, Soares LE, Santo AM, Martin AA, Nociti FH., Jr Mineral distribution and CLSM analysis of secondary caries inhibition by fluoride/MDPB-containing adhesive system after cariogenic challenges. J Dent. 2009;37:307–14. doi: 10.1016/j.jdent.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 35.Feng Li, Fang Li, Dan Wu, Sai Ma, et al. The effect of an antibacterial monomer on the antibacterial activity and mechanical properties of a pit-and-fissure sealant. J Am Dent Assoc. 2011;142:184–93. doi: 10.14219/jada.archive.2011.0062. [DOI] [PubMed] [Google Scholar]
- 36.Naorungroj S, Wei HH, Arnold RR, Swift EJ, Jr, Walter R. Antibacterial surface properties of fluoride-containing resin-based sealants. J Dent. 2010;38:387–91. doi: 10.1016/j.jdent.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 37.Xu X, Wang Y, Ling L. Synthesis and characterization of antibacterial dental monomers. Polym Prep. 2008;49:885–6. [Google Scholar]
- 38.Wang Y, Wen ZT, Liao S, Chen L, Xu X. Antibacterial activity and mechanical properties of antibacterial dental composite. J Dent Res. 2011;90(special issue):2505. [Google Scholar]
- 39.Xu X, Wang Y, Liao S, Wen ZT, Fan Y. Synthesis and characterization of antibacterial dental monomers and composites. J Biomed Mater Res Part B. 2012;100B:1151–62. doi: 10.1002/jbm.b.32683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu R, Wang Y, Fan Y, Hagan J, Xu X. Biodegradation and bonding strength of antibacterial fluoride-releasing bonding agents. J Dent Res. 2011;90(special issue):2366. [Google Scholar]
- 41.Matalon S, Peretz B, Sidon R, Weiss EI, Slutzky H. Antibacterial properties of pit and fissure sealants combined with daily fluoride mouth rinse. Pediatr Dent. 2010;32:9–13. [PubMed] [Google Scholar]
- 42.Wang Y, Samoei GK, Lallier TE, Xu X. Synthesis and Characterization of New Antibacterial Fluoride-Releasing Monomer and Dental Composite. ACS Macro Lett. 2013;2:59–62. doi: 10.1021/mz300579y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu X, Wang Y, Balagam B, Peter I, Mitchell JC, Ferracane JL. Formulation and characterization of fluoride-releasing bioactive sealants. J Dent Res. 2009;88(special issue):296. [Google Scholar]
- 44.Xu X, Wang Y, Wu R, Fan Y, Hagan J. Formulation of novel antibacterial fluoride-releasing dentin bonding agent. J Dent Res. 2010;89(special issue):2058. [Google Scholar]


