Abstract
The evolution of resistance to parasites has been the focus of numerous theoretical studies and several mechanisms, ranging from innate to acquired immune responses, have been considered. Life-history theory predicts that long-lived species should invest more resources into maintenance and immunity than short-lived species. Here, we provide further theoretical and empirical support for this hypothesis. First, an analysis of the evolution of the persistence of immune protection in a theoretical framework accounting for maternal transfer of immunity reveals that longer-lived hosts are expected to invest in more persistent intragenerational and transgenerational immune responses. Controlling for phylogenetic structure and for the confounding effect of catabolic activity, we further showed that immunoglobulin half-life and longevity are positively correlated in mammal species. Our study confirms that persistence of immunity has evolved as part of elaborate anti-parasitic defence strategies.
Keywords: host–parasite interactions, life-history theory, immune system, maternal antibodies
1. Introduction
There is a large body of work focusing on the evolution of defence strategies against parasites (reviewed in [1]). A general theoretical prediction is that host lifespan can have a huge impact on the evolution of defence strategies [2]: long-lived hosts should invest more resources into acquired immunity than short-lived hosts [3–4]. In other words, long-lived hosts have more to lose than short-lived ones and are predicted to invest relatively more into survival and immunity. Although some attention has been given to the evolution of the persistence of immune protection [3], previous theoretical studies focused on a simplified host life cycle and overlooked the importance of the transfer of immunity across generations (see [4]). This temporary protection, achieved in vertebrates through the transfer of immunoglobulins, has been shown to be critical for early-life survival (reviewed in [5]). Because producing immunoglobulins is considered a physiologically demanding process, it has been suggested that the evolution of persistent transgenerational immunity is constrained by life-history traits such as lifespan [6]. In addition to investing in more persistent immune responses in adults [3], longer-lived species would thus be expected to display strong and durable immunity in both exposed adults and newborns. Building on a theoretical framework we developed previously [4], we study the evolution of the persistence of immune protection in maternally protected and recovered individuals. We further test the resulting prediction with a comparative approach examining the persistence of immunoglobulins in mammals.
2. Material and methods
(a). Evolutionary model
We model a host population composed of individuals with different epidemiological status (see the electronic supplementary material, S1 for mathematical details): susceptible (S), infected (I), recovered (R) or passively protected by maternal immunity (M). The parasite is characterized by its horizontal transmission rate β and its virulence α. Recovery occurs at a rate γ, and recovered individuals can transfer their immunity passively at a rate θ to their offspring. We assume that immunity can be lost by R individuals at a rate δR and by maternally protected individuals at a rate δM. These rates of loss of immunity are related to the half-life of immune protection (thalf) by the relation: thalf,i = ln(2)/δi, where i = M or R.
All hosts are assumed to reproduce irrespective of their epidemiological status at a density-dependent rate λ = r(1−κN) (with N = M + S + I + R, the total population size) and to die from natural causes at a rate μ. This system has been detailed elsewhere [4]; a general invasion criterion can be obtained corresponding to the average number of offspring produced by a rare mutant host in the resident population (see details in the electronic supplementary material, S1). We will assume a cost of the persistence of the protection (δi) on the reproductive rate of its host of the form 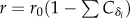 with
with 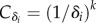 , where i = M or R.
, where i = M or R.
Under the above assumptions, it is possible to determine the evolutionary outcome of this system using the analysis presented in [4]. In the following, we first consider a case in which the persistence of immunity evolves independently in recovered and maternally protected individuals. Then, we allow these two traits to evolve when they are linked to each other.
(b). Persistence of antibodies in mammal species
We collected data on the persistence of immunoglobulin G (IgG) for 19 species of mammal (see figure 1a and electronic supplementary material, S2). We recovered the longevity of those species from the AnAge database [7]. However, we discarded the longevity value of the database for humans, as it is probably influenced by recent advances in medicine and thus less evolutionarily meaningful. We replaced it by the mean expected longevity for a population of hunter–gatherers [8].
Figure 1.
(a) Phylogenetic associations between the 19 species of mammal included in the comparative analysis. (b) Half-life of immunoglobulin G as a function of longevity in 19 species of mammals.
We also collected data from the literature on the basal metabolic rate (BMR) as a marker of catabolic activity. Because higher BMRs are indicative of higher catabolic activity, species with high BMR may be expected to display lower persistence of immunoglobulins. This information could only be obtained for 17 of the 19 species of our dataset (see the electronic supplementary material, S2). The analysis including this variable was restricted to these species.
We used generalized least-square models accounting for phylogenetic information to analyse the data. Phylogenetic information for the 19 species was obtained from the Interactive Tree of Life (figure 1a). Because the phylogenetic tree had no branch length, we used a Grafen correlation matrix [9]. When necessary, model selection was performed using Akaike information criterion (AIC). All statistical analyses were performed using the software R and the package ‘ape’ [10].
3. Results
(a). Evolutionary model
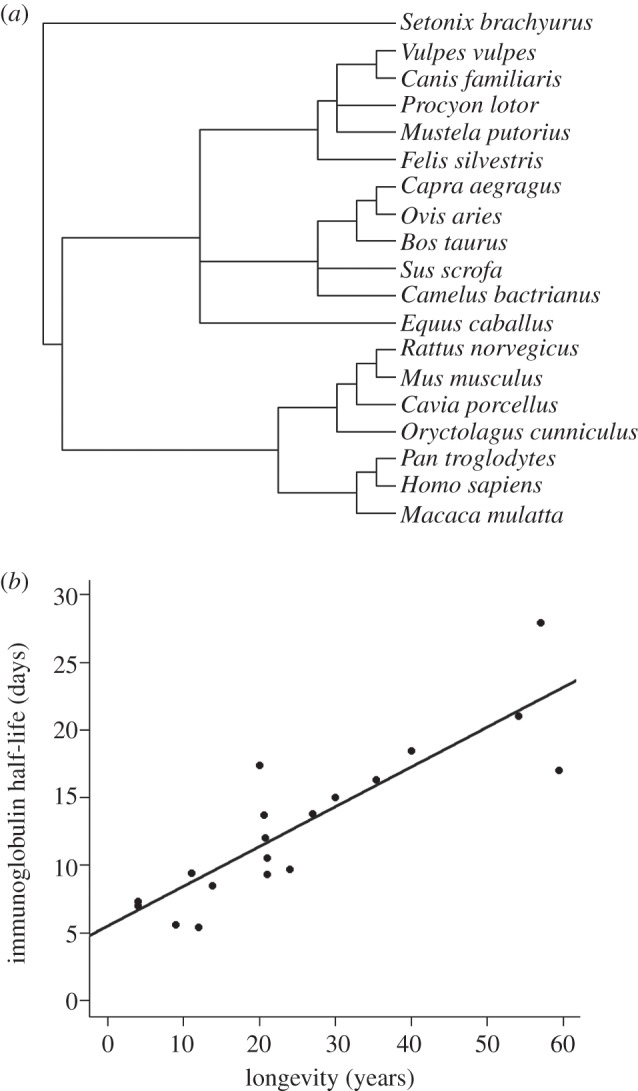
Similar to Miller et al. [3], we found that the evolutionarily stable persistence of immune protection is an increasing and saturating function of life expectancy (figure 2a) in recovered individuals (δR, black curve). Moreover, investment follows a similar pattern in maternally protected individuals when it evolves independently (δM, grey curve). Additionally, persistence is always predicted to be longer in recovered than in maternally protected individuals when the corresponding traits evolve independently (figure 2a). Note that modifications of the shape of the cost function can affect the evolutionary outcome. In particular, if we use the trade-off function used by Miller et al. [3], we can observe bistability of the evolution of persistence. Yet, even in this case, we generally expect a positive association between host lifespan and evolutionarily stable levels of immune protection.
Figure 2.
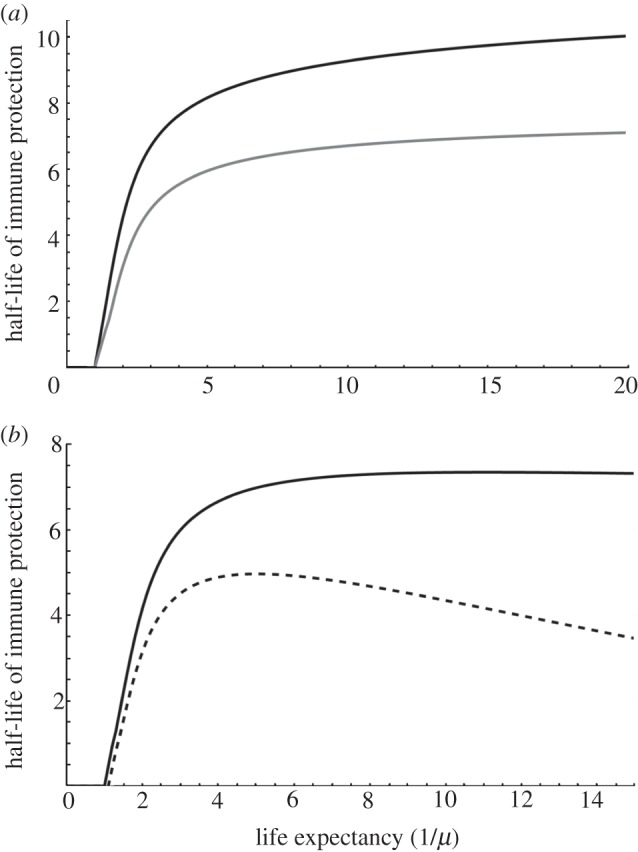
Effect of the host life expectancy on the evolutionarily stable half-life of immune protection. (a) Persistence in recovered (δR, black curve; 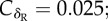 δM = 0.1) and maternally protected (δM, grey curve;
δM = 0.1) and maternally protected (δM, grey curve; 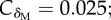 δR = 0.1) individuals are separate traits. (b) Persistence relies on a single trait (δ;
δR = 0.1) individuals are separate traits. (b) Persistence relies on a single trait (δ; 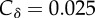 ), but the decay of protection is slowed down in R individuals according to τ = 1/5 (plain line) or τ = 1/500 (dashed line). Default parameter values: r0 = 1.5; κ = 0.1; k = 1/2; α = 3; β = 3; γ = 0.5.
), but the decay of protection is slowed down in R individuals according to τ = 1/5 (plain line) or τ = 1/500 (dashed line). Default parameter values: r0 = 1.5; κ = 0.1; k = 1/2; α = 3; β = 3; γ = 0.5.
Decay of immune protection is the result of a balance between protein catabolism and recycling mechanisms based on receptors specific for either IgG in mammals (FcRn; [11]) or IgY in birds (FcRY; [12]). We thus modified the previous model so that δM = δ and δR = δτ, with δ (an evolving trait) describing the common decay process in M and R individuals, and τ (a fixed parameter) the ability of recovered individuals to reduce this decay by constantly synthesizing immune compounds. The evolutionarily stable investment in half-life depends on how effectively R individuals can slow down the decay (figure 2b). If τ is high enough, the investment in half-life is an increasing and saturating function of lifespan (solid curve). When τ decreases and R individuals remain protected for an extended period, investment in half-life displays a maximum for intermediate values of life expectancies (dashed curve).
(b). Comparative analysis
When considering the full dataset of 19 species of mammals, the half-life of IgG appears to be strongly correlated with the longevity of the species (t = 6.48, p < 0.001; figure 1b). On the restricted dataset for which BMR values could be obtained, the best model based on AIC retains this same effect of the longevity of the host and an additive effect of the BMR (table 1). There is nevertheless a noticeable difference in the relative size of the effects of longevity and BMR in this model (longevity: slope = 0.238 ± 0.031, p < 0.001; BMR: slope = 0.024 ± 0.004, p < 0.001).
Table 1.
Degrees of freedom (d.f.), AIC and ΔAIC values for the different models tested in the comparative analysis.
| model | d.f. | AIC | ΔAIC |
|---|---|---|---|
| half-life ∼ longevity | 4 | 94.34 | 2.77 |
| half-life ∼ BMR | 4 | 97.30 | 5.73 |
| half-life ∼ longevity + BMR | 5 | 91.57 | 0 |
| half-life ∼ longevity × BMR | 6 | 108.35 | 16.78 |
4. Discussion
Life-history theory predicts a stronger investment in acquired immune defences for long-lived species [2–4,13]. Here, we provide theoretical and empirical support for this argument. We show that the evolutionarily stable investment in the duration of immune protection is expected to vary from non-persistent specific immunity in short-lived species up to rather persistent specific immunity in long-lived species. Our prediction is in accordance with the outcome of a comparative analysis of data on the half-life of one of the main effectors of the acquired immune response in mammals, IgG. Our analysis reveals that, when controlling for phylogeny, longevity of a species is a strong predictor of the half-life of its antibodies. Moreover, this effect is still largely significant when a covariate describing the catabolic activity of the species is added in the model. In other words, although differences in catabolism may partly account for differences in the persistence of antibodies, the pace of life is an important determinant of the observed pattern.
Here, we investigated the evolution of the duration of the immune protection acquired upon recovery or through the transfer of maternal immunity. However, even long after the decay of specific immune compounds, re-infection with the same pathogen allows sensitized memory cells to produce a secondary immune response. This secondary response needs less time to be mounted and is more efficient at clearing the parasite. This corresponds to an increased recovery rate upon secondary infection, leading to a decreased immune period that may in turn reduce the benefits of long-lived protection. In this model, we also considered the situation where only one parasite was circulating in the host population. Considering several strains may have consequences for the persistence of immune protection [4]. This may for instance be mediated by the existence of cross-protective mechanisms. Less-specific effectors (than for instance antibodies) may provide protection against several parasites at once, which may thus increase the benefits of an increased persistence of such acquired molecules.
We have also shown that empirical data support the prediction of a correlation between life expectancy and investment in immunity. Our analysis further suggests that long persistence of immunoglobulins could have evolved regardless of differences in metabolism. The existence of active mechanisms protecting immunoglobulins from normal catabolism [11–12] also leaves room for a separate evolution of persistence and catabolism. There are, however, several methodological problems with the analysis of IgG half-lives recovered from the literature. Immunological techniques used are not standardized and to what extent this influences the estimation of the half-life of the antibody is not known. The half-lives we used in our analysis were also obtained in different species exposed to different pathogens, which may result in differently persisting antibodies. Various antigens have indeed been shown to lead to various half-lives of protection [14], which may be mediated by differences in the half-life of the immunoglobulin. There is thus a need for studies where the exposure of individuals is controlled. Finally, we showed that the half-life of antibodies and the longevity of individuals are strongly correlated in mammalian species, a result in accordance with studies of the investment in other immune traits in mammals [15]. Similar data were not available for other taxa although there seems to be some evidence for variability in the half-life of antibodies among birds, with slow-living species displaying longer-lived antibodies ([6] and references therein). Overall, our study suggests that the persistence of transgenerational immunity represents an integral part of evolved defence strategies that may have influenced the persistence of individual immune protection.
Acknowledgements
The authors acknowledge financial support from the Centre National de la Recherche Scientifique (CNRS). T.B. acknowledges support from the Agence Nationale de la Recherche (ANR) grant no. 11 BSV7 003 ‘EVEMATA’, and S.G. from European Research Council (ERC) starting grant no. 243054 ‘EVOLEPID’.
References
- 1.Boots M, Best A, Miller MR, White A. 2009. The role of ecological feedbacks in the evolution of host defence: what does theory tell us? Phil. Trans. R. Soc. B 364, 27–36 10.1098/rstb.2008.0160 (doi:10.1098/rstb.2008.0160) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ricklefs RE, Wikelski M. 2002. The physiology/life-history nexus. Trends Ecol. Evol. 17, 462–468 10.16/S0169-5347(02)02578-8 (doi:10.16/S0169-5347(02)02578-8) [DOI] [Google Scholar]
- 3.Miller MR, White A, Boots M. 2007. Host life span and the evolution of resistance characteristics. Evolution 61, 2–14 10.1111/j.1558-5646.2007.00001.x (doi:10.1111/j.1558-5646.2007.00001.x) [DOI] [PubMed] [Google Scholar]
- 4.Garnier R, Boulinier T, Gandon S. 2012. Coevolution between maternal transfer of immunity and other resistance strategies against pathogens. Evolution 66, 3067–3078 10.1111/j.1558-5646.2012.01665.x (doi:10.1111/j.1558-5646.2012.01665.x) [DOI] [PubMed] [Google Scholar]
- 5.Boulinier T, Staszewski V. 2008. Maternal transfer of antibodies: raising immuno-ecology issues. Trends Ecol. Evol. 23, 282–288 10.1016/j.tree.2007.12.006 (doi:10.1016/j.tree.2007.12.006) [DOI] [PubMed] [Google Scholar]
- 6.Garnier R, Ramos R, Staszewski V, Militão T, Lobato E, González-Solís J, Boulinier T. 2012. Maternal antibody persistence: a neglected life-history trait with implications from albatross conservation to comparative immunology. Proc. R. Soc. B 279, 2033–2041 10.1098/rspb.2011.2277 (doi:10.1098/rspb.2011.2277) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Magalhães JP, Costa J. 2009. A database of vertebrate longevity records and their relation to other life-history traits. J. Evol. Biol. 22, 1770–1774 10.1111/j.1420-9101.2009.01783.x (doi:10.1111/j.1420-9101.2009.01783.x) [DOI] [PubMed] [Google Scholar]
- 8.Kaplan H, Hill K, Lancaster J, Hurtado AM. 2000. A theory of human life-history evolution: diet, intelligence, and longevity. Evol. Anthropol. 9, 156–185 (doi:10.1002/1520-6505(2000)9:4<156::AID-EVAN5>3.0.CO;2-7) [DOI] [Google Scholar]
- 9.Grafen A. 1989. The phylogenetic regression. Phil. Trans. R. Soc. Lond. B 326, 119–157 10.1098/rstb.1989.0106 (doi:10.1098/rstb.1989.0106) [DOI] [PubMed] [Google Scholar]
- 10.Paradis E. 2006. Analyses of phylogenetics and evolution with R. New York, NY: Springer [Google Scholar]
- 11.Ghetie V, Ward ES. 2000. Multiple roles for the major histocompatibility complex class I-related receptor FcRn. Annu. Rev. Immunol. 18, 739–766 10.1146/annurev.immunol.18.1.739 (doi:10.1146/annurev.immunol.18.1.739) [DOI] [PubMed] [Google Scholar]
- 12.West AP, Herr AB, Bjorkman PJ. 2004. The chicken yolk sac IgY receptor, a functional equivalent of the mammalian MHC-related Fc receptor, is a phospholipase A2 receptor homolog. Immunity 20, 601–610 10.1016/S1074-7613(04)00113-X (doi:10.1016/S1074-7613(04)00113-X) [DOI] [PubMed] [Google Scholar]
- 13.Boots M, Bowers RG. 2004. The evolution of resistance through costly acquired immunity. Proc. R. Soc. Lond. B 271, 715–723 10.1098/rspb.2003.2655 (doi:10.1098/rspb.2003.2655) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amanna IJ, Carlson NE, Slifka MK. 2007. Duration of humoral immunity to common viral and vaccine antigens. N. Engl. J. Med. 357, 1903–1915 10.1056/NEJMoa066092 (doi:10.1056/NEJMoa066092) [DOI] [PubMed] [Google Scholar]
- 15.Previtali MA, Ostfeld RS, Keesing F, Jolles AE, Hanselmann R, Martin LB. 2012. Relationship between pace of life and immune responses in wild rodents. Oikos 121, 1483–1492 10.1111/j.1600-0706.2012.020215.x (doi:10.1111/j.1600-0706.2012.020215.x) [DOI] [Google Scholar]


