Abstract
Odours that accumulate from roosting can attract predators and increase predation risk. Consequently, selection should favour strategies that allow prey to evade detection by predators, including changing roosts. Insectivorous bats that roost in tree hollows regularly switch roosts and roost in different sized groups, strategies that would alter the accumulation of roost odours and are hypothesized to reduce predation risk. We experimentally manipulated the amount and refresh rate of roosting odour cues at 90 artificial bat roosts in Sydney, Australia, to test the hypothesis that odours increase predator visitation. Predators visited roosts with bat faeces significantly more often than untreated control roosts. Roosts with small amounts of faeces mimicking sites used by solitary bats had the greatest rate of visitation. This suggests that bats roosting alone, rather than in groups, have a greater likelihood of disturbance or predation. Roost switching probably decreases the predictability of finding occupied roosts; however, we show that all roosts (those currently or recently occupied) were visited by predators, suggesting generalist urban predators readily investigate potential roosts. This is the first demonstration that bat odours are attractive to predators that use olfactory cues, showing that bats are at risk of predation in visually cryptic roosts.
Keywords: bat roost, predation risk, urban ecology, Rattus rattus, nest predation
1. Introduction
The detection of prey-generated cues by predators represents the first, and arguably most influential, stage in the sequence of behaviours that culminates in the killing and consumption of prey [1]. As a result, both predators and prey have developed a suite of strategies thought to reduce the predictability of their presence and hence likelihood of detection by their enemies, respectively [2]. For example, the odours from faeces, hair, feathers and eggs that accumulate at nest or roost sites are long-lasting and potentially potent advertisements of nest or roost locations, placing individual prey at risk from predators [3]. The selection pressure to avoid detection is likely to drive changes in prey activity, mobility and behaviour [4,5]. This scenario is the context for the ‘shell-games’ hypothesis [6], which states that predators continually search for elusive prey, while prey move to evade predation. This hypothesis has rarely been tested.
Insectivorous bats, which roost solitarily or communally in tree hollows and other structures that are accessible to a range of potential predators [7,8], may conform to the shell-games hypothesis. For example, tree-roosting bats frequently switch between a pool of suitable roosts [9], either as a group or as individuals, despite the thermoregulatory advantages of roosting in an already occupied roost [9–11]. This tactic has been proposed to reduce parasite loads, and maintain social relationships, but may also be a strategy for predator evasion [9], a hypothesis that has never been empirically tested. Bats change their behaviour in response to the presence of predators, for example, predatory birds, snakes and other mammals [7,12]; however, how this influences their detection by predators is unknown. Given that bats defaecate in roosts, roost switching would reduce guano accumulation that is potentially attractive to scent-hunting animals, and reduce the predictability of their presence at any given roost to assist in predator evasion.
We seeded artificial roosts with guano, in suburban Sydney, New South Wales, Australia, to examine potential predation risk to roosting bats to test the hypothesis that changes in roost switching and group size influences visitation by scent-hunting predators. Bat faeces or guano was used to provide a cue and simulate an active roost. We predicted that roost visitation by predators would be influenced by our methods to mimic roost switching and changes in group size. If frequent switching reduces predator visitation, we predicted that roosts which were re-used on consecutive nights would have greater visitation than those only used once. Furthermore, we predicted that group roosts would have greater visitation than roosts only occupied by one individual, as a group roost advertises a greater cue for detection.
2. Material and methods
Bat roost visitation was monitored in Sydney Harbour National Park, Sydney, Australia, during the breeding season for the local bat assemblage, which comprises species that roost in tree hollows and caves. To simulate a roost, bat guano was collected opportunistically from individual bats trapped around Sydney and added to artificial roosts. Collected guano was weighed and divided by the number of individuals from which it was collected. On average (n = 8), approximately 0.1 g of faeces was produced in 1 day by an individual, and as such 0.1 g was used to represent the use of a roost by a solitary bat. To simulate 10 bats using a roost, 1 g of faeces was used (see the electronic supplementary material).
We used 90 artificial roosts (n = 30 solitary, 30 group and 30 control roosts; refer to the electronic supplementary material for more details) in our study. Faeces were deployed to simulate roosts of differing group size (solitary or group) and longevity (single use or ‘switched’, and re-used on consecutive days or ‘stayed’). The level of visitation by potential predatory species to these roosts was compared with control roosts. The odour cue (0.1, 1 g or water = control) was placed inside the roost entrance. Half of all treatments (15 replicates) were re-applied daily for 5 days, to represent the re-use of the roost (‘re-used’ treatment). In the other half, the faeces were only applied on the first day to mimic switching (‘switched’ treatment). Visitation was monitored using motion censored infrared video cameras mounted outside the roost for 5 days. Roost visitors were detected using indirect signs of visitation at the roost entrance, or directly recorded via motion censored infrared cameras (refer to the electronic supplementary material for more details).
We used a contingency analysis to test whether bat faeces-scented roosts (pooled across all solitary and group roosts) were visited more often than control roosts. The number of roosts visited per treatment was compared using Cox's proportional hazards modelling through which we could examine the effect of amount and cue renewal treatments. We calculated Kaplan–Meier survival curves to plot visitation functions by treatment, and by site (to determine whether each site had similar visitation rates). Log-rank tests were used to compare survival curves among treatments, assigning significance where p < 0.01 to account for multiple comparisons. All data have been deposited in Dryad (doi:10.5061/dryad.r01j0).
3. Results
Potential predators visited significantly more faeces-scented than control roosts (two-tailed Fisher's exact test p = 0.04). Ringtail possums Pseudocheirus peregrinus were the most common roost visitor accounting for 40 per cent of all visits (including re-visits; figure 1). Introduced black rats Rattus rattus and native birds, mainly pied currawongs Strepera graculina, were also more commonly recorded at faeces-scented than control roosts (two-tailed Fisher's exact test p = 0.04).
Figure 1.
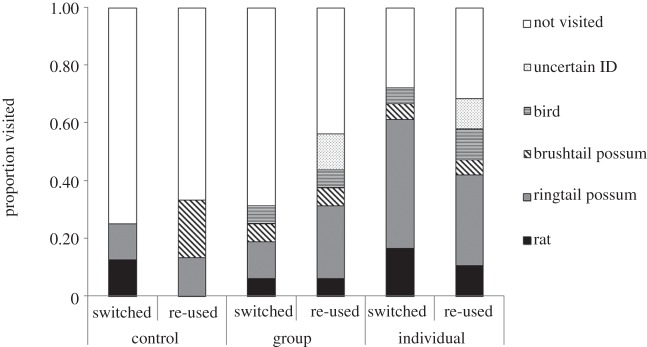
The proportion of roosts visited by each species and the proportion of roosts not visited. Multiple independent visits to individual roosts are represented here. The first visitation only was used in the analysis. Birds include the Australian raven Corvus coronoides and pied currawong Strepera graculina; brushtail possums Trichosurus vulpecula; ringtail possums Pseudocheirus peregrinus and the black rat Rattus rattus.
Simulated group size also influenced the number of visits (table 1), but there was no effect of odour cue renewal (table 1). Log-rank tests showed that potential predators visited roosts with only 0.1 g of guano (reflecting solitary use by bats) more than control roosts (χ2 = 6.76, d.f. = 1, p = 0.004; figure 2) but not roosts with 1 g of guano (χ2 = 2.6, d.f. = 1, p = 0.11; figure 2). However, there was no difference in the number of group and control roosts visited (χ2 = 1.01, d.f. = 1, p = 0.32; figure 2), and there was no difference in roost visitation between sites used (χ2 = 3.74, d.f. = 2, p = 0.15).
Table 1.
Cox's proportional hazards model—effect likelihood ratio tests. Italic value is significant at α = 0.05.
| factor | d.f. | χ2 | p-value |
|---|---|---|---|
| group size | 2 | 6.19 | 0.045 |
| renewal | 1 | 1.19 | 0.276 |
| renewal × group size | 2 | 1.44 | 0.487 |
Figure 2.
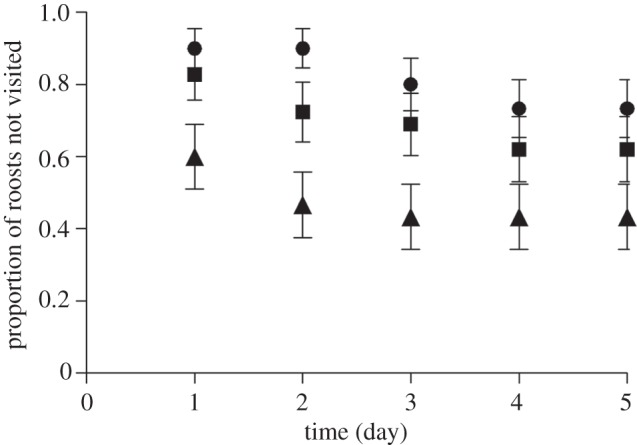
Proportion of roosts not visited, i.e. ‘survived’ (± s.e.) for observations over a 5 day period. All species visiting roosts are included in this analysis. A roost was removed from the analysis and considered ‘disturbed’ after the first visitation. Circles denote control, squares denote group and triangles denote individual roosts.
4. Discussion
We found that even tiny quantities of faeces at an artificial roost significantly increased the likelihood of visitation by potential predators within a few days. The addition of faeces from just a single bat increased predator visitation to roosts by 30 per cent after 1 day, which increased to 44 per cent after 2 days. In natural settings, bats are thought to select roosts that probably restrict predator access [13], but recent significant declines in bat populations have been linked to predation at roost sites [14]. Our results indicate that faecal odours may facilitate rapid roost detection by predators, regardless of switching tactics. Although roost switching may make it difficult to predict the location of an occupied roost from 1 day to the next, our simulation of this strategy suggests that it is not reducing visitation by potential predators. It is surprising that roosts with more guano (reflecting larger groups) received lower visitation; however, this result suggests that roosting with other bats may reduce predation risk. Furthermore, while parasite avoidance [15] and sociality [11,16] may be strong drivers of bat roost switching behaviour, our experiment is the first to demonstrate that predation risk probably contributes to patterns of roosting behaviour of bats.
Predation on roosting bats by generalist urban predators including rats and possums may be a more significant threat than currently considered [17]. We anticipated the extensive visitation to roosts by black rats, as they are frequently arboreal, notorious nest raiders, and implicated in the decline of bats in New Zealand [14,17], and possibly other islands [18]. Rats were strongly attracted to guano, regardless of the amount present. However, ringtail possums were, unexpectedly, the most frequent visitor to roosts seeded with bat faeces. Why these animals would be attracted to bat odour is not immediately clear as they are considered to be primarily folivorous [19], but they do depredate artificial birds’ nests in urban bushland [20]. Given that these potential predators appear able to rapidly find roosts with guano, our results may explain the decline of bats and other roosting or nesting species in the presence of scent-hunting predators [14,21]. Roosting animals such as bats may therefore be at a high risk as we show that roosts are rapidly detected, suggesting that protection of multiple roosting sites is needed in areas where these species are under threat owing to a loss of roosting opportunities. While our data suggest that roost switching may not be effective in reducing visitation by potential predators, we show that contrary to expectations, larger group size reduced predation risk, and should be investigated further as a predator evasion strategy by roosting species such as bats.
Acknowledgements
We thank M. Scott for fieldwork assistance. This research was supported by student grants to C.T. provided by the Ku-ring-gai Bat Conservation Society and an ARC Discovery grant no. DP0881455. This work was conducted by permission from the OEH (licence no. S10860) and Animal Care and Ethics Committee no. 08/153B. We thank M. R. Brigham and B. M. Fenton for their useful comments on the manuscript.
References
- 1.Endler JA. 1991. Interactions between predators and prey. In Behavioural ecology: an evolutionary approach (eds Krebs, J, Davies N.), pp. 169–196 Oxford, UK: Blackwell [Google Scholar]
- 2.Dawkins R, Krebs JR. 1979. Arms races between and within species. Proc. R. Soc. Lond. B 205, 489–511 10.1098/rspb.1979.0081 (doi:10.1098/rspb.1979.0081) [DOI] [PubMed] [Google Scholar]
- 3.Hughes NK, Price CJ, Banks PB. 2010. Predators are attracted to the olfactory signals of prey. PLoS ONE 5, e13114. 10.1371/journal.pone.0013114 (doi:10.1371/journal.pone.0013114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hughes NK, Banks PB. 2010. Interacting effects of predation risk and signal patchiness on activity and communication in house mice. J. Anim. Ecol. 79, 88–97 10.1111/j.1365-2656.2009.01630.x (doi:10.1111/j.1365-2656.2009.01630.x) [DOI] [PubMed] [Google Scholar]
- 5.Banks PB. 2000. Nonlinearity in the predation risk of prey mobility. Proc. R. Soc. Lond. B 267, 1621–1625 10.1098/rspb.2000.1187 (doi:10.1098/rspb.2000.1187) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitchell WA, Lima SL. 2002. Predator–prey shell games: large-scale movement and its implications for decision-making by prey. Oikos 99, 249–259 10.1034/j.1600-0706.2002.990205.x (doi:10.1034/j.1600-0706.2002.990205.x) [DOI] [Google Scholar]
- 7.Fenton MB, Rautenbach IL, Smith SE, Swanepoel CM, Grosell J, van Jaarsveld J. 1994. Raptors and bats: threats and opportunities. Anim. Behav. 48, 9–18 10.1006/anbe.1994.1207 (doi:10.1006/anbe.1994.1207) [DOI] [Google Scholar]
- 8.Sparks DW, Simmons MT, Gummer CL, Duchamp JE. 2003. Disturbance of roosting bats by woodpeckers and raccoons. Northeast Nat. 10, 105–108 10.1656/1092-6194(2003)010[0105:DORBBW]2.0.CO;2 (doi:10.1656/1092-6194(2003)010[0105:DORBBW]2.0.CO;2) [DOI] [Google Scholar]
- 9.Lewis SE. 1995. Roost fidelity of bats: a review. J. Mammal. 76, 481–496 10.2307/1382357 (doi:10.2307/1382357) [DOI] [Google Scholar]
- 10.Kunz TH. 1982. Roosting ecology of bats. In Ecology of bats (ed. Kunz TH.), pp. 1–55 New York, NY: Penum Press [Google Scholar]
- 11.Willis CKR, Brigham RM. 2007. Social thermoregulation exerts more influence than microclimate on forest roost preferences by a cavity-dwelling bat. Behav. Ecol. Sociobiol. 62, 97–108 10.1007/s00265-007-0442-y (doi:10.1007/s00265-007-0442-y) [DOI] [Google Scholar]
- 12.Boyles JG, Storm JJ. 2007. Avoidance of predator chemical cues by bats: an experimental assessment. Behaviour 144, 1019–1032 10.1163/156853907781871806 (doi:10.1163/156853907781871806) [DOI] [Google Scholar]
- 13.Lausen CL, Barclay RMR. 2002. Roosting behaviour and roost selection of female big brown bats (Eptesicus fuscus) roosting in rock crevices in southeastern Alberta. Can. J. Zool. 80, 1069–1076 10.1139/z02-086 (doi:10.1139/z02-086) [DOI] [Google Scholar]
- 14.O'Donnell CFJ, Christie JE, Hitchmough RA, Lloyd B, Parsons S. 2010. The conservation status of New Zealand bats, 2009. N. Z. J. Zool. 37, 297–311 10.1080/03014223.2010.513395 (doi:10.1080/03014223.2010.513395) [DOI] [Google Scholar]
- 15.Reckardt K, Kerth G. 2007. Roost selection and roost switching of female Bechstein's bats (Myotis bechsteinii) as a strategy of parasite avoidance. Oecologia 154, 581–588 10.1007/s00442-007-0843-7 (doi:10.1007/s00442-007-0843-7) [DOI] [PubMed] [Google Scholar]
- 16.Kerth G, Konig B. 1999. Fission, fusion and nonrandom associations in female Bechstein's bats (Myotis bechsteinii). Behaviour 136, 1187–1202 10.1163/156853999501711 (doi:10.1163/156853999501711) [DOI] [Google Scholar]
- 17.Pryde MA, O'Donnell CFJ, Barker RJ. 2005. Factors influencing survival and long-term population viability of New Zealand long-tailed bats (Chalinolobus tuberculatus): implications for conservation. Biol. Conserv. 126, 175–185 10.1016/j.biocon.2005.05.006 (doi:10.1016/j.biocon.2005.05.006) [DOI] [Google Scholar]
- 18.Hoye G. 2011. The status of microbats on Norfolk Island, southwest Pacific. In The biology and conservation of Australasian bats (eds Law B, Eby P, Lunney D, Lumsden IF.), pp. 297–307 Mosman, Australia: Royal Zoological Society of NSW [Google Scholar]
- 19.Pahl L. 1987. Feeding-behavior and diet of the common ringtail possum, Pseudocheirus peregrinus, in Eucalyptus woodlands and Leptospermum thickets in southern Victoria. Aust. J. Zool. 35, 487–506 10.1071/ZO9870487 (doi:10.1071/ZO9870487) [DOI] [Google Scholar]
- 20.Matthews A, Dickman CR, Major RE. 1999. The influence of fragment size and edge on nest predation in urban bushland. Ecography 22, 349–356 10.1111/j.1600-0587.1999.tb00572.x (doi:10.1111/j.1600-0587.1999.tb00572.x) [DOI] [Google Scholar]
- 21.Harris D. 2009. Review of negative effects of introduced rodents on small mammals on islands. Biol. Invasions 11, 1611–1630 10.1007/s10530-008-9393-0 (doi:10.1007/s10530-008-9393-0) [DOI] [Google Scholar]


