Abstract
Many amphibian lineages show terrestrialization of their reproductive strategy and breeding is partially or completely independent of water. A number of causal factors have been proposed for the evolution of terrestrialized breeding. While predation has received repeated attention as a potential factor, the influence of other factors such as habitat has never been tested using appropriate data or methods. Using a dataset that comprises 180 amphibian species from various East African habitats, we tested whether species occurring in different habitats show different patterns of terrestrialization in their breeding strategy. We recovered a significant association between terrestrialized breeding strategies and forest habitats. In general, forest seems to act as a facilitator, providing a permissive environment for the evolution of terrestrialized breeding strategies. However, while terrestrial oviposition is strongly correlated with lowland and montane forest habitat, complete terrestrial development is significantly correlated with montane forest only, indicating different selective pressures acting at different steps towards complete terrestrial development.
Keywords: direct development, Eastern Arc Mountains, reproductive mode, topography, viviparity
1. Introduction
Variations in life-history traits are known to be strongly associated with habitat [1–3]. This is evident from strategies adopted by individuals in a population along environmental gradients [4,5] and, on a broader scale, among taxa dispersed along altitudinal or latitudinal gradients or across habitats [6,7]. Investigating the ecological factors associated with the distribution of organisms with differing life-history strategies provides an opportunity to elucidate selective factors favouring particular life-history strategies in different environments.
Among major groups of vertebrates, amphibians exhibit by far the greatest diversity of reproductive strategies and have departed in many ways from the ancestral state of aquatic eggs and larvae that metamorphose into a more or less terrestrial adult [8]. For anurans alone, 39 reproductive modes have been described that have different combinations of traits, including oviposition site, developmental characters, larval habitat and the degree of parental care [8–10]. Thirty of the 39 described modes are characterized by some degree of terrestrial reproduction.
Globally, extant amphibian assemblages display differences in life-history strategies, possibly as an adaptive response to local conditions [11]. A number of hypotheses have been put forward to explain the various modes of terrestrial reproduction in amphibians in general and particularly in anurans. Lutz [12] and Tihen [13] suggested that the driving factor for the evolution of terrestrial egg deposition was predation on aquatic eggs and larvae, and plasticity in life-history traits as a response to predation is now well documented [5,14,15]. Others stressed the influence of the physical environment on the evolution of terrestrial reproductive modes in amphibians (e.g. topography [16]; forest habitats [17]). Several recent studies have found a correlation between the diversity of reproductive modes in amphibians and the amount of rainfall, with more terrestrialized reproductive modes generally being present in more humid areas [18,19].
We analysed the distribution of amphibian species and their reproductive strategies across the lowland and highlands of East Africa, a region with a diverse array of habitats, including the Eastern Arc Mountains with montane grasslands and forests, and a broad range of different lowland habitats [20]. The high diversity of species, varying reproductive strategies, and different habitat types in East Africa makes it a suitable system for testing the influence of habitat on the evolution of terrestrialization of reproductive strategies. More specifically, we tested whether terrestrialized breeding strategies are evenly distributed or significantly associated with particular environments.
2. Material and methods
(a). Species sampling and breeding biology
We assembled a dataset of 166 anuran and 14 caecilian species of the East African coastal lowlands and the Eastern Arc Mountain chain, based on species lists and field survey data (see the electronic supplementary material). We assigned species to one of four habitat types—lowland forest, lowland non-forest, montane forest and montane grasslands—based on information from IUCN [21], Poynton et al. [22] and our own assessment of the taxa (see the electronic supplementary material).
Information on breeding biology was taken from the literature, particularly Channing & Howell [23] and the global amphibian assessment database [21], and references therein. We used a three state coding scheme to categorize breeding biology: 0—aquatic eggs and larvae, 1—terrestrial eggs and aquatic larvae, 2—complete terrestrial development.
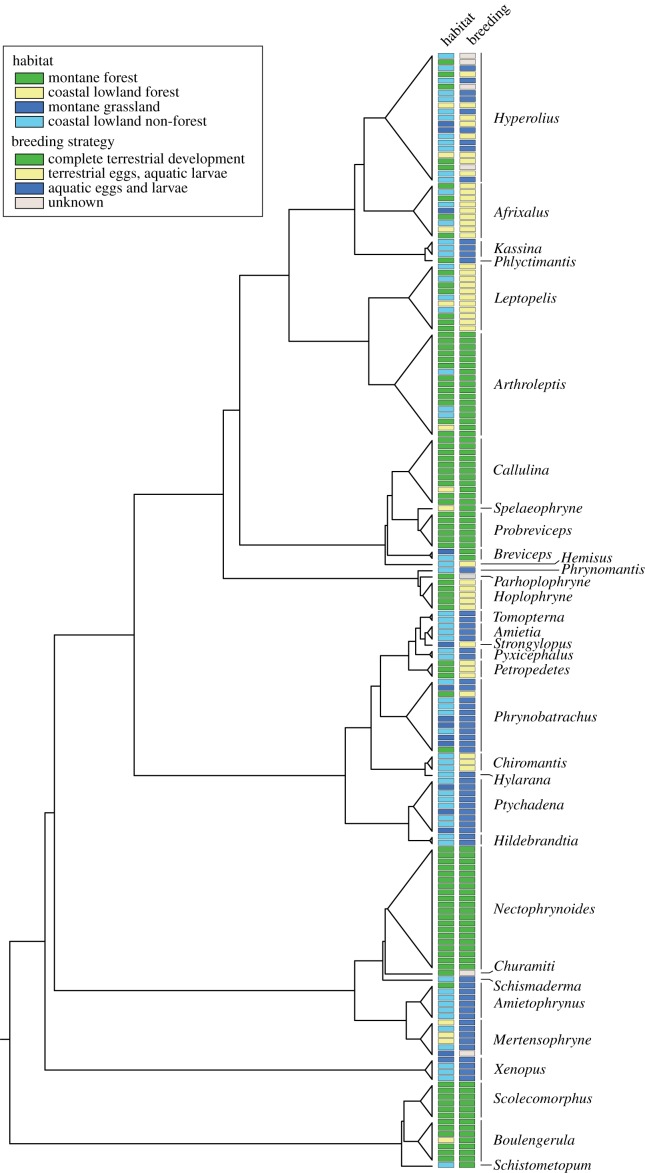
Of the 180 amphibians included, 64 are predominantly non-forest coastal lowland species, 11 coastal lowland forest species, 90 montane forest species and 15 montane grassland species (see figure 1 and electronic supplementary material). Sixty species were categorized as aquatic, 42 as semi-terrestrial and 71 as completely terrestrial breeders. The breeding biology of seven species was unknown (see figure 1 and electronic supplementary material).
Figure 1.
Phylogeny and phylogenetic distribution of habitat preference and breeding biology of East African amphibians. (Online version in colour.)
(b). Comparative analysis of breeding biology
We assembled a phylogeny for all East African taxa (see the electronic supplementary material for details). Correlates of breeding strategy and habitat types were identified using a phylogenetic generalized least-squares approach [25] using the package APE [26] in R v. 2.13.0 [27]. The regression models correct for phylogenetic non-independence by implementing a Brownian motion (BM), a Pagel's lambda (λ) or an Ornstein–Uhlenbeck (OU) error structure. Akaike Information Criterion (AIC) scores of each regression were compared (models with ΔAIC > 2 were deemed as acceptable alternative models). A number of different analyses were performed to explore potential bias in the data (see the electronic supplementary material).
Our coding system for the breeding biology of amphibians is based on two traits: place of egg deposition and larval habitat. To test whether the evolution of these two traits is correlated with a particular environment, any habitat recovered as having a significant correlation with breeding strategy was carried forward, and correlated evolution was tested using the DISCRETE module in BayesTraits [28]. Both likelihood and Bayesian approaches were implemented, and likelihood ratio (LR) and Bayes factor (BF) scores of models where habitat and life-history traits evolve dependently or independently of each other were compared. LR scores follow a χ2 distribution with 4 d.f., and a difference in BF scores greater than 10 was considered as strong evidence in favour of one model over the other (see the electronic supplementary material for model settings).
The sequence alignment, phylogeny and all comparative analysis datasets were deposited in the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.8f74d [24].
3. Results
Habitat type and breeding biology contain a phylogenetic signal (λ = 0.635 and λ = 0.985, respectively). Regression models that incorporate a λ error structure outperformed the BM and OU models, with an AIC score of 46.735 over the BM and OU scores of 93.847 and 51.005, respectively. The λ model shows that, against non-forest lowland habitats, lowland and montane forests have a significant, positive effect on the terrestrialization of breeding biology. Montane grasslands have no effect on terrestrialized breeding, indicating that altitude as such does not appear to be associated with terrestrialized reproduction (table 1).
Table 1.
Phylogenetic generalized least-squares regression implementing a Pagel's lambda model of evolution to test the effect of habitat on breeding biology.
| coefficient ± s.e. | t-value | p-value | |
|---|---|---|---|
| Pagel's lambda model; λ = 0.635, AIC = 46.735 | |||
| intercept | 1.204±0.773 | 1.557 | 0.121 |
| coastal lowland forest | 0.256±0.071 | 3.582 | <0.001 |
| montane forest | 0.230±0.052 | 4.429 | <0.001 |
| montane grassland | 0.030±0.061 | 0.489 | 0.625 |
Because both types of forest have a positive effect on terrestrialization of breeding strategy, both were carried forward to the BayesTraits analysis to test for correlated evolution of habitat and either terrestrial oviposition or terrestrial larval development (including direct development, ovoviviparity and viviparity). LR and log-BF tests demonstrate significant correlations between terrestrial egg-laying and both montane and lowland forest habitat (LR = 36.221, p < 0.001, BF = 22.454 and LR = 10.922, p < 0.05, BF = 11.696, respectively; table 2). Furthermore, the likelihood analyses reveal that montane forest is also significantly correlated with terrestrial larval development (LR = 12.512, p < 0.05, although this conclusion is not supported by the Bayesian analysis, BF =−1.776; table 2), whereas both likelihood and Bayesian analyses indicate no correlation between terrestrial larval development and lowland forest (LR = 0.154, p = 0.997, BF = 4.125). The BayesTraits analyses robustly indicate that forest in general is linked to the evolution of terrestrial egg deposition. Additional, somewhat more equivocal evidence suggests that the evolution of terrestrial larval development is associated specifically with montane, but not with lowland forest. These results remain robust even when excluding newly discovered species and also when excluding viviparous and ovoviviparous species, all of which are predominately found in montane forest areas (see the electronic supplementary material).
Table 2.
Correlated evolution of breeding strategy and habitat in BayesTraits-DISCRETE showing log likelihood scores and harmonic means for independent and dependent evolution of traits.
| log likelihood |
likelihood ratio | p-value | MCMC harmonic mean |
Bayes factor | |||
|---|---|---|---|---|---|---|---|
| independent | dependent | independent | dependent | ||||
| terrestrial egg—montane forest | −140.556 | −122.445 | 36.221 | <0.001 | −145.416 | −134.189 | 22.454 |
| terrestrial egg—coastal lowland forest | −92.491 | −87.029 | 10.922 | <0.05 | −104.587 | −98.739 | 11.696 |
| terrestrial larva—montane forest | −100.574 | −94.318 | 12.512 | <0.05 | −107.237 | −108.125 | −1.776 |
| terrestrial larva—coastal lowland forest | −52.509 | −52.432 | 0.154 | 0.997 | −71.978 | −69.916 | 4.125 |
4. Discussion
Many amphibian species worldwide show partly or fully terrestrialized modes of reproduction. However, until now the link between habitat and terrestrialization of amphibian life history had not been assessed quantitatively within a comparative phylogenetic and geographical framework. Our analysis recovered forest as the best predictor of the distribution of amphibians with terrestrialized reproductive modes in East Africa. This suggests that forest may play a role in the evolution and maintenance of terrestrialized reproductive modes, assuming a stable association between species and their habitat throughout their evolutionary history. This study does not support or reject hypotheses on the precise causal factors that drive the evolution of different breeding strategies, but it is the first study to quantify the trend observed in previous studies that terrestrial forms of breeding are associated with particular environments [16,17].
Terrestrial egg-laying in East Africa is strongly correlated with forest habitat of any kind, which suggests that common biotic and/or abiotic factors of low- and highland forests promote terrestrial egg-laying. Humidity has recently been shown to influence the occurrence of terrestrial breeders [5,18,19]. Forest may be instrumental in providing humidity levels permissive for the evolution of terrestrial oviposition, e.g. owing to a lower risk of egg desiccation. At the same time complete terrestrial development is associated with montane forest only, suggesting selective factors that are unique to that environment. Topographic complexity and the availability of aquatic breeding sites are different in lowland and montane forests, and might explain the observed differences in developmental habitat. Montane forest habitats are generally characterized by a paucity of standing bodies of water and, at least at times, by swift-flowing streams, both of which might exert strong selective pressures against aquatic larvae and thus promote complete terrestrial development (including viviparity and ovoviviparity; [29]). Interestingly, dragonflies, damselflies and water beetles (whose larvae are important predators of amphibian larvae) show similar patterns of terrestrial breeding specialization in relation to montane forest habitats [30–32]. We conclude that terrestrially breeding East African amphibians have strong affinities with forests, particularly montane forests, and we predict that analyses in other regions will produce broadly similar results.
Acknowledgements
This research was supported by the Swiss National Science Foundation (31003A-133067) to S.P.L. and a Volkswagen Postdoctoral Fellowship, a Putnam Expedition grant (Museum of Comparative Zoology, Harvard University) and an ESF ‘Frontiers of Speciation Research’ grant to H.M. We thank the Tanzania Commission for Science and Technology (COSTECH research permit RCA 2007-153, RCA 2004-335-ER-98-13, RCA 2009-306-NA-2009-201), the Tanzania Wildlife Research Institute (TAWIRI), the Wildlife Division for issuing necessary permits, and David Gower and Terry Ord for helpful comments on early drafts.
References
- 1.MacArthur RH, Wilson EO. 1967. The theory of island biogeography. Princeton, NJ: Princeton University Press [Google Scholar]
- 2.Stearns SC. 1980. A new view of life-history evolution. Oikos 35, 266–281 10.2307/3544434 (doi:10.2307/3544434) [DOI] [Google Scholar]
- 3.Via S, Lande R. 1985. Genotype-environment interaction and the evolution of phenotypic plasticity. Evolution 39, 505–522 10.2307/2408649 (doi:10.2307/2408649) [DOI] [PubMed] [Google Scholar]
- 4.Morrison C, Hero J-M. 2003. Geographic variation in life-history characteristics of amphibians: a review. J. Anim. Ecol. 72, 270–279 10.1046/j.1365-2656.2003.00696.x (doi:10.1046/j.1365-2656.2003.00696.x). [DOI] [Google Scholar]
- 5.Touchon JC, Warkentin KM. 2008. Reproductive mode plasticity: aquatic and terrestrial oviposition in a treefrog. Proc. Natl Acad. Sci. USA 105, 7495–7499 10.1073/pnas.0711579105 (doi:10.1073/pnas.0711579105). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stearns SC, Koella JC. 1986. The evolution of phenotypic plasticity in life-history traits—predictions of reaction norms for age and size at maturity. Evolution 40, 893–913 10.2307/2408752 (doi:10.2307/2408752) [DOI] [PubMed] [Google Scholar]
- 7.Purcell J. 2011. Geographic patterns in the distribution of social systems in terrestrial arthropods. Biol. Rev. 86, 475–491 10.1111/J.1469-185x.2010.00156.X (doi:10.1111/J.1469-185x.2010.00156.X). [DOI] [PubMed] [Google Scholar]
- 8.Duellman WE, Trueb L. 1986. Biology of amphibians. New York, NY: McGrawn-Hill [Google Scholar]
- 9.Haddad C, Prado C. 2005. Reproductive modes in frogs and their unexpected diversity in the Atlantic forest of Brazil. Bioscience 55, 207–217 10.1641/0006-3568(2005)055[0207:RMIFAT]2.0.CO;2 (doi:10.1641/0006-3568(2005)055[0207:RMIFAT]2.0.CO;2) [DOI] [Google Scholar]
- 10.Wells KD. 2007. The ecology and behavior of amphibians. Chicago, IL: University of Chicago Press [Google Scholar]
- 11.Ernst R, Keller A, Landburg G, Grafe TU, Linsenmair KE, Rödel M-O, Dziock F. 2012. Common ancestry or environmental trait filters: cross-continental comparisons of trait–habitat relationships in tropical anuran amphibian assemblages. Glob. Ecol. Biogeogr. 21, 704–715 10.1111/j.1466-8238.2011.00719.x (doi:10.1111/j.1466-8238.2011.00719.x) [DOI] [Google Scholar]
- 12.Lutz B. 1948. Ontogenetic evolution in frogs. Evolution 2, 29–39 10.2307/2405613 (doi:10.2307/2405613) [DOI] [PubMed] [Google Scholar]
- 13.Tihen J. 1960. Comments on the origin of the amniote egg. Evolution 14, 528–531 10.2307/2406002 (doi:10.2307/2406002) [DOI] [Google Scholar]
- 14.Vonesh JR. 2005. Egg predation and predator-induced hatching plasticity in the African reed frog, Hyperolius spinigularis. Oikos 110, 241–252 10.1111/j.0030-1299.2005.13759.x (doi:10.1111/j.0030-1299.2005.13759.x) [DOI] [Google Scholar]
- 15.Touchon JC, Gomez-Mestre I, Warkentin KM. 2006. Hatching plasticity in two temperate anurans: responses to a pathogen and predation cues. Can. J. Zool. 84, 556–563 10.1139/Z06-58 (doi:10.1139/Z06-58) [DOI] [Google Scholar]
- 16.Goin OB, Goin CJ. 1962. Amphibian eggs and montane environment. Evolution 16, 364–371 10.2307/2406285 (doi:10.2307/2406285) [DOI] [Google Scholar]
- 17.Poynton J. 1964. Relationship between habitat and terrestrial breeding in amphibians. Evolution 18, 131. 10.2307/2406429 (doi:10.2307/2406429) [DOI] [Google Scholar]
- 18.da Silva FR, Almeida-Neto M, do Prado VHM, Haddad CFB, de Cerqueira Rossa-Feres D. 2012. Humidity levels drive reproductive modes and phylogenetic diversity of amphibians in the Brazilian Atlantic Forest. J. Biogeogr. 39, 1720–1732 10.1111/j.1365-2699.2012.02726.x (doi:10.1111/j.1365-2699.2012.02726.x) [DOI] [Google Scholar]
- 19.Gomez-Mestre I, Pyron RA, Wiens JJ. 2012. Phylogenetic analyses reveal unexpected patterns in the evolution of reproductive modes in frogs. Evolution 66, 3687–3700 10.1111/j.1558-5646.2012.01715.x (doi:10.1111/j.1558-5646.2012.01715.x) [DOI] [PubMed] [Google Scholar]
- 20.Mittermeier RA, Gil RP, Hoffman M, Pilgrim J, Brooks T, Mittermeier CG, Lamoreux J, Fonseca GAB. 2005. Hotspots revisited: earth's biologically richest and most endangered terrestrial ecoregions, 2 edn Boston, MA: University of Chicago Press [Google Scholar]
- 21.IUCN 2011. Conservation international and natureserve. Global amphibian assessment. See http://www.iucnredlist.org/amphibians
- 22.Poynton JC, Loader SP, Sherratt E, Clarke BT. 2007. Amphibian diversity in East African biodiversity hotspots: altitudinal and latitudinal patterns. Biodivers. Conserv. 16, 1103–1118 10.1007/s10531-006-9074-1 (doi:10.1007/s10531-006-9074-1). [DOI] [Google Scholar]
- 23.Channing A, Howell KM. 2006. Amphibians of East Africa, p. 418 Ithaca, NY: Cornell University Press [Google Scholar]
- 24.Müller H, Liedtke HC, Menegon M, Beck J, Ballesteros-Mejia L, Nagel P, Loader SP. 2013. Data from: forests as promoters of terrestrial life history strategies in East African amphibians. 10.5061/dryad.8f74d (doi:10.5061/dryad.8f74d) [DOI] [PMC free article] [PubMed]
- 25.Martins EP, Hansen TF. 1997. Phylogenies and the comparative method: a general approach to incorporating phylogenetic information into the analysis of interspecific data. Am. Nat. 149, 646–667 10.1086/286013 (doi:10.1086/286013) [DOI] [Google Scholar]
- 26.Paradis E, Claude J, Strimmer K. 2004. APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20, 289–290 10.1093/bioinformatics/btg412 (doi:10.1093/bioinformatics/btg412) [DOI] [PubMed] [Google Scholar]
- 27.R Core Team 2012. R: a language and enviroment for statistical computing. Vienna, Austria: R foundation for statistical computing; See http://www.R-project.org/ [Google Scholar]
- 28.Pagel M. 1994. Detecting correlated evolution on phylogenies: a general method for the comparative analysis of discrete characters. Proc. R. Soc. Lond. B 255, 37–45 10.1098/rspb.1994.0006 (doi:10.1098/rspb.1994.0006) [DOI] [Google Scholar]
- 29.Campbell JA, Duellman WE. 2000. New species of stream-breeding hylid frogs from the northern versant of the highlands of Oaxaca, Mexico. Sci. Pap., Nat. Hist. Mus., The University of Kansas, 16, 1–28 [Google Scholar]
- 30.Willey RL. 1955. A terrestrial damselfly nymph (Megapodagrionidae) from New Caledonia. Psyche 62, 137–144 10.1155/1955/39831 (doi:10.1155/1955/39831). [DOI] [Google Scholar]
- 31.Watson JAL. 1982. A truly terrestrial dragonfly larva from Australia (Odonata, Corduliidae). J. Aust. Entomol. Soc. 21, 309–311 10.1111/j.1440-6055.1982.tb01826.x (doi:10.1111/j.1440-6055.1982.tb01826.x) [DOI] [Google Scholar]
- 32.Balke M, Hendrich L. 1996. A new species of the terrestrial water beetle genus Geodessus Brancucci (Coleoptera: Dytiscidae), sieved from leaf litter in southern India. Aquat. Insects 18, 91–99 10.1080/01650429609361607 (doi:10.1080/01650429609361607) [DOI] [Google Scholar]