Abstract
Emperor penguins Aptenodytes forsteri are able to survive the harsh Antarctic climate because of specialized anatomical, physiological and behavioural adaptations for minimizing heat loss. Heat transfer theory predicts that metabolic heat loss in this species will mostly depend on radiative and convective cooling. To examine this, thermal imaging of emperor penguins was undertaken at the breeding colony of Pointe Géologie in Terre Adélie (66°40′ S 140° 01′ E), Antarctica in June 2008. During clear sky conditions, most outer surfaces of the body were colder than surrounding sub-zero air owing to radiative cooling. In these conditions, the feather surface will paradoxically gain heat by convection from surrounding air. However, owing to the low thermal conductivity of plumage any heat transfer to the skin surface will be negligible. Future thermal imaging studies are likely to yield further insights into the adaptations of this species to the Antarctic climate.
Keywords: metabolic heat loss, thermal imaging, thermoregulation, Antarctic
1. Introduction
Emperor penguins, Aptenodytes forsteri, experience one of the most severe environments on Earth. It is the only species of bird breeding during the Antarctic winter, where air temperature may reach as low as −40°C and wind speed as high as 40 m s−1 [1]. Males spend four months without feeding during pairing and incubation. Their thermoneutral zone extends from −10°C to +20°C, and during incubation core temperature is maintained around 36.9°C [1–3]. Emperor penguins are able to survive Antarctic conditions owing to specialized anatomical, physiological and behavioural adaptations for minimizing heat loss [4]. Penguin plumage provides more than 80 per cent of total insulation [5], and is highly resistant to wind penetration [6,7]. In most birds, plumage is able to resist the flow of heat, such that surface temperature is normally a few degrees above ambient temperature [8]. However, under certain conditions animal pelage can cool to below air temperature [9,10]. The aim of this study was, therefore, to determine surface temperature variation in free ranging emperor penguins, and to predict the direction and magnitude of heat fluxes from different body parts. This will improve our understanding of the effect of weather and climate on the energetics of this species.
2. Material and methods
Thermal imaging of emperor penguins was undertaken at the breeding colony of Pointe Géologie, Dumont d'Urville, in Terre Adélie (66°40′ S 140° 01′ E), Antarctica from 4 June to 29 June 2008. A thermal imaging camera (P25 FLIR Systems) recorded infrared images, and digital photographs were also taken (K10, Pentax) at a minimum distance of 10 m (figure 1a). Air temperature, relative humidity and wind speed were recorded at 1 min intervals and cloud cover (oktas, [11]) at 3 h intervals at Dumont d'Urville meteorological station. Air temperature Ta (°C) and wind speed, u (m s−1) at the colony (height = 1 m) was adjusted from station readings Tmet and umet according to: Ta = 0.919 × Tmet and u = 0.561 × umet [12].
Figure 1.
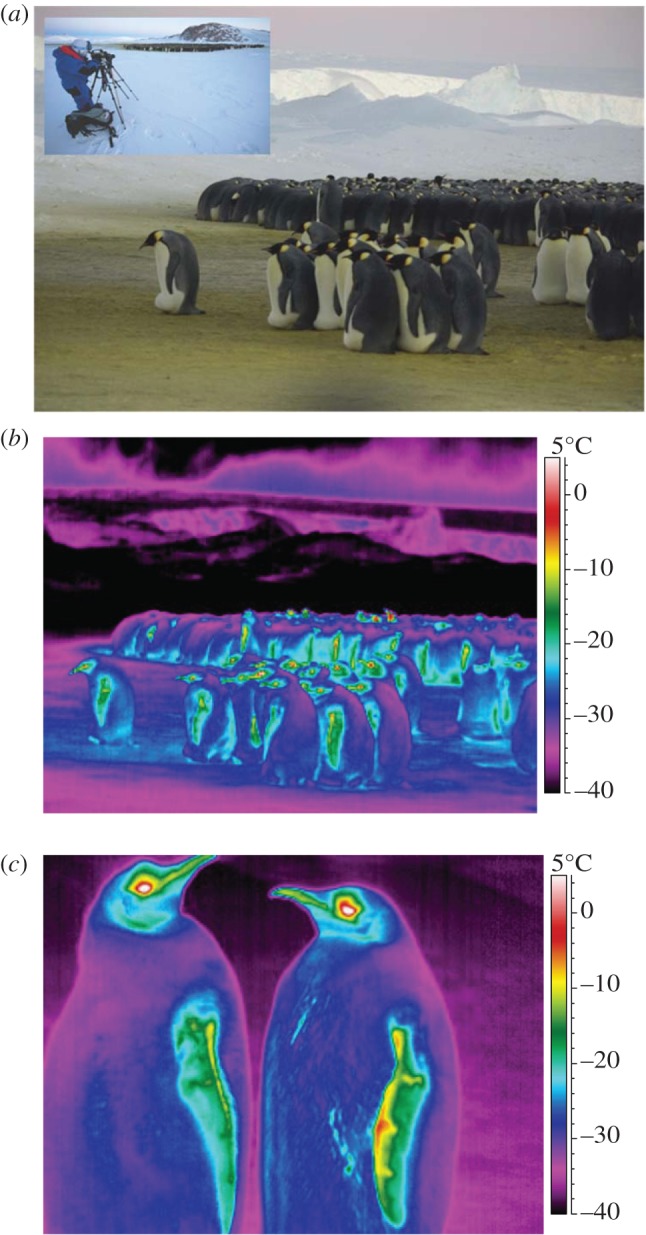
(a) Emperor penguin colony and thermal imaging (inset). (b) Thermal image of isolated and huddling penguins (Tair =−21.0°C, RH = 42%, u = 1.0 m s−1 and cloud cover = 0 oktas). (c) Close-up images of the head and flippers (Tair =−21.8°C, RH = 43%, u = 3.0 m s−1 and cloud cover = 2 oktas). (Online version in colour.)
Surface temperatures were obtained for 40 males that were separated by at least one body length (figure 1b). Individuals were selected that were stationary or shuffling (no feet exposed) or walking slowly (feet seen). Mean surface temperature of different body parts (dorsal, ventral, flipper, head and feet: emissivity = 0.98) was determined using image analysis software (ThermaCAM Reporter v. 7.0 see [13]). Surface temperature of surrounding ice (emissivity = 0.97) was determined from three spot measurements taken at one body length from the bird.
A heat transfer model was used to estimate the direction and relative magnitude of heat fluxes from different body regions (see the electronic supplementary material). Statistics were computed using IBM SPSS v. 19.
3. Results
The mean air temperature ± standard error was −17.6 ± 0.71°C, relative humidity 44.4 ± 1.69%, cloud cover 1.5 ± 0.15 oktas and wind speed 2.3 ± 0.26 m s−1. The mean temperature of the ice was −29.1 ± 0.11°C. On 3 days when air temperature was below −20°C, ice and sky temperature over-ranged beyond −45°C (below the recording range of the camera). The corresponding surface temperature of dorsal, ventral, head, flippers and feet were −23.2 ± 0.94°C, −21.8 ± 1.06°C, −18.8 ± 1.02°C, −17.0 ± 0.92°C and −16.8 ± 1.31°C, respectively. There was a significant difference in surface temperature between different body parts (generalized linear model, GLM F4,155 = 9.78, p < 0.001). Dorsal and ventral surface temperatures were similar (Tukey's test p = 0.95), but were significantly lower than the surface temperature of head, flippers and exposed feet (p ≤ 0.01 in all cases). Close-up images revealed that almost all the external body surfaces were below freezing, with the exception of the eye region (figure 1c). Only the inner edges of the upper and lower mandibles were around 10°C above air temperature, but outer regions were close to ambient. Overall, the mean bill temperature was −17.3 ± 1.17°C and was within 0.23 ± 0.73°C of air temperature.
The temperature gradient between plumage surface and air temperature varied between different body parts (F4,156 = 52.6, p < 0.001, r2 = 0.58). Mean dorsal and ventral surface temperatures were 4–4.8°C below ambient, whereas the head, flippers and feet were 0.4–1.9°C above air temperature (figure 2a).
Figure 2.
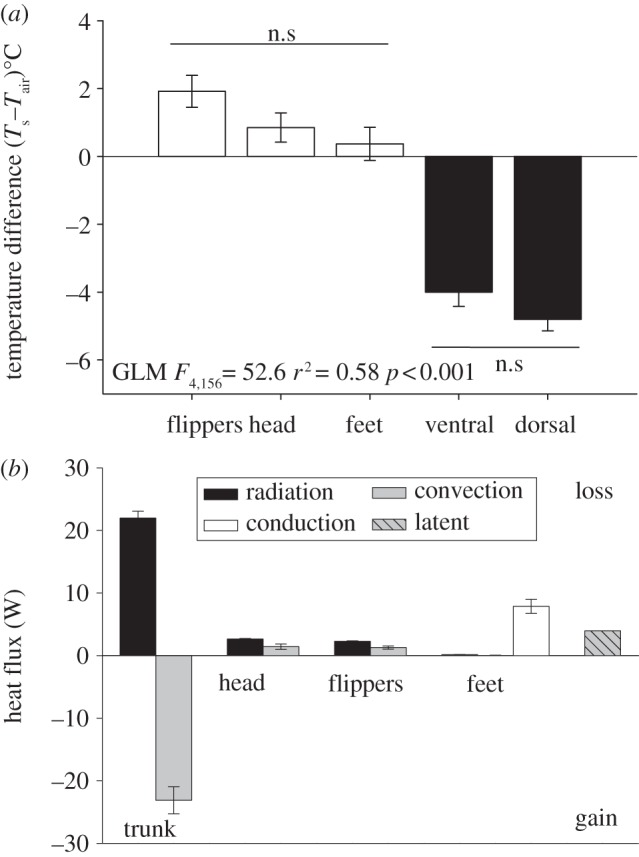
(a) Mean (±s.e.) temperature difference between surface (Ts) and air (Tair) for different body parts of emperor penguins (n = 40). Horizontal lines indicate no significant (n.s.) difference between body regions. (b) Corresponding surface heat fluxes from different parts of the body. Positive and negative values represent heat loss and gain from the surface, respectively.
A GLM was used to account for temperature variation of each body part, where incubation status and activity (stationary versus moving) were entered as factors. Time, air and ice temperature, wind speed, and cloud cover were covariates. Relative image size (number of pixels) was entered as a covariate to account for measurement distance. There were only four incubating birds that exposed their feet, and so incubation was not included in this model. The above variables explained most of the variance in surface temperature (partial η2 = 0.87–0.94, p < 0.001). Air temperature was the only significant explanatory variable for dorsal, ventral and head temperature, and surface temperature was positively correlated with air temperature (η2 = 0.37–0.60 p < 0.001 in all cases). However, flipper and feet temperature were positively correlated with air temperature (flipper: η2 = 0.47, p < 0.001, feet: η2 = 0.47, p < 0.007) and negatively correlated with wind speed (flipper: η2 = 0.37, p < 0.04, feet: η2 = 0.34, p < 0.03).
The heat transfer model (see the electronic supplementary material) showed that radiative heat loss was greatest from the body trunk 22.0 ± 1.08 W, followed by head 2.6 ± 0.10 W, flipper 2.3 ± 0.07 W and feet 0.15 ± 0.03 W (figure 2b). Convective heat losses for head, flipper and feet were small and averaged 1.4 ± 0.44, 1.3 ± 0.24 and 0.02 ± 0.03 W, respectively. The trunk surface was predicted to gain 23.1 ± 2.16 W by convection from surrounding warmer air. Conduction from the feet averaged 7.9 ± 1.12 W and latent heat loss was constant at 4.0 W (figure 2b).
Assuming small convective heat gains are not transmitted to the skin, total heat loss was estimated to be 41.6 ± 1.75 W. Total heat loss from the trunk, head, flippers and feet averaged 22.0 ± 1.08, 4.0 ± 0.52, 3.6 ± 0.29 and 8.0 ±1.16 W, respectively. Therefore, on average 50 per cent of metabolic heat was lost from the trunk, 40 per cent from head, flippers and feet together and 10 per cent through latent heat loss.
4. Discussion
Infrared thermography provided a remote and non-invasive temperature measurement well-suited to a species such as the emperor penguin. Thick plumage has excellent insulation and only unfeathered regions (feet, eye and bill) and sparsely feathered flippers showed heat loss from the body interior. Heat loss in penguins is minimized by counter-current heat exchange systems through arterio-venous networks in the head, axillae and legs. In particular, the post-orbital rete mirabile functions as a heat exchanger in the eye, nasal passages and jaw muscles [14]. Emperor penguins have relatively small bills in proportion to their body size, and small beaks have been selected to minimize heat loss [15]. Surface temperature of the pedal phalanges and webs were well below freezing. However, their thick scaly skin affords good protection from radiative cooling and contact with ice. Previously tarsus temperature has been recorded to be a few degrees above 0°C [16,17].
The proximal edge of the flipper was relatively warm, reflecting the fact that blood vessels here are less well insulated [14,18,19]. However, the humeral arterial plexus is particularly well adapted for the efficient redirection of heat to the core. Among penguins, the emperor penguin has the greatest number of arteries in this region, providing efficient counter-current heat exchange [20].
Temperature is a function of the mean kinetic energy of molecules in a system and determines the direction of heat flux, whereas heat is a function of the total kinetic energy of all molecules in a system. Temperature is, therefore, dependent on the sum of heat fluxes at the surface. The radiative temperature of the sky is 10–20°C below air temperature for an overcast and clear sky, respectively [11]. Therefore, in this study, penguins were radiating to a sky in excess of −40°C (figure 1b). Thermographic measurements are usually made where surroundings are at air temperature and, therefore, plumage is close to ambient [13]. Most emperor penguin surfaces were lower than air temperature and well below freezing. This phenomenon is due to extreme radiative cooling of the surface, removing heat to the extent that the internal energy of the system (temperature) is less than that of air. Measurements on sheep exposed to a clear night sky have shown the fleece to be at least 2.5°C below air temperature. Experiments on pigeons, Columba livia, have recorded temperature differences of 5°C [9,10]. On a clear night, a sheep's fleece can accumulate water (and release latent heat) by condensation when the fleece temperature drops below the dew point of air [9]. Ice will form when the surface cools below the frost point. In this study, the plumage was on average just above frost point (−24.2°C) and no ice was seen on feathers. Snow/ice on the backs of animals have however been reported during blizzards [3].
The temperature variation of feathered regions was mostly explained by air temperature alone while flipper and feet temperatures were also dependent on wind speed. Wind is unable to penetrate penguin plumage at low wind speeds, explaining the fact that metabolic rate remains constant below 5 m s−1 [1]. Measurements were made in relatively moderate conditions for Antarctica in order to protect the camera. Free ranging emperor penguins have a metabolic rate of around 1.5 W kg−1 or 45 W for a 30 kg penguin [21]. Our heat transfer model (see the electronic supplementary material), therefore, provided a realistic estimate of metabolic heat loss, and showed that radiative heat loss dominated in clear and relatively calm conditions. The trunk comprised 80 per cent of total surface area and more than half of their metabolic heat production was lost largely through radiation. Radiative cooling also occurred from the head and flippers, contributing 10 per cent of heat loss from each of these body parts. However, head and flippers were important sites of heat exchange, as their radiative heat flux density was 63–65 W m−2 compared with only 47 W m−2 from the well insulated trunk.
The cool trunk was predicted to gain around 20 W by convective warming from the surrounding air. Although convective heat transfer to the plumage surface will occur, it is expected that little of this heat will reach the skin. Penguin plumage has a low thermal conductivity [22] and even with strong solar warming changes in avian skin temperature are minimal [23,24]. Despite higher flipper and head temperature, their estimated convective heat loss was small. In windy cloudy conditions, we would expect the feather surface to be above air temperature, leading to large convective heat losses if penguins do not huddle [12]. Emperor penguins lost around 20 per cent of their metabolic heat by conduction when they rested on both feet. However, these higher rates of heat loss may be transient while walking. By resting on their tarsometatarsus joint, emperor penguins were predicted to reduce conduction to no more than 5 per cent of metabolic heat loss, similar to minimum rates of heat loss in other species [25]. In conclusion, thermal imaging clearly showed that the surface temperature and energy balance of emperor penguins is dependent on spatial variation in their insulation and its interaction with environmental conditions.
Acknowledgements
All procedures were approved by the Ethical Committee of the IPEV (French Polar Institute Paul Emile Victor) and by the Scientific Committee of the IPEV, following the Scientific Committee for Antarctic Research Code of conduct.
Thanks to support of the Dumont d'Urville expedition members in 2008, especially Luc Piard and Cyril Nahon who repaired the battery of the thermal imaging camera. Margaret Reilly, Hunterian Zoology Museum kindly provided the penguin specimen for the heat transfer model.
References
- 1.Le Maho Y, Delclitte P, Chatonnet J. 1976. Thermoregulation in fasting emperor penguins. Am. J. Physiol. 231, 913–922 [DOI] [PubMed] [Google Scholar]
- 2.Pinshow B, Fedak MA, Battles DR, Schmidt-Nielsen K. 1976. Energy-expenditure for thermoregulation and locomotion in emperor penguins. Am. J. Physiol. 231, 903–912 [DOI] [PubMed] [Google Scholar]
- 3.Gilbert C, Le Maho Y, Perret M, Ancel A. 2007. Body temperature changes induced by huddling in breeding male emperor penguins. Am. J. Physiol. Regul. Int. Comp. Physiol. 292, R176–R185 10.1152/ajpregu.00912.2005 (doi:10.1152/ajpregu.00912.2005) [DOI] [PubMed] [Google Scholar]
- 4.Prévost J. 1961. Ecologie du Manchot Empereur. Paris, France: Expéditions Polaires Françaises: Hermann Press [Google Scholar]
- 5.Jarman M. 1973. Experiments on the emperor penguin, Aptenodytes forsteri, in various thermal environments. Bull. Br. Antartic. Surv. 33–34, 57–63 [Google Scholar]
- 6.Taylor JRE. 1986. Thermal insulation of the down and feathers of Pygoscelid penguin chicks and the unique properties of penguin feathers. Auk 103, 160–168 [Google Scholar]
- 7.Stahel CD, Nicol SC, Walker GJ. 1987. Heat-production and thermal-resistance in the little penguin Eudyptula minor in relation to wind-speed. Physiol. Zool. 60, 413–423 [Google Scholar]
- 8.McCafferty DJ, Moncrieff JB, Taylor IR, Boddie GF. 1998. The use of IR thermography to measure the radiative temperature and heat loss of a barn owl (Tyto alba). J. Therm. Biol. 23, 311–318 10.1016/S0306-4565(98)00022-9 (doi:10.1016/S0306-4565(98)00022-9) [DOI] [Google Scholar]
- 9.McArthur AJ. 1991. Thermal-radiation exchange, convection and the storage of latent-heat in animal coats. Agric. Forest Meterol. 53, 325–336 10.1016/0168-1923(91)90050-Z (doi:10.1016/0168-1923(91)90050-Z) [DOI] [Google Scholar]
- 10.Leger J, Larochelle J. 2006. On the importance of radiative heat exchange during nocturnal flight in birds. J. Exp. Biol. 209, 103–114 10.1242/jeb.01964 (doi:10.1242/jeb.01964) [DOI] [PubMed] [Google Scholar]
- 11.Monteith JL, Unsworth MH. 1990. Principles of environmental physics. London, UK: Edward Arnold [Google Scholar]
- 12.Gilbert C, Robertson G, Le Maho Y, Ancel A. 2008. How do weather conditions affect the huddling behaviour of emperor penguins? Polar Biol. 31, 163–169 10.1007/s00300-007-0343-6 (doi:10.1007/s00300-007-0343-6) [DOI] [Google Scholar]
- 13.McCafferty DJ, Gilbert C, Paterson W, Pomeroy PP, Thompson D, Currie J, Ancel A. 2011. Estimating metabolic heat loss in birds and mammals by combining infrared thermography with biophysical modelling. Comp. Biochem. Physiol. A Mol. Integ. Physiol. 158, 337–345 10.1016/j.cbpa.2010.09.012 (doi:10.1016/j.cbpa.2010.09.012) [DOI] [PubMed] [Google Scholar]
- 14.Frost PGH, Siegfried WR, Greenwood PJ. 1975. Arteriovenous heat exchange systems in the jackass penguin Spheniscus demersus. J. Zool. (Lond.) 175, 231–242 10.1111/j.1469-7998.1975.tb01398.x (doi:10.1111/j.1469-7998.1975.tb01398.x) [DOI] [Google Scholar]
- 15.Symonds MRE, Tattersall GJ. 2010. Geographical variation in bill size across bird species provides evidence for Allen's rule. Am. Nat. 176, 188–197 10.1086/653666 (doi:10.1086/653666) [DOI] [PubMed] [Google Scholar]
- 16.Goldsmith R, Sladen WJL. 1961. Temperature regulation of some Antarctic penguins. J. Physiol. Lond. 157, 251–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prévost J, Sapin-Jaloustre J. 1964. A propos des premieres mesures de topographie thermique chez les Spheniscides de la Terre Adelie. Oiseau 34, 52–90 [Google Scholar]
- 18.Trawa G. 1970. Note preliminaire sur la vascularisation des membres des Spheniscides de Terre Adelie. Oiseau. RFO 40, 142–156 [Google Scholar]
- 19.Despin B, Le Mayo Y, Schmitt M. 1978. Measures de temperatures peripheriques par thermographic infrarouge chez le manchot de Humbolt (Spheniscus humbolti). Oiseau. RFO 48, 151–158 [Google Scholar]
- 20.Thomas DB, Fordyce RE. 2012. Biological plasticity in penguin heat-retention structures. Anat. Rec. Advan. Integ. Anat. Evol. Biol. 295, 249–256 10.1002/ar.21538 (doi:10.1002/ar.21538) [DOI] [PubMed] [Google Scholar]
- 21.Ancel A, Visser H, Handrich Y, Masman D, Le Maho Y. 1997. Energy saving in huddling penguins . Nature 385, 304–305 10.1038/385304a0 (doi:10.1038/385304a0) [DOI] [Google Scholar]
- 22.Dawson C, Vincent JFV, Jeronimidis G, Rice G, Forshaw P. 1999. Heat transfer through penguin feathers. J. Theor. Biol. 199, 291–295 10.1006/jtbi.1999.0959 (doi:10.1006/jtbi.1999.0959) [DOI] [PubMed] [Google Scholar]
- 23.Marder J. 1973. Body temperature regulation in the brown necked raven (Corvus corax ruficollis). II. Thermal changes in the plumage of ravens exposed to solar radiation. Comp. Biochem. Physiol. 45, 431–440 10.1016/0300-9629(73)90450-7 (doi:10.1016/0300-9629(73)90450-7) [DOI] [PubMed] [Google Scholar]
- 24.Pinshow B, Welch WR. 1980. Winter breeding in emperor penguins: a consequence of the summer heat. Condor 82, 159–163 10.2307/1367469 (doi:10.2307/1367469) [DOI] [Google Scholar]
- 25.Van Sant MJ, Bakken GS. 2006. Thermoregulation on the air-water interface. II. Foot conductance, activity metabolism and a two-dimensional heat transfer model. J. Therm. Biol. 31, 491–500 10.1016/j.jtherbio.2006.05.002 (doi:10.1016/j.jtherbio.2006.05.002) [DOI] [Google Scholar]


