Abstract
Objective
To compare functional brain differences in tremor dominant (TD) and non-tremor dominant (NTD) subtypes of Parkinson’s disease (PD) using functional magnetic resonance imaging (fMRI).
Design
fMRI activation in TD and NTD patient groups who performed a grip task was compared using voxel-wise analysis. Significantly different brain areas between patient groups were examined with a region of interest (ROI) analysis to compare patients with healthy controls. Voxel-based morphometry was used to determine macroscopic differences in grey and white matter volume between patient groups.
Setting
University research institution.
Participants
Twenty drug-naïve patients with PD (10 TD and 10 NTD) and 20 healthy controls.
Main outcome measures
Blood oxygenation level dependent activation and percent signal change.
Results
Robust findings across both voxel-wise and ROI analyses showed that compared to TD patients, NTD patients had reduced activation in ipsilateral dorsolateral prefrontal cortex (DLPFC), and internal and external globus pallidus (GPi and GPe). ROI analyses confirmed that NTD patients had reduced activity in ipsilateral DLPFC, GPi, and GPe compared to TD patients and control subjects. TD patients had increased activity in contralateral DLPFC compared to NTD patients and controls. These results could not be explained by differences in grey or white matter volume.
Conclusions
Reduced fMRI BOLD activity occurs in the prefrontal cortex and globus pallidus of NTD PD patients compared to both TD PD patients and controls, suggesting that fMRI is a promising technique to understand activation differences in subtypes of PD.
Keywords: fMRI, Parkinson’s disease, BOLD, tremor, phenotype
Introduction
The cardinal motor features of Parkinson’s disease (PD) are bradykinesia, rigidity, and tremor. Within the general diagnosis of PD, distinct clinical subtypes have been identified based in part on the age of onset, the predominant motor sign (e.g. tremor dominant [TD], non-tremor dominant akinetic-rigid [NTD]), and the clinical course of the disease.1, 2 Previous studies have shown that the TD variant has a slower rate of progression and less deterioration of health related quality of life.3–5
Post-mortem analysis of the brains of patients with PD further supports the classification based on specific clinical features. Post-mortem confirmation of PD is based on evidence of specific inclusion bodies which develop as spindle- or thread like Lewy neurites in cellular processes and in the form of globular Lewy bodies in neuronal cell bodies.6 Patients with the NTD phenotype of PD have been shown to have a significantly higher mean overall Lewy body score than patients with TD PD, and more specifically show significantly more cortical Lewy bodies in the frontal regions of the brain than TD patients.7 PD is also characterized post-mortem by substantia nigra compacta dopamine neuronal loss and dopamine deficiency in specific nuclei of the basal ganglia,8 with NTD patients having reduced dopamine levels in globus pallidus.9 Using in vivo functional magnetic resonance imaging (fMRI), drug-naïve patients with PD have reduced activity in the thalamus, primary motor cortex (M1), supplementary motor area (SMA), and in all nuclei of the basal ganglia when compared to healthy controls.10 Also, fMRI activity in nuclei of the basal ganglia correlates with specific symptoms such as bradykinesia and tremor in drug-naïve PD.11 However, to date no direct comparison of brain activation in drug-naïve PD patients with a TD versus NTD phenotype has been performed. The current study was designed to examine functional and structural differences using fMRI and voxel-based morphometry in drug-naïve NTD versus TD PD patients. We hypothesized that patients with NTD PD would show both cortical and basal ganglia activation deficits compared to patients with TD PD. We further test the hypothesis that areas that show reduced activation in NTD patients relative to TD patients will also be reduced in NTD patients relative to control subjects.
Patients and Methods
Twenty drug-naïve PD patients who have never been treated with dopaminergic medications participated in the study (Table 1). The Mini-Mental State Examination score was greater than 26 for each subject. All patients were diagnosed with PD by a movement disorder neurologist and met the PD Society Brain Bank diagnostic criteria.12, 13 A group diagnosis approach was used, such that the diagnosis of each patient was confirmed from video tape by 7 other movement disorders neurologists in the practice at Rush University Medical Center. Ten subjects were in the TD group and 10 subjects were in the NTD group. Criterion for inclusion in the TD group was the presence of rest tremor of 2 from the motor section of the Unified Parkinson’s Disease Rating Scale (UPDRS) in either the head-neck region or in at least one upper or lower extremity. Criterion for inclusion in the NTD group was the presence of no rest tremor in the head-neck region or in any upper extremity. Diagnosis in all patients was reconfirmed two years after MRI testing by reviewing patient charts documented by the movement disorders neurologist. Consistent diagnosis was maintained over two years. Two years following testing, all but 3 patients with PD (1 NTD patient and 2 TD patients) had started either dopamine agonists or levodopa and each of these patients had a positive response to drug therapy. Twenty healthy aged matched control subjects also participated (mean age = 58, number of males = 10). The controls were healthy volunteers from the Chicago-land area and did not have a prior history of neurological or psychiatric disease. UPDRS motor scores for all controls were 0. The first patient was enrolled 3/22/2007, and the last patient was enrolled 10/02/2010. The first control subject was enrolled 5/7/2007 and the last control subject was enrolled 10/19/2010. All subjects gave written informed consent consistent with the Declaration of Helsinki, which was approved by the Institutional Review Boards at Rush University Medical Center and the University of Illinois at Chicago.
Table 1.
Patient Characteristics
| Patient | Age | Gender | Time since diagnosis (months) |
Handedness | Hand Tested |
HY Stage |
Total Motor UPDRS |
Motor UPDRS Rest Tremor | Motor UPDRS Action Tremor |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H/N | LUE | RUE | LLE | RLE | Total | LUE | RUE | ||||||||
| Non-Tremor Dominant | |||||||||||||||
| NTD 1 | 47 | F | 1 | R | R | I | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| NTD 2 | 57 | M | 4 | L | R | II | 25 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| NTD 3 | 69 | M | 2 | R | L | II | 18 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| NTD 4 | 45 | F | 19 | R | L | II | 18 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| NTD 5 | 57 | M | 18 | R | L | II | 18 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| NTD 6 | 55 | F | 9 | R | R | II | 11 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| NTD 7 | 50 | M | 3 | R | R | II | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| NTD 8 | 44 | M | 1 | L | L | I | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 |
| NTD 9 | 66 | F | 10 | R | R | II | 27 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| NTD 10 | 47 | M | 5 | R | R | I | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mean (1sd) |
53.7 (8.7) |
7.2 (6.7) |
15.3 (6.9) |
0 (0) |
0 (0) |
0 (0) |
0 (0) |
.1 (.31) |
.1 (.31) |
.5 (.7) |
.5 (.52) |
||||
| Tremor Dominant | |||||||||||||||
| TD 1 | 72 | M | 4 | R | L | II | 32 | 1 | 2 | 1 | 2 | 0 | 6 | 1 | 1 |
| TD 2 | 55 | F | 19 | R | L | I | 12 | 0 | 2 | 0 | 1 | 0 | 3 | 2 | 0 |
| TD 3 | 55 | M | 33 | L | R | II | 31 | 0 | 0 | 2 | 0 | 0 | 2 | 0 | 1 |
| TD 4 | 58 | F | 8 | R | R | II | 16 | 0 | 0 | 2 | 0 | 0 | 2 | 1 | 1 |
| TD 5 | 64 | M | 3 | R | L | II | 25 | 0 | 2 | 0 | 1 | 0 | 3 | 0 | 0 |
| TD 6 | 70 | F | 2 | R | L | II | 9 | 0 | 2 | 0 | 0 | 0 | 2 | 1 | 0 |
| TD 7 | 56 | F | 2 | R | R | II | 12 | 0 | 0 | 2 | 0 | 0 | 2 | 0 | 0 |
| TD 8 | 47 | M | 14 | R | R | II | 28 | 0 | 0 | 2 | 0 | 0 | 2 | 1 | 2 |
| TD 9 | 47 | F | 18 | R | R | II | 10 | 0 | 1 | 2 | 0 | 0 | 3 | 0 | 0 |
| TD 10 | 49 | M | 8 | R | R | II | 21 | 1 | 1 | 2 | 0 | 0 | 4 | 1 | 2 |
| Mean (1sd) |
57.3 (8.9) |
11.1 (9.99) |
19.6 (8.9) |
.2 (.42) |
1.0 (.94) |
1.3 (.94) |
.4 (.67) |
0 (0) |
2.9 (1.2) |
.7 (.67) |
.7 (.82) |
||||
| t-test | p=.37 | p=.31 | p=.24 | p=.15 | p<.005 | p<.001 | p=.08 | p=.33 | p<.001 | p=.52 | p=.52 | ||||
Abbreviations: NTD = non tremor dominant; TD = tremor dominant; HY = Hoehn and Yahr; H/N = head and neck; RUE = right upper extremity; LUE = left upper extremity; RLE = right lower extremity; LLE = left lower extremity.
Subjects produced force against a fiber-optic force transducer (Aither Engineering, Lanham, MA), with patients using their most affected hand and controls using the hand to maintain a similar hand dominance ratio as the patient groups (Figure 1A). Images were collected using a quadrature volume head coil inside a 3T MR Scanner (GE Healthcare 3T94 Excite 2.0, Waukesha, WI). The subject’s head was stabilized using adjustable padding. The functional images were obtained using a T2*-sensitive, single shot, gradient-echo echo-planar pulse sequence (echo-time 25 ms; time to repeat 2,500 ms; flip angle 90°; field of view 200 mm2; imaging matrix 64×64; 42 axial slices at 3 mm thickness; 0 mm gap). The anatomical images were obtained using a T1-weighted, fast spoiled gradient-echo pulse sequence (echo-time 1.98 ms; TR 9 ms; flip angle 25°; field of view 240 mm2; imaging matrix 256×256; 120 axial slices at 1.5 mm thickness; 0 mm gap).
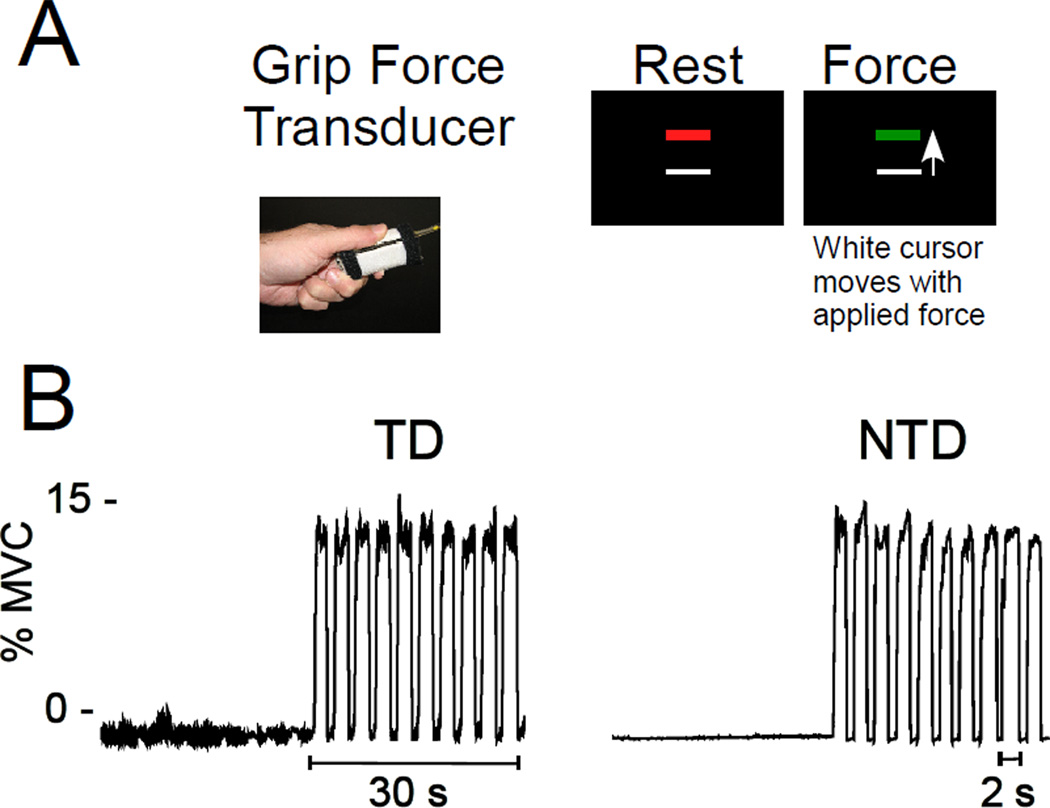
Figure 1. Grip Force fMRI Paradigm.
A. Grip force transducer used to produce isometric force and visual display seen by the subject at rest (red bar) and during force production (green bar). B. Representative force traces from a TD (left) and NTD (right) subject.
The fMRI methods are consistent with previous work.10, 11 The fMRI experiment was a block design paradigm of four 30s task blocks and five 30s rest blocks. During rest blocks, subjects fixated on a stationary red target without producing force. During task blocks, subjects performed 2-s pulse-hold contractions followed by 1s of rest (Figure 1B).10 The target bar represented 15% of the maximum voluntary contraction (MVC). A white force cursor was displayed on the screen that moved vertically related to the force produced by the subject. Each force pulse began as the target bar turned green and remained green for 2 s. The force pulse ended when the target bar turned red for 1 s, indicating rest. There were10 pulses per task block.
After the force output was collected four points were marked for each pulse: onset of force, beginning and end of the sustained force period, and offset of force.14 Based on these marked points, four force variables were calculated: 1) mean force amplitude, 2) duration of force, 3) rate of change of increasing force, and 4) rate of change of decreasing force. Force data were analyzed in order to compare behavioral performance between groups. The difference in the group means was analyzed using a one-way between-subjects ANOVA for each dependent measure. All tests were evaluated as significant at P < .05.
AFNI, the public domain software (http://afni.nimh.nih.gov/afni/), was used to analyze fMRI data. Before analysis, fMRI data were transposed for those subjects that used their left-hand so that the left and right hemispheres in all datasets were contralateral and ipsilateral to the tested hand, respectively. Head motion was less than 1 mm in x, y and z directions for all subjects. A voxel-wise analysis was performed on the fMRI data to identify group differences in blood oxygenation level dependent (BOLD) activation between the NTD and TD patient groups. Motion-corrected individual datasets were normalized by dividing the instantaneous signal in each voxel at each point in the time series by the mean signal in that voxel across the scan. A Gaussian filter was applied to the resultant datasets (full width at half maximum, FWHM, at 3.3 mm). Then, the time series data were regressed to a simulated hemodynamic response function for the task sequence. Before group analysis, each subject’s anatomical and functional dataset was transformed to standardized space using the normalized anatomical dataset as a template. The output data for the tasks were analyzed using a mixed-effects, two-way ANCOVA with patient group (TD, NTD) as the fixed factor and subject as the random factor. We analyzed the TD versus NTD comparison with and without covariates. We included action tremor scores from Table 1 as one covariate and bradykinesia minus resting tremor as the other covariate. For the bradykinesia covariate, we summed bradykinesia items from the UPDRS (questions 23, 24, 25, 26, 29, 31) and subtracted summed resting tremor (question 20) values for both TD and NTD groups. Adding the covariates to the model did not alter the findings and the same areas were identified using either approach. Following the ANCOVA, this yielded the estimated difference in the patient group means for task minus rest. These data were corrected for Type 1 error using a Monte Carlo simulation model (AFNI, Alphasim). Datasets were thresholded at t < 3.8 (P < .005) with a minimum activation cluster volume of 205 µL (P < .05, corrected). Since nuclei in the basal ganglia are small, an uncorrected t-threshold of t < 3.8 (P < .005) was used.
Brain regions with activation differences between the NTD and TD groups were compared to healthy controls using a region of interest (ROI) analysis. Percent signal change (PSC) data were acquired consistent with previous work. 10 The ROIs were determined based on the voxel wise analysis. One-way ANOVAs were performed to compare PSC within ROIs in patients with TD and NTD with healthy control participants. Significant main effects were examined using Tukey honestly significant difference post-hoc tests.
To ensure that any differences in voxel-wise functional activation between NTD and TD were not due to changes in brain structure between patient groups, voxel-based morphometry (VBM) analyses were implemented using SPM8 in Matlab 7.10 and Freesurfer v 5.0.0. The New Segment procedure described in the SPM8 Manual with enhanced preprocessing methods and modeling parameters based on the VBM study of Pereira et al.15 was used as the model for analysis. A voxel-wise statistical analysis was performed by implementing the general linear model with comparison between the TD and the NTD groups using a two sample t-test. Areas of volumetric variation had to meet a statistical threshold of P < .05, corrected for multiple comparisons using the false discovery rate method (FDR), and a minimum cluster size of 10 voxels.
Results
Table 1 shows patient characteristics of both group of patients. There were no significant differences between patient groups in age (t = 0.91, df = 18, P = .37) or UPDRS motor score (t = 1.03, df = 18, P = .32). Figure 1B shows a force trace from a representative PD patient in the TD (left) and NTD (right) groups. Despite having a visible rest tremor, the TD patient was able to perform the task as well as the NTD patient. At the group level, a one way ANOVA showed no differences between NTD, TD, and control groups in mean force (F(2,37) = 0.32, P = .731), rate of force increase (F(2,37) = 2.38, P = .106), or rate of force decrease (F(2,37) = 0.60, P = .554). There was a main group effect for duration of force (F(2,37) = 5.22, P = .010). Post-hoc tests revealed that this was due to longer force duration in the TD group compared to controls (P< = .013), but there was no significant difference between NTD and TD groups. Overall, these data suggest that the behavioral performance is not driving any differences observed in the fMRI data.
Voxel-wise analysis of the BOLD signal revealed reduced activation in the NTD group compared to the TD group in several cortical and subcortical areas: bilateral dorsolateral prefrontal cortex (DLPFC), contralateral pre-supplementary motor area (SMA), ipsilateral inferior parietal lobule (IPL), ipsilateral precuneus, contralateral lingual gyrus, contralateral caudate, contralateral internal and external globus pallidus (GPi, GPe), and ipsilateral thalamus (Table 2, Figure 2A and 2B). There were no areas that showed increased activity in the NTD compared to the TD group. Table 1 shows that one of the NTD patients had a rest tremor score of 1 on the right lower extremity. Removing this subject did not alter the findings in the paper.
Table 2.
Talairach coordinates for significant regions in the voxel-wise comparison of TD > NTD
| Region | Center of Mass |
|||
|---|---|---|---|---|
| x | y | z | ||
| DLPFC | Ipsilateral | 33.1 | 40.8 | 31.3 |
| DLPFC | Contralateral | −40.8 | 39.3 | 22.9 |
| Pre-SMA | Contralateral | −14.1 | 6.8 | 64.7 |
| Caudate | Contralateral | −12.0 | 21.4 | −2.2 |
| GPi | Contralateral | −18.3 | −11.3 | 4.3 |
| GPe | Contralateral | −26.1 | −15.1 | 3.6 |
| Thalamus | Ipsilateral | 1.8 | −22.6 | 7.7 |
| Supramarginal gryus/IPL | Ipsilateral | 49.3 | −43.1 | 34.9 |
| Lingual gyrus | Contralateral | −21.7 | −65.0 | 2.6 |
| Precuneus | Ipsilateral | 7.9 | −73.4 | 28.1 |
Abbreviations: DLPFC = dorsolateral prefrontal cortex; SMA = supplementary motor area; GPi = internal segment globus pallidus; GPe = external segment globus pallidus; IPL = inferior parietal lobule.
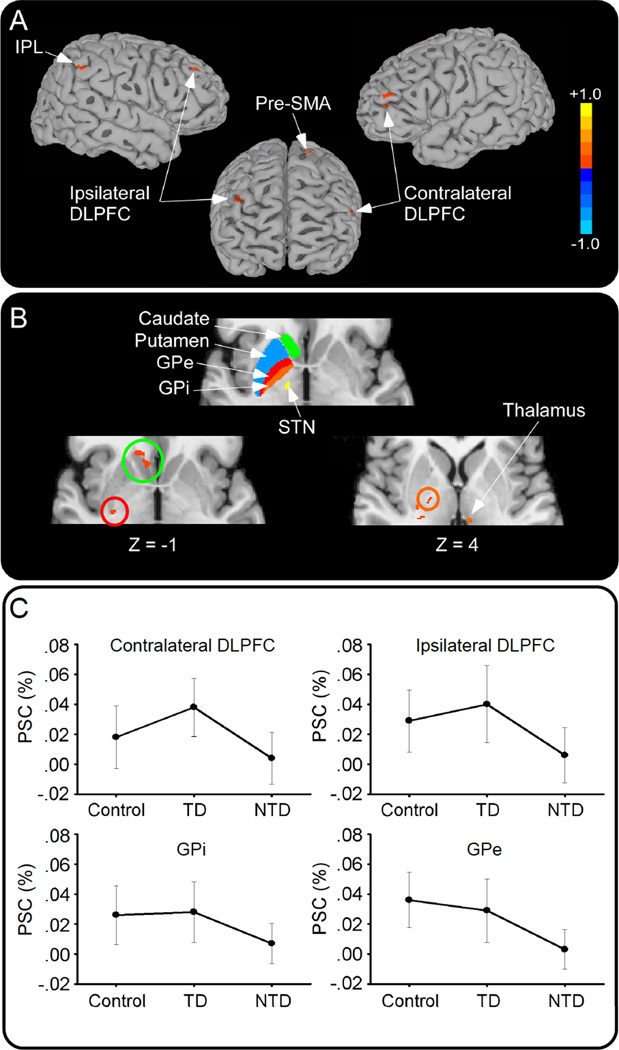
Figure 2. Brain Activation in TD and NTD Groups.
Voxel-wise fMRI analysis of TD minus NTD groups. A. Reduced activity in inferior parietal lobule (IPL), bilateral dorsolateral prefrontal cortex (DLPFC), and pre-supplementary motor area (pre-SMA) in NTD<TD patients. B. Top panel shows the color coded Basal Ganglia Human Area Template 22 that was used to identify the location of basal ganglia activation. Reduced activation is shown for contralateral caudate (green circle), external globus pallidus (GPe; red circle), internal globus pallidus (GPi; orange circle), and ipsilateral thalamus in NTD<TD. C. Percent signal change (PSC) in controls, TD and NTD in contralateral and ipsilateral DLPFC (top) and GPi and GPe (bottom).
VBM analysis showed no differences in grey or white matter volume either cortically or subcortically between NTD and TD patient groups that could have accounted for the between patient group differences found in the functional voxel-wise analysis. An uncorrected threshold at P < .001 did not reveal any differences between groups for the regions shown in Table 2.
Follow-up ROI analysis of the areas listed in Table 2 confirmed findings from the voxel-wise analysis for the NTD and TD comparison in bilateral DLPFC, contralateral GPi and GPe, ipsilateral thalamus, IPL, and precuneus (Table 3). In each of these areas, PSC was lower in NTD patients compared to TD patients. NTD patients showed lower PSC in ipsilateral DLPFC, GPi and GPe compared to controls (Figure 2C). TD patients were only different from controls in one area, contralateral DLPFC, where PSC was higher in TD patients compared to controls (Figure 2C, Table 3). Thus, bilateral DLPFC, contralateral GPi and GPe, ipsilateral thalamus, IPL, and precuneus are areas that show robust differences between TD and NTD patient groups across both voxel-wise and ROI analyses.
Table 3.
One-way ANOVA results from the ROI analysis
| ROI | ANOVA | Tukey post-hoc | |||
|---|---|---|---|---|---|
| F | P | Control vs. NTD |
Control vs. TD |
NTD vs. TD |
|
| DLPFC contralateral | 7.678 | .002 | .186 | .028 | .001 |
| DLPFC ipsilateral | 6.578 | .004 | .028 | .354 | .003 |
| Pre-SMA contralateral | 2.035 | .145 | -- | -- | -- |
| Caudate contralateral | 2.767 | .076 | -- | -- | -- |
| GPi contralateral | 4.19 | .023 | .039 | .901 | 0.036 |
| GPe contralateral | 11.68 | .000 | .000 | .577 | 0.006 |
| Thalamus ipsilateral | 3.827 | .031 | .081 | .693 | .032 |
| Supramarginal gryus/IPL ipsilateral | 3.318 | .047 | .485 | .189 | .039 |
| Lingual gyrus contralateral | 1.645 | .207 | -- | -- | -- |
| Precuneus ipsilateral | 4.607 | .016 | .085 | .435 | .014 |
Abbreviations: ROI = region of interest; ANOVA = analysis of variance; NTD = non-tremor dominant; TD = tremor dominant; DLPFC = dorsolateral prefrontal cortex; SMA = supplementary motor area; GPi = internal segment globus pallidus; GPe = external segment globus pallidus; IPL = inferior parietal lobule. Bold type indicates significant result at P< .05.
Discussion
The current study examined functional and structural differences using fMRI and VBM in drug-naïve TD and NTD patients with PD. Follow-up analysis using an ROI approach was performed to confirm between patient group differences and to examine how these between-patient group differences related to a healthy control group. Robust findings across both analysis methods showed significant differences both cortically (prefrontal cortex) and subcortically (globus pallidus) between patients with NTD PD and TD PD, with NTD patients showing reduced activation. Compared to controls, NTD patients always showed reduced activation, whereas TD patients were either not significantly different than controls or in the case of contralateral DLPFC, showed higher activation. These results could not be explained by differences in grey or white matter volume. Therefore, BOLD changes in the prefrontal cortex and basal ganglia differ in early stage PD patients clustered into NTD and TD groups.
In the cortex, NTD patients showed reduced bilateral prefrontal cortical activation in bilateral DLPFC compared to TD patients. NTD patients also had lower PSC compared to controls in ipsilateral DLPFC. One possibility is that the presence of resting tremor in the TD group could not explain these findings since higher resting activity in the TD group could reduce task related BOLD signal change in the TD group thus minimizing any between group differences. Another possibility is that DLPFC activity was higher in the TD group to suppress tremor since DLPFC has been related to inhibiting force output.14 We compared the BOLD activity with and without covariates that included action tremor and bradykinesia minus resting tremor, and the findings were similar for DLPFC and all other areas with and without the covariates. Thus, while the presence of rest tremor in the TD group and absence of rest tremor in the NTD group led to between group differences in the BOLD signal, these group findings are robust to the UPDRS values as covariates.
Prior post-mortem analysis of brain structure in PD has shown significantly higher mean overall Lewy body score for NTD PD patients than for TD PD patients, particularly in the prefrontal regions of the cortex.7 NTD PD patients show more bradykinesia than TD patients and minimal tremor at disease onset.7 It is not clear whether this is due to pathological differences in brain structure between phenotypes at the outset of the disease, or whether this is due to adaptive changes from symptom differences between phenotypes. To resolve this question, it would be necessary to perform correlational analyses between Lewy body deposition and measures of bradykinesia. Nevertheless, brain changes observed post-mortem seem to support clinical subtyping of patients into either NTD or TD PD phenotypes. Since the current study examined TD and NTD patients relatively early in the disease process prior to starting dopaminergic medication, our findings suggest that grouping patients into TD and NTD based on motoric features reveals changes in the BOLD signal in areas such as DLPFC. Whether these changes in the BOLD signal in the DLPFC have any relation to cortical Lewy body deposition is beyond the current study and caution should be taken in attempting to relate these findings without further inquiry.
The task used in the current study was chosen because it requires robust activation of frontal cortical regions and parietal cortical regions, including M1, dorsal and ventral premotor cortex, SMA, DLPFC, IPL, superior parietal lobule, and anterior cingulate cortex.16, 17 However, in the current study only DLPFC in the prefrontal cortex was different between patient groups in both voxel wise and ROI analysis suggesting that this prefrontal area is robustly sensitive to differences in patients clustered into TD and NTD groups. DLPFC has been suggested to play an important role in working memory and executive function. Indeed, the DLPFC activation has previously been shown to be sensitive to changes in learning in early-stage PD, with activation being normal at baseline but declining to sub-normal levels after 2 years.18 Kikuchi et al.19 used single-photon emission computed tomography to show hypoperfusion in DLPFC, SMA and insular cortex in PD. However, only hypoperfusion in DLPFC and insular cortex was correlated with disease severity leading the authors to suggest that DLPFC and insular cortex may play key roles in specific symptoms of impairment at advanced stages, such as impaired working memory, postural instability and autonomic dysfunction. The results of the current study suggest that disease severity alone may not be the driving feature of this difference, and that symptom specific differences could be another factor that should be considered. That is, patients with a NTD subtype, even in the earliest stages of the disease may show greater deficits in frontal cortical areas compared to patients with a TD subtype.
Subcortically, the current study showed reduced BOLD activation in GPi, GPe and thalamus in NTD versus TD (Table 2 and Table 3). Since the basal ganglia have established connections with the DLPFC, it could be that the cortical findings are due to changes in the basal ganglia or it could be that these cortical and subcortical findings are not directly related. The reduced activation in GPi in the NTD group was in the ventral part of the GPi, and this is location and pattern of findings for the BOLD signal are consistent with previous post-mortem findings of reduced dopamine in the ventral part of the GPi in NTD PD compared to TD PD 9. It is also consistent with the previous finding in a group of drug-naïve PD patients with mixed phenotype that found higher tremor scores on the UPDRS are associated with higher PSC in GPi.11 In the previous study by Prodoehl et al.11, there was a negative correlation between disease severity and PSC in all other BG nuclei and thalamus, and bradykinesia was the symptom that most consistently predicted BOLD activation in these regions. In a different study moderate PD patients were tested following a 12-hr withdrawal from medication, and it was found that pallidal dopamine depletion correlated with clinical tremor severity, and that GPi, GPe, and putamen were transiently active during the onset of tremor episodes.20 Our findings extend this work by showing that early stage, drug-naïve patients clustered into a NTD group had reduced BOLD signal compared to those patients clustered into a TD group in specific nuclei of the basal ganglia (GPi, GPe) as well as the thalamus. Further, our findings suggest that group differences in basal ganglia activation between patients with PD and healthy controls may be driven in part by patients with motoric features consistent with the NTD group rather than the TD group.
Comparing PD patients to healthy controls, only one area in the ROI analysis showed significantly increased activation in PD. Contralateral DLPFC had a significantly higher PSC in TD patients compared to controls. Although rest tremor in PD has been associated with increased metabolism in the thalamus, subthalamus, pons, and premotor-cortical network, suggesting an increased functional activity of thalamo-motor projections21, what underlies increased DLPFC activation in TD patients deserves further study. Additionally, future study of symptom-specific differences with patients in a more advanced stage of the disease process should examine cognitive changes that might accompany the functional activation deficits found in the current study. Since the patients included in this study were drug-naive, it could be argued that patients with atypical parkinsonism were included, particularly in the NTD group. To minimize this possibility, reconfirmation of the diagnosis was made two years after MRI testing was performed. Also, after two years, all but three patients had started dopamine therapy and each patients was responding positively to medication giving us confidence in the findings.
In conclusion, the present study confirmed that fMRI differences in the basal ganglia and cortex between patients with PD and control subjects are primarily due to patients with the NTD subtype rather than the TD subtype. These findings suggest that objective measures of brain function may be useful in future genotype-phenotype analyses and targeted therapeutic trials focused on PD subtypes.
Acknowledgments
Financial Disclosure: This work was supported by grants from the National Institutes of Health (R01-NS-52318, R01-NS-58487, R01-NS-40902, R01-NS-28127), the Michael J. Foundation for Parkinson Research, and a Parkinson Research Center grant from the Parkinson’s Disease Foundation.
We thank the staff at the Section for Movement Disorders in the Department of Neurological Sciences at Rush University Medical Center, Chicago IL, and the patients for their time and commitment to this research.
Financial Disclosures of all Authors for the past 12 months
Drs. Vaillancourt and Corcos have received funding from the National Institutes of Health (NIH; R01-NS-52318, R01-NS-58487, R01-NS-40902, R01-NS-28127) and the Michael J. Foundation for Parkinson Research. Dr. Comella has served as a consultant for Allergan, Merz, Ipsen, Esai, and Boehringer, and has received royalties from Kluwer publishing and Cambridge publishing. Dr. Comella has received research grants that go to her institution from Boehringer, Ipsen, Merz, and NIH and her institution has also received support from a Parkinson Research Center grant from the Parkinson’s Disease Foundation.
Footnotes
Potential conflict of interest: None reported.
Author Contributions
Janey Prodoehl, PhD: data acquisition, drafting and revising manuscript for content, study concept and design, data analysis, interpretation of data.
Peggy Planetta, PhD: data acquisition, data analysis, interpretation of data, revising manuscript for content.
Ajay Kurani, MS: data analysis, interpretation of data, revising manuscript for content.
Cynthia Comella, MD: data acquisition, revising manuscript for content, interpretation of data.
Daniel Corcos, PhD: revising manuscript for content, study concept and design, interpretation of data.
David Vaillancourt, PhD: study supervision, data acquisition, drafting and revising manuscript for content, study concept and design, statistical analysis, interpretation of data, obtaining funding.
References
- 1.Jellinger KA, Paulus W. Clinico-pathological correlations in Parkinson's disease. Clin Neurol Neurosurg. 1992;94(Suppl):S86–S88. doi: 10.1016/0303-8467(92)90033-y. [DOI] [PubMed] [Google Scholar]
- 2.Jankovic J, McDermott M, Carter J, et al. Variable expression of Parkinson's disease: a base-line analysis of the DATATOP cohort. The Parkinson Study Group. Neurology. 1990 Oct;40(10):1529–1534. doi: 10.1212/wnl.40.10.1529. [DOI] [PubMed] [Google Scholar]
- 3.Zetusky WJ, Jankovic J, Pirozzolo FJ. The heterogeneity of Parkinson's disease: clinical and prognostic implications. Neurology. 1985;35(4):522–526. doi: 10.1212/wnl.35.4.522. [DOI] [PubMed] [Google Scholar]
- 4.Rajput AH, Pahwa R, Pahwa P, Rajput A. Prognostic significance of the onset mode in parkinsonism. Neurology. 1993;43(4):829–830. doi: 10.1212/wnl.43.4.829. [DOI] [PubMed] [Google Scholar]
- 5.Ransmayr G, Poewe W, Plorer S, Gerstenbrand F, Leidlmair K, Mayr U. Prognostic implications of the motor symptoms of Parkinson's disease with respect to clinical, computertomographic and psychometric parameters. J Neural Transm. 1986;67(1–2):1–14. doi: 10.1007/BF01243355. [DOI] [PubMed] [Google Scholar]
- 6.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003 Mar-Apr;24(2):197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 7.Selikhova M, Williams DR, Kempster PA, Holton JL, Revesz T, Lees AJ. A clinico-pathological study of subtypes in Parkinson's disease. Brain. 2009 Nov;132(Pt 11):2947–2957. doi: 10.1093/brain/awp234. [DOI] [PubMed] [Google Scholar]
- 8.Hornykiewicz O. Biochemical aspects of Parkinson's disease. Neurology. 1998 Aug;51(2 Suppl 2):S2–S9. doi: 10.1212/wnl.51.2_suppl_2.s2. [DOI] [PubMed] [Google Scholar]
- 9.Rajput AH, Sitte HH, Rajput A, Fenton ME, Pifl C, Hornykiewicz O. Globus pallidus dopamine and Parkinson motor subtypes: clinical and brain biochemical correlation. Neurology. 2008 Apr 15;70(16 Pt 2):1403–1410. doi: 10.1212/01.wnl.0000285082.18969.3a. [DOI] [PubMed] [Google Scholar]
- 10.Spraker MB, Prodoehl J, Corcos DM, Comella CL, Vaillancourt DE. Basal ganglia hypoactivity during grip force in drug naive Parkinson's disease. Hum Brain Mapp. 2010 Dec;31(12):1928–1941. doi: 10.1002/hbm.20987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prodoehl J, Spraker M, Corcos D, Comella C, Vaillancourt D. Blood oxygenation level-dependent activation in basal ganglia nuclei relates to specific symptoms in de novo Parkinson's disease. Mov Disord. 2010 Oct 15;25(13):2035–2043. doi: 10.1002/mds.23360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hughes AJ, Ben-Shlomo Y, Daniel SE, Lees AJ. What features improve the accuracy of clinical diagnosis in Parkinson's disease: a clinicopathologic study. 1992. Neurology. 2001 Nov;57(10 Suppl 3):S34–38. [PubMed] [Google Scholar]
- 13.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases [see comments] Journal of Neurology, Neurosurgery, and Psychiatry. 1992;55(3):181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spraker MB, Corcos DM, Vaillancourt DE. Cortical and subcortical mechanisms for precisely controlled force generation and force relaxation. Cereb Cortex. 2009 Nov;19(11):2640–2650. doi: 10.1093/cercor/bhp015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pereira JM, Xiong L, Acosta-Cabronero J, Pengas G, Williams GB, Nestor PJ. Registration accuracy for VBM studies varies according to region and degenerative disease grouping. Neuroimage. 2010 Feb 1;49(3):2205–2215. doi: 10.1016/j.neuroimage.2009.10.068. [DOI] [PubMed] [Google Scholar]
- 16.Vaillancourt DE, Thulborn KR, Corcos DM. Neural Basis for the Processes that Underlie Visually-Guided and Internally-Guided Force Control in Humans. Journal of Neurophysiology. 2003 Jul 2;90(5):3330–3340. doi: 10.1152/jn.00394.2003. [DOI] [PubMed] [Google Scholar]
- 17.Vaillancourt DE, Yu H, Mayka MA, Corcos DM. Role of the basal ganglia and frontal cortex in selecting and producing internally guided force pulses. Neuroimage. 2007 Jul 1;36(3):793–803. doi: 10.1016/j.neuroimage.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carbon M, Reetz K, Ghilardi MF, Dhawan V, Eidelberg D. Early Parkinson's disease: longitudinal changes in brain activity during sequence learning. Neurobiol Dis. 2010 Feb;37(2):455–460. doi: 10.1016/j.nbd.2009.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kikuchi A, Takeda A, Kimpara T, et al. Hypoperfusion in the supplementary motor area, dorsolateral prefrontal cortex and insular cortex in Parkinson's disease. J Neurol Sci. 2001 Dec 15;193(1):29–36. doi: 10.1016/s0022-510x(01)00641-4. [DOI] [PubMed] [Google Scholar]
- 20.Helmich RC, Janssen MJ, Oyen WJ, Bloem BR, Toni I. Pallidal dysfunction drives a cerebellothalamic circuit into Parkinson tremor. Ann Neurol. 2011 Feb;69(2):269–281. doi: 10.1002/ana.22361. [DOI] [PubMed] [Google Scholar]
- 21.Jellinger KA. Post mortem studies in Parkinson's disease--is it possible to detect brain areas for specific symptoms? J Neural Transm Suppl. 1999;56:1–29. doi: 10.1007/978-3-7091-6360-3_1. [DOI] [PubMed] [Google Scholar]
- 22.Prodoehl J, Yu H, Little DM, Abraham I, Vaillancourt DE. Region of interest template for the human basal ganglia: comparing EPI and standardized space approaches. Neuroimage. 2008 Feb 1;39(3):956–965. doi: 10.1016/j.neuroimage.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]