Abstract
Sexual selection, the suite of processes that lead to differential mating success among individuals, probably influences the evolutionary trajectory of populations. Because sexual selection often shifts traits away from their survival optima, strong sexual selection pressures are thought to increase potential for population extinction, especially during environmental change. Sexual selection pressures may also increase the opportunity for speciation by accelerating the generation of pre-zygotic isolation among populations. These relationships remain largely untested experimentally. Here, we allow populations of baker's yeast, Saccharomyces cerevisiae, to evolve for approximately 250 generations with altered sex ratios in order to test the effect of the strength of sexual selection on the fate of populations. We find that populations experiencing stronger sexual selection are less able to adapt to a novel environment compared with populations experiencing weaker sexual selection or no sex, and that strong sexual selection erases the benefits of sexual reproduction. This pattern persists when fitness is assayed in a closely related environment. We also identify a trend that may suggest the beginning of pre-zygotic isolation between populations experiencing stronger sexual selection, though this is not statistically significant. These results highlight the importance of sexual selection in shaping macroevolutionary patterns and biodiversity.
Keywords: sexual selection, Saccharomyces cerevisiae, extinction, experimental evolution, adaptation
1. Introduction
Sexual selection describes the collection of evolutionary processes that lead to differential mating success among individuals. As sexual selection can select for traits that decrease survival, the presence of sexual selection can move a phenotype away from its ecological optimum [1]. This can have detrimental effects for the organism, either by increasing conspicuousness [2], imposing energetic costs of production and maintenance of elaborate traits [3,4] or permitting sexual conflict [5]. This has led to the theoretical prediction that populations experiencing strong sexual selection may be at an increased risk of population decline or extinction [6], especially during environmental change [7]. Theory also predicts that strong sexual selection coupled with changes in mating preferences between populations can generate pre-zygotic isolation well before genetic or ecological incompatibilities lead to reproductive isolation, thus increasing the potential for speciation [8].
Previous studies have examined the relationship between sexual selection and some measure of extinction risk [9,10] or speciation potential [11,12] in a comparative framework with equivocal results. Ultimately, such studies rely on correlational data and cannot demonstrate causal links between variation in the strength of sexual selection and differential extinction or speciation.
To test these predictions, we allowed replicate populations of baker's yeast, Saccharomyces cerevisiae, to evolve under different sexual selective strengths to determine the effect of sexual selection on the evolutionary trajectory of populations. Yeast is highly suitable for experimental evolution studies [13] and is especially amenable to studies of sexual selection [14]. Diploid yeast can be induced to undergo sporulation (meiosis), generating four spores, two of each mating type: MATa and MATα. During mating, haploid yeast of opposite mating types respond to mating pheromones secreted by the other type and consistently mate with stronger pheromonal signallers [15].
By altering the mating skew, we manipulated sexual selective strength [5,14] in four populations in each of three treatments: strong sexual selection, weak sexual selection and used asexual populations to control for the effects of sex [16]. In our weak selection group, haploids were combined at approximately a 1 : 1 ratio of MATa : MATα cells at the time of mating. In the strong selection group, this ratio was 1 : 10, creating a greater mating skew and thus greater opportunity for sexual selection to act.
Extinction risk was assayed two ways. First, we examined the rate of adaptation in the medium in which the populations were evolved, a low glucose–high salt medium that is known to be physiologically stressful to yeast [16,17]. Second, we examined the rate of adaptation to a novel but related environment containing sucrose instead of glucose, as theory predicts that sexual selection enhances extinction risk during environmental change [7]. Changing the primary carbon source from glucose to sucrose imposed the additional cost of invertase production, an enzyme necessary to liberate glucose from sucrose [18]. If sexual selection increases extinction risk, adaptation should be hindered in the strong selection treatment compared with the weak selection treatment in both cases.
Speciation potential was assayed by measuring mating efficiency between populations within a treatment. If sexual selection increases speciation potential, we expect slower mating between crosses of strong selection populations compared with weak selection or asexual populations, as this may be an indication of pre-zygotic isolation.
2. Material and methods
(a). Construction of the yeast strain
We constructed a S. cerevisiae strain from a woodland isolate, that had MATa- and MATα-specific promoters attached to antibiotic resistance genes or genes encoding amino acids deleted in the genetic background [19], using transformations and crosses to laboratory strains with desired constructs. This allowed us to select for each cell type in the yeast life cycle (see the electronic supplementary material, table S1) and created a heterozygous strain allowing for genetic diversity in offspring of sexual reproduction.
(b). Experimental evolution
Populations were grown via serial transfer for 4 days in medium that selected for diploids. Populations were sporulated, digested to release spores and pure cultures of both mating types were obtained. MATa and MATα cells were combined in varying ratios to mate; the number of MATa cells was held constant across treatments, creating an equal number of mated diploids and increasing the effective population size of the strong selection treatment. The cycle was repeated nine times, approximately 250 mitotic generations, with an effective population size of approximately 3.7 × 106. The experiment was terminated as sporulation rates began to decrease. Asexual populations were stored at 4°C during the sexual phase (see figure 1 and the electronic supplementary material).
Figure 1.
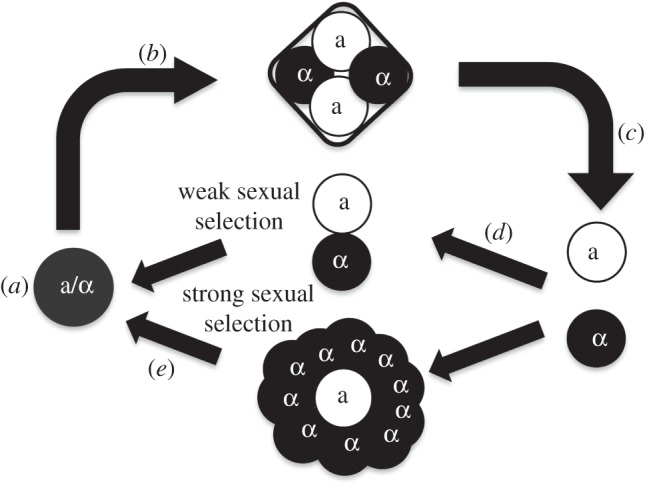
Experimental cycle. (a) Diploid populations grow. (b) Sexual populations sporulate. (c) MATa and MATα cells are selected separately. (d) MATa and MATα cells are combined in ratios that determine the strength of sexual selection. (e) Haploids mate to form diploids.
(c). Fitness estimates
Following the study of Lenski et al. [20], evolved populations were competed against a marked version of the ancestor in replicate. We used the number of colonies present on plated samples before and after growth to estimate Malthusian parameters for the evolved and ancestral populations. Relative fitness was calculated as the ratio between these two quantities. Fitness values greater than 1 represent an increase in fitness relative to the ancestral population.
(d). Mating propensity assays
Populations frozen after six cycles (approx. 180 generations) were reconstituted and separated into MATa and MATα cultures, which were combined and allowed to mate for 3 h. Proportion mated was determined by counting the number of diploids after 3 h and dividing by the maximum proportion of cells available to mate.
(e). Statistical analyses
Differences in fitness were assessed using ANOVA with cycle as a continuous variable, treatment as a fixed variable, population as a random variable nested within treatment type and interactions between the effects. All analyses were performed in JMP for Mac v. 9.0.2.
3. Results
Fitness relative to the ancestral population was estimated for all 12 populations after one, three, six and nine cycles (approx. 30, 90, 180 and 250 mitotic generations) in two media types (figure 2). In our model, the cycle by treatment interaction indicates whether the rate of change in fitness differs by treatment. In glucose medium, this interaction was significant (p = 0.023), indicating that the rate of fitness increase differed among the three groups. Treatment and cycle were also significant (p = 0.010, p < 0.001, respectively; electronic supplementary material, table S2), though these single effects must be interpreted with caution, as the slopes of the lines are probably different. When fitness was assayed in sucrose medium, the interaction term was again significant (p = 0.005), as were treatment and cycle (p = 0.001, p < 0.001, respectively; electronic supplementary material, table S3).
Figure 2.
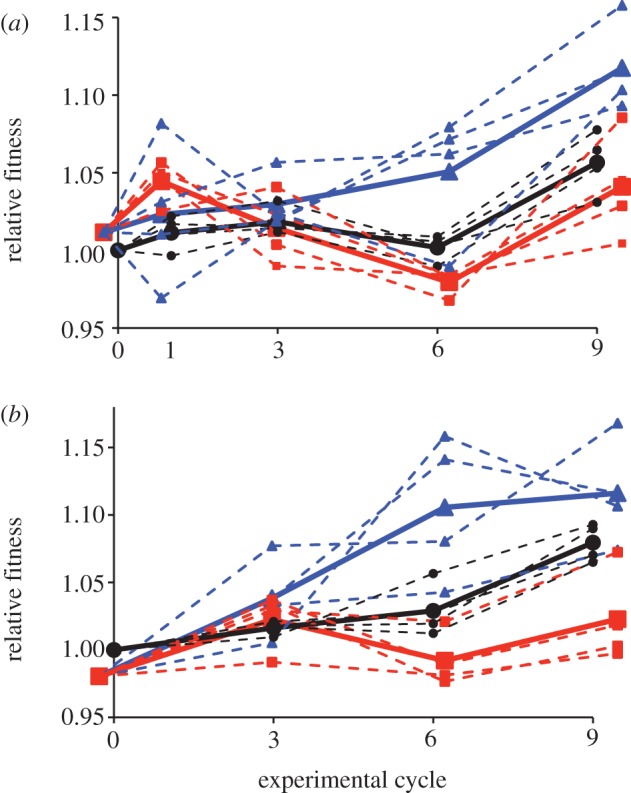
Relative fitness in (a) glucose and (b) sucrose media. Solid lines denote average for each treatment; dashed lines, replicate populations within each treatment. Black, asexual; blue, weak sexual selection; red, strong sexual selection.
Interestingly, when two of the strong sexual selection groups were crossed with each other, the proportion mated after 3 h appeared to be depressed (average proportion mated: 18.2%) compared with crosses between weak selection (25.3%) and asexual (22.5%) treatments, although these differences were not statistically significant (p = 0.227).
4. Discussion
We found a reduction in the rate of adaptation in populations of yeast exposed to strong sexual selection compared with those exposed to weak or no sexual selection. Specifically, weak sexual selection populations adapted most efficiently to a harsh environment and strong sexual selection populations most poorly, with asexual populations generally between these extremes. This pattern was maintained during an environmental change (i.e. switching from glucose to sucrose media). Importantly, these results cannot be explained by differences in effective population size between the sexual treatments, as the strong sexual selection treatment had a larger effective population size (see the electronic supplementary material).
Sexual populations of yeast adapt faster than asexual populations [16], and although some theory predicts populations experiencing strong sexual selection may suffer a fitness deficit compared with those experiencing weaker selection, it is of particular interest that strong sexual selective pressures appeared to erase the benefits of sex. Furthermore, while the asexual populations could only adapt via new mutations, the sexual populations had the advantage of additional genetic variation from new allelic combinations (the founding ancestor was heterozygous, thus producing recombinant offspring each mating cycle), suggesting that the effect of strong sexual selection was powerful indeed.
We hypothesize that the alleles favoured by selection to make a cell an attractive mating partner (increased pheromone production [15]) are not necessarily the same as those favoured for growth in the harsh new environment. Increased pheromone production comes at a fitness cost [4]. Thus, in the strong sexual selection treatment, the balance of these competing selective pressures may have been tipped towards the alleles for being an attractive mate and hindered the rate of adaptation. A recent analytical model shows that pheromone level may be a signal of genetically fitter partners, who are able to bear the cost of its production [21]. Our study suggests that it is possible that mutations that increase pheromonal signalling are separate from those that increase growth. In a well-adapted population, pheromone signalling may be an indicator of genetic fitness, but in a novel environment in the presence of strong sexual selection, our study suggests it is not.
In addition, we found that crosses between populations experiencing strong sexual selection showed a trend suggesting they were less likely to mate than crosses between populations that experienced little or no sexual selection. This trend towards pre-zygotic isolation, though not statistically significant, is in the predicted direction and notable given the relatively few number of generations the populations evolved.
Our results suggest that strong sexual selection pressures hinder adaptation, possibly influence population decline and extinction, and may increase the potential of pre-zygotic isolation. This insight may shed some light on patterns of biodiversity and could be used to inform conservation practices, as strong sexual selection may decrease persistence of populations exposed to novel habitats.
Acknowledgements
This work was supported by a Dintersmith Fellowship to L.P.R. from the W&M Charles Center, a Plumeri Fund grant to J.P.S. and NSF-DEB 0820969 to H.A.M. We thank J.-N. Jasmin and C. Boone for access to strain constructs, O. Kerscher for generously allowing use of his laboratory and the colony-counting elves, RKM, ACO and JS. We also thank D. Greig for helpful comments on the manuscript.
References
- 1.Andersson MB. 1994. Sexual selection. Princeton, NJ: Princeton University Press [Google Scholar]
- 2.Ryan MJ, Tuttle MD, Rand AS. 1982. Bat predation and sexual advertisement in neotropic anuran. Am. Nat. 119, 136–139 10.1086/283899 (doi:10.1086/283899) [DOI] [Google Scholar]
- 3.Vehrencamp SL, Bradbury JW, Gibson RM. 1989. The energetic cost of display in male sage grouse. Anim. Behav. 38, 885–896 10.1016/S0003-3472(89)80120-4 (doi:10.1016/S0003-3472(89)80120-4) [DOI] [Google Scholar]
- 4.Smith C, Greig D. 2010. The cost of sexual signaling in yeast. Evolution 64, 3114–3122 10.1111/j.1558-5646.2010.01069.x (doi:10.1111/j.1558-5646.2010.01069.x) [DOI] [PubMed] [Google Scholar]
- 5.Holland B, Rice WR. 1999. Experimental removal of sexual selection reverses intersexual antagonistic coevolution and removes a reproductive load. Proc. Natl Acad. Sci. USA 96, 5083–5088 10.1073/pnas.96.9.5083 (doi:10.1073/pnas.96.9.5083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lande R. 1980. Sexual dimorphism, sexual selection, and adaptation in polygenic characters. Evolution 34, 292–305 10.2307/2407393 (doi:10.2307/2407393) [DOI] [PubMed] [Google Scholar]
- 7.Tanaka Y. 1996. Sexual selection enhances population extinction in a changing environment. J. Theor. Biol. 180, 197–206 10.1006/jtbi.1996.0096 (doi:10.1006/jtbi.1996.0096) [DOI] [PubMed] [Google Scholar]
- 8.Panhuis TM, Butlin R, Zuk M, Tregenza T. 2001. Sexual selection and speciation. Trends Ecol. Evol. 16, 364–371 10.1016/S0169-5347(01)02160-7 (doi:10.1016/S0169-5347(01)02160-7) [DOI] [PubMed] [Google Scholar]
- 9.Donze J, Moulton MP, Labisky RF, Jetz W. 2004. Sexual plumage differences and the outcome of game bird (Aves: Galliformes) introductions on oceanic islands. Evol. Ecol. Res. 6, 595–606 [Google Scholar]
- 10.Morrow EH, Pitcher TE. 2003. Sexual selection and the risk of extinction in birds. Proc. R. Soc. Lond. B 270, 1793–1799 10.1098/rspb.2003.2441 (doi:10.1098/rspb.2003.2441) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arnqvist G, Edvardsson M, Friberg U, Nilsson T. 2000. Sexual conflict promotes speciation in insects. Proc. Natl Acad. Sci. USA 97, 10 460–10 464 10.1073/pnas.97.19.10460 (doi:10.1073/pnas.97.19.10460) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gage MJG, Parker GA, Nylin S, Wiklund C. 2002. Sexual selection and speciation in mammals, butterflies and spiders. Proc. R. Soc. Lond. B 269, 2309–2316 10.1098/rspb.2002.2154 (doi:10.1098/rspb.2002.2154) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Replansky T, Koufopanou V, Greig D, Bell G. 2008. Saccharomyces sensu stricto as a model system for evolution and ecology. Trends Ecol. Evol. 23, 494–501 10.1016/j.tree.2008.05.005 (doi:10.1016/j.tree.2008.05.005) [DOI] [PubMed] [Google Scholar]
- 14.Rogers DW, Greig D. 2009. Experimental evolution of a sexually selected display in yeast. Proc. R. Soc. B 276, 543–549 10.1098/rspb.2008.1146 (doi:10.1098/rspb.2008.1146) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackson CL, Hartwell LH. 1990. Courtship in Saccharomyces cerevisiae: both cell types choose mating partners by responding to the strongest pheromone signal. Cell 63, 1039–1051 10.1016/0092-8674(90)90507-B (doi:10.1016/0092-8674(90)90507-B) [DOI] [PubMed] [Google Scholar]
- 16.Goddard MR, Charles H, Godfray J, Burt A. 2005. Sex increases the efficacy of natural selection in experimental yeast populations. Nature 434, 636–640 10.1038/nature03405 (doi:10.1038/nature03405) [DOI] [PubMed] [Google Scholar]
- 17.Samani P, Bell G. 2010. Adaptation of experimental yeast populations to stressful conditions in relation to population size. J. Evol. Biol. 23, 791–796 10.1111/j.1420-9101.2010.01945.x (doi:10.1111/j.1420-9101.2010.01945.x) [DOI] [PubMed] [Google Scholar]
- 18.Gore J, Youk H, van Oudenaarden A. 2009. Snowdrift game dynamics and facultative cheating in yeast. Nature 459, 253–256 10.1038/nature07921 (doi:10.1038/nature07921) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tong AHY, et al. 2001. Systematic genetic analysis with ordered arrays of yeast deletion mutants. Science 294, 2364–2368 10.1126/science.1065810 (doi:10.1126/science.1065810) [DOI] [PubMed] [Google Scholar]
- 20.Lenski RE, Rose MR, Simpson SC, Tadler SC. 1991. Long-term experimental evolution in Escherichia coli. I. Adaptation and divergence during 2,000 generations. Am. Nat. 138, 1315–1341 10.1086/285289 (doi:10.1086/285289) [DOI] [Google Scholar]
- 21.Tazzyman SJ, Seymour RM, Pomiankowski A, Greig D. 2012. Mate choice among yeast gametes can purge deleterious mutations. J. Evol. Biol. 25, 1463–1471 10.1111/j.1420-9101.2012.02539.x (doi:10.1111/j.1420-9101.2012.02539.x) [DOI] [PubMed] [Google Scholar]


