Abstract
Figs (Ficus) have a reciprocally obligate mutualism with tiny, short-lived (1–2 days) fig wasps (Agaonidae). The small size and short life of these pollinators is expected to make them more vulnerable to climate change than their larger and longer-lived hosts. We experimentally tested the thermal tolerances of four species of adult female fig wasp from equatorial Singapore. The results suggest that an increase of 3°C or more above the current temperatures experienced across much of the equatorial tropics would markedly decrease the active adult lifespan of all four species. Fig plants are the centre of an intricate web of specialist and generalist animals. Unless fig wasps can acclimate or adapt to warmer temperatures in time, these responses may disrupt the mutualism, potentially affecting multiple trophic levels.
Keywords: climate warming, fig wasps, Ficus, mutualism, thermal tolerance
1. Introduction
The potential impact of anthropogenic climate change on plant–pollinator mutualisms remains poorly understood, particularly in the tropics [1]. Specialized pollination relationships, in which a plant species has few alternative pollinators, are expected to be more vulnerable than generalized ones [2,3]. In the extreme case of obligate, one-to-one relationships (one pollinator to one plant), neither species will survive climatic conditions that are unsuitable for the more sensitive partner [2]. The figs (Ficus, Moraceae) are a pantropical genus of 755 species of woody plants and their ecological importance as a food resource for diverse tropical frugivores has been well documented [4,5]. They have reciprocally obligate pollination relationships with tiny (1–2 mm), short-lived (1–2 days adult lifespan), fig wasps (Agaonidae). The different physiologies of the two partners are expected to respond very differently to climate change, destabilizing the mutualism in the short-run and possibly leading to co-extinctions in the long-run [3,6]. Numerous vertebrates (more than 1200 spp.) consume at least some figs, while some birds, bats and primates depend on them for a large fraction of their diets [5]. In addition, some fig species are important pioneers for reforestation efforts, while both planted and spontaneous fig species play a significant role in supporting urban wildlife [7,8]. Given the ecological importance of fig plants, it is important to understand the vulnerability of their pollinators.
This study was carried out in equatorial Singapore, which has a climate of extreme equability, with negligible seasonality in either rainfall or temperature [9]. Singapore experiences mean daily and mean maximum temperatures of 27°C and 31°C, respectively, with an extreme range (since 1929) of 19.4–36.0°C [9]. These conditions are expected to select for a narrow range of thermal tolerance and thus a high sensitivity to the predicted warming in the lowland tropics [10].
2. Material and methods
Four species of adult female fig wasps collected from Singapore were used: Valisia malayana (pollinator of Ficus grossularioides), Ceratosolen constrictus hewitti (F. fistulosa), Ceratosolen appendiculatus (F. variegata) and Eupristina verticillata (F. microcarpa), all in the family Agaonidae. Syconia (4333 wasps, 240 syconia) were haphazardly collected and adult female fig wasps left to emerge at room temperature for 1 h 30 min, so that they were the same age and to minimize acclimation. Experiments were run in a Sanyo growth chamber MLR-351H and were ended when all wasps had died or became irreversibly immobile. No data were censored. Kaplan–Meier survival analysis was used to obtain median lifespan duration (LD) estimates, and Cox's proportional hazard model with hazard ratios was used to obtain mortality rates (MRs). Temperature treatments for V. malayana, C. c. hewitti and E. verticillata were 25°C, 26°C, 28°C, 30°C, 32°C, 34°C, 36°C and 38°C at 85% relative humidity. Survival was recorded at 2 h intervals, except that above 36°C the pollinators died in less than 2 h so their states were recorded earlier. Ceratosolen appendiculatus temperature treatments were 26°C, 28°C, 30°C, 32°C and 34°C at 85% relative humidity and survival was recorded at 0, 6, 12 and then at 12 h intervals. Valisia malayana was also tested at 60% and 85% relative humidity at 32°C, 34°C, 36°C and 38°C, with survival recorded at 2 h intervals. Syconia on the same tree but different branches were used for within-species comparisons. Wasps from six syconia of V. malayana, two of C. c. hewitti and four of C. appendiculatus were tested at 28°C and 85% relative humidity, and survival recorded at 0, 6, 12 and then at 12 h intervals. All data have been deposited in Dryad (http://dx.doi.org/10.5061/dryad.hj7h2), see electronic supplementary material.
3. Results
The LD for all four species decreased steadily with increasing temperature (figure 1a). At 27°C, the mean daily temperature in Singapore, the LD of our study species ranged from 11 to 24 h. At 31°C, LD decreased sharply from 6 to 18 h (figure 1a), and at 34°C, all four species survived 6 h or less. The MR of all species increased with temperature, but at different rates, with E. verticillata displaying the highest overall hazard ratio, with wasps 2.6 times more likely to die with every 2°C increase in temperature, compared with 1.4 times in C. appendiculatus (table 1). There was no significant difference in LD or MR between 85% (average for Singapore) and 60% (low for Singapore) relative humidity treatments (n = 1504, HR = 1.0, p = 0.72; figure 1b and table 1). There were significant between-brood MR differences for all three species tested: V. malayana (n = 418, Wald test = 132, d.f. = 5, p < 0.001), C. c. hewitti (n = 315, Wald test = 14, d.f. = 1, p < 0.001) and C. appendiculatus (n = 268, Wald test = 24, d.f. = 3, p < 0.001). Median LD estimates differed in V. malayana and C. c. hewitti but not C. appendiculatus (table 2).
Figure 1.
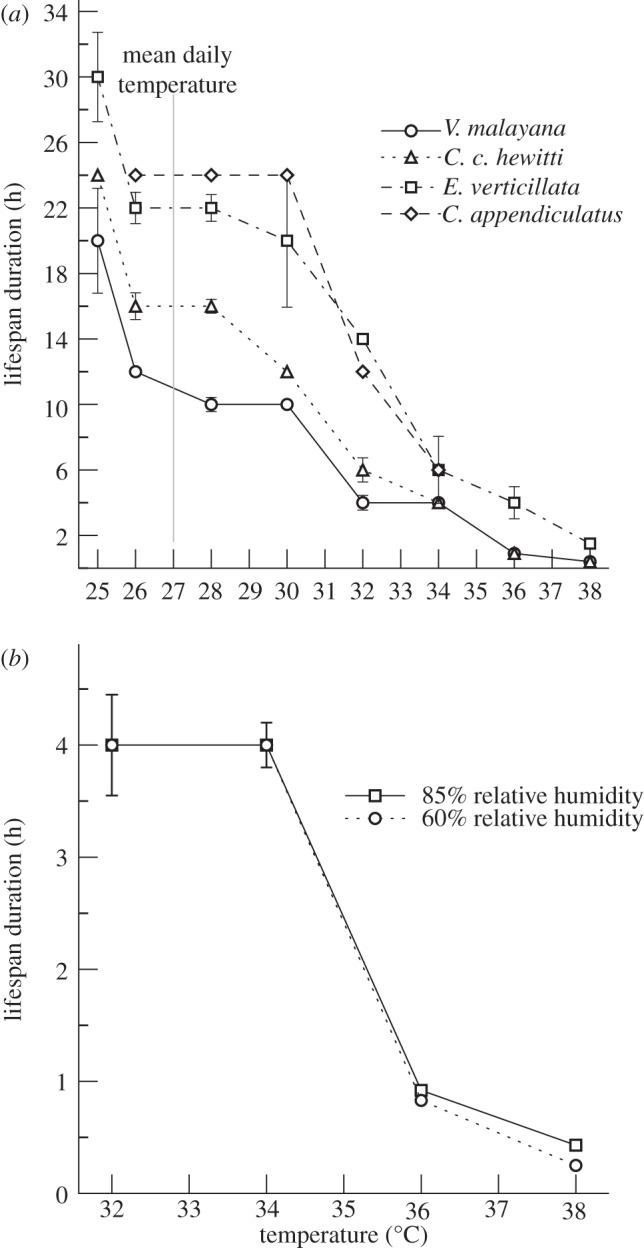
Median lifespan duration with 95% CIs for temperature treatments for (a) Valisia malayana, Ceratosolen c. hewitti, Ceratosolen appendiculatus and Eupristina verticillata at 85% relative humidity and (b) Valisia malayana at 60% and 85% relative humidity.
Table 1.
Temperature and humidity effects on mortality rate. Cox's proportional hazards model (hazard ratio, HR), regression coefficient (coeff.) and standard error (s.e.) for (a) Valisia malayana, Ceratosolen c. hewitti, Ceratosolen appendiculatus and Eupristina verticillata from 26°C to 38°C at 85% relative humidity and (b) Valisia malayana at 32°C, 34°C, 36°C and 38°C crossed with 60% and 85% relative humidity.
| no. of wasps | HR | coeff. | s.e. | p-value | |
|---|---|---|---|---|---|
| (a) temperature | |||||
| Valisia malayana | 1923 | 1.6 | 0.49 | 0.01 | <0.001 |
| Ceratosolen c. hewitti | 757 | 1.9 | 0.64 | 0.03 | <0.001 |
| Ceratosolen appendiculatus | 763 | 1.4 | 0.36 | 0.02 | <0.001 |
| Eupristina verticillata | 201 | 2.6 | 0.94 | 0.07 | <0.001 |
| (b) humidity | |||||
| humidity 60% (baseline group) | 689 | 1.0 | 0 | — | — |
| humidity 85% | 815 | 1.0 | −0.02 | 0.06 | 0.72 |
Table 2.
Median lifespan estimate and mortality rate of wasps emerging from different syconia at 28°C and 85% relative humidity. Median lifespan estimate (h) derived from Kaplan–Meier analysis. Cox's proportional hazards model (hazard ratio, HR), regression coefficient (coeff), standard error (s.e.) and Wald test for Valisia malayana, Ceratosolen c. hewitti and Ceratosolen appendiculatus.
| no. of wasps | lifespan estimate (h) | mortality rate |
|||||
|---|---|---|---|---|---|---|---|
| HR | coeff. | s.e. | p-value | Wald test | |||
| V. malayana | |||||||
| A (baseline group) | 41 | 24 | 1.0 | 0 | — | — | W = 132, d.f. = 5, p < 0.001 |
| B | 88 | 12 | 2.7 | 0.98 | 0.19 | >0.001 | |
| C | 37 | 24 | 1.3 | 0.30 | 0.22 | 0.20 | |
| D | 101 | 24 | 0.7 | −0.43 | 0.19 | 0.02 | |
| E | 77 | 12 | 0.6 | −0.51 | 0.20 | 0.01 | |
| F | 74 | 24 | 0.5 | −0.79 | 0.20 | >0.001 | |
| C. c. hewitti | |||||||
| A (baseline group) | 111 | 12 | 1.0 | 0 | — | — | W = 14, d.f. = 1, p < 0.001 |
| B | 204 | 24 | 0.6 | 0.64 | 0.12 | >0.001 | |
| C. appendiculatus | |||||||
| A (baseline group) | 41 | 24 | 1.0 | 0 | — | — | W = 24, d.f. = 3, p < 0.001 |
| B | 62 | 24 | 0.6 | −0.47 | 0.20 | 0.02 | |
| C | 87 | 24 | 1.1 | 0.10 | 0.19 | 0.60 | |
| D | 78 | 24 | 0.5 | −0.61 | 0.20 | <0.001 | |
4. Discussion
Much of lowland southeast Asia currently experiences mean daily temperatures of around 27°C and daily maxima in the 30–36°C range [11]. The IPCC's report [12] projects a regional warming of 2.2–3.0°C (25–75% quartile values from 21 models) for the moderate A1B greenhouse gas scenario by 2080–2099 and approximately 2.6–3.5°C for the A2 scenario. An increase in mean temperature of 3.0°C would result in a decrease in fig wasp LD in three of the four species with C. c. hewitti displaying the largest decrease at 25 per cent (figure 1a). However, mean daily temperatures are averaged over 24 h and daytime temperatures are more relevant for day-dispersing wasps that live less than 24 h. The mean daytime temperature (7.00–19.00) of Singapore for 2010 was 29°C [13] and an increase in 3.0°C, which is expected by 2100 [12], would reduce the LD of day-dispersing V. malayana, C. c. hewitti, C. appendiculatus and E. verticillata by 33–60% (figure 1a). Night-dispersing species will experience lower absolute temperatures, but a similar degree of warming [14].
Reduced LD and increased MR for fig wasps, some of which disperse tens of kilometres to receptive host figs [15], would mean that wasps would have less time to locate a receptive host syconium and pollinate and/or oviposit in the hundreds of florets within it [16]. The potential consequences include: (i) declines in the populations of fig wasps; (ii) reduced pollination services leading to fig population declines; (iii) reduced fig populations reducing food abundance for frugivores, resulting in secondary population declines. Besides supporting a huge range of vertebrates, a single host fig can support up to 30, more or less host-specific, non-pollinating fig wasp species [17]. Sustained collapses in the fig–fig wasp mutualism could thus affect multiple trophic levels.
Pollinator species with a wide latitudinal range will have been exposed to higher thermal extremes over their evolutionary history than those that occur only in the equatorial lowlands, and might be expected to have a higher degree of thermal tolerance [10]. Indeed, the thermal tolerances of the four species of fig wasp studied here appear to correspond to the ranges of their host figs, with the least thermally tolerant, V. malayana (F. grossularioides), restricted to the aseasonal tropics, and the most thermally tolerant, E. verticillata (F. microcarpa) and C. appendiculatus (F. variegata), ranging from Queensland, Australia to south Japan [4]. Ceratosolen c. hewitti (F. fistulosa) has an intermediate range. Rainfall and humidity may also influence wasp survival ([16,18] but see [19]), but the humidity treatments in this study did not affect LD and MR significantly.
Developing fig syconia are able to use evaporative cooling to avoid temperatures that would be lethal to fig wasp larvae [20]. Even within the same tree, however, syconia are exposed to different microclimates, whereas wasps from a single syconium may have several mothers. Individual wasps may, therefore, vary in both their opportunities for temperature acclimation during development and in the genetic component of thermal tolerance. We did not assess either in this study, but the significant variation in thermal tolerance among wasp broods from the same host tree suggests that one or both may be significant. The literature suggests that the ability of insects to acclimate to higher temperatures may be limited [21], but the short generation times of fig wasps (approx. one month) would favour rapid evolution if heritable genetic variation in heat tolerance exists. It is also important to note that we assessed only the ability of wasps to move. Both pollination and oviposition are complex behaviours in fig wasps and their success may be more sensitive to warming. Moreover, in a Neotropical pseudoscorpion a 3.5°C warming had only a small impact on survival but rendered the females sterile [22].
The three genera of fig wasps we tested represent three widely divergent clades [23], suggesting that our results can be generalized to other fig wasps. This implies that fig populations, not just in Singapore but in the entire aseasonal tropics, could be extremely vulnerable to anthropogenic global warming. Because of their ecological importance, any loss of fig species or reduction in their abundance would be of major conservation concern. We hope this study will encourage more research.
Acknowledgements
We thank the following people for helpful comments on earlier drafts: Rhett Harrison, Koh Lian Pin, Thomas Wanger and Steve Compton. For help on analysis, we thank Alex Cook and Chong Kwek Yan and National Parks Board (Singapore) for permits to collect material.
References
- 1.Corlett RT. 2011. Impacts of warming on tropical lowland rainforests. Trends Ecol. Evol. 26, 606–613 10.1016/j.tree.2011.06.015 (doi:10.1016/j.tree.2011.06.015) [DOI] [PubMed] [Google Scholar]
- 2.Gilman RT, Fabina NS, Abbott KC, Rafferty NE. 2011. Evolution of plant–pollinator mutualism in response to climate change. Evol. Appl. 5, 2–16 10.1111/j.1752-4571.2011.00202.x (doi:10.1111/j.1752-4571.2011.00202.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koh LP, Dunn RR, Sodhi NS, Colwell RK, Proctor HC, Smith VS. 2004. Species coextinctions and the biodiversity crisis. Science 305, 1632–1634 10.1126/science.1101101 (doi:10.1126/science.1101101) [DOI] [PubMed] [Google Scholar]
- 4.Berg CC, Corner EJH. 2005. Flora Malesiana series. I. Seed plants. Leiden, Netherlands: National Herbarium Nederland [Google Scholar]
- 5.Shanahan M, So S, Compton SG, Corlett RT. 2001. Fig-eating by vertebrate frugivores: a global review. Biol. Rev. 76, 529–572 10.1017/S1464793101005760 (doi:10.1017/S1464793101005760) [DOI] [PubMed] [Google Scholar]
- 6.Harrison RD. 2000. Repercussions of El Niño: drought causes of the extinction and the breakdown of mutualism in Borneo. Proc. R. Soc. Lond. B 267, 911–915 10.1098/rspb.2000.1089 (doi:10.1098/rspb.2000.1089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuaraksa C, Elliott S, Hossaert-Mckey M. 2012. The phenology of dioecious Ficus spp. tree species and its importance for forest restoration projects. Forest Ecol. Manage. 265, 82–93 10.1016/j.foreco.2011.10.022 (doi:10.1016/j.foreco.2011.10.022) [DOI] [Google Scholar]
- 8.Corlett RT. 2006. Figs (Ficus, Moraceae) in urban Hong Kong, South China. Biotropica 38, 116–121 [Google Scholar]
- 9.National Environment Agency 2011. Weather Statistics. See http://app2.nea.gov.sg/weather_statistics.aspx (accessed July 2012). [Google Scholar]
- 10.Deutsch CA, Tewksbury JJ, Heuy RB, Sheldon KS, Ghalambor CK, Haak DC, Martin PR. 2008. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl Acad. Sci. USA. 105, 6668–6672 10.1073/pnas.0709472105 (doi:10.1073/pnas.0709472105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Weather Service 2012. Global temperature time series. See http://www.cpc.ncep.noaa.gov/products/global_monitoring/temperature/global_temp_accum.shtml (accessed July 2012) [Google Scholar]
- 12.IPCC 2007. Climate change 2007: IPCC fourth assessment report. Cambridge, UK: Cambridge University Press [Google Scholar]
- 13.Singapore Meterological Service 2010. Day mean temperature (7 am–7 pm) Changi Met. Station Singapore: National Environment Agency [Google Scholar]
- 14.Harrison RD, Rasplus J-Y. 2006. Dispersal of fig pollinators in Asian tropical rain forests. J. Trop. Ecol. 22, 631–639 10.1017/S0266467406003488 (doi:10.1017/S0266467406003488) [DOI] [Google Scholar]
- 15.Ahmed S, Compton SG, Butlin RK, Gilmartin PM. 2009. Wind-borne insects mediate directional pollen transfer between desert fig trees 160 kilometers apart. Proc. Natl Acad. Sci. USA. 106, 20 343–20 347 10.1073/pnas.0902213106 (doi:10.1073/pnas.0902213106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunn DW, Yu DW, Ridley J, Cook JM. 2008. Longevity, early emergence and body size in a pollinating fig wasp-implications for stability in a fig-pollinator mutualism. J. Anim. Ecol. 77, 927–935 10.1111/j.1365-2656.2008.01416.x (doi:10.1111/j.1365-2656.2008.01416.x) [DOI] [PubMed] [Google Scholar]
- 17.Hawkins BA, Compton SG. 1992. African fig wasp communities: undersaturation and latitudinal gradients in species richness. J. Anim. Ecol. 61, 361–372 10.2307/5328 (doi:10.2307/5328) [DOI] [Google Scholar]
- 18.Warren M, Robertson MP, Greeff JM. 2010. A comparative approach to understanding factors limiting abundance patterns and distributions in a fig tree–fig wasp mutualism. Ecography 33, 148–158 10.1111/j.1600-0587.2009.06041.x (doi:10.1111/j.1600-0587.2009.06041.x) [DOI] [Google Scholar]
- 19.Ghara M, Borges RM. 2010. Comparative life-history traits in a fig wasp community: implications for community structure. Ecol. Entomol. 35, 139–148 10.1111/j.1365-2311.2010.01176.x (doi:10.1111/j.1365-2311.2010.01176.x) [DOI] [Google Scholar]
- 20.Patino S, Herre EA, Tyree MT. 1994. Physiological determinants of Ficus fruit temperature and implications for survival of pollinator wasp species: comparative physiology through an energy budget approach. Oecologia 100, 13–20 10.1007/BF00317125 (doi:10.1007/BF00317125) [DOI] [PubMed] [Google Scholar]
- 21.Overgaard J, Kristensen TN, Mitchell KA, Hoffmann AA. 2011. Thermal tolerance in widespread and tropical Drosophila species: does phenotypic plasticity increase with latitude? Am. Nat. 178, S80–S96 10.1086/661780 (doi:10.1086/661780) [DOI] [PubMed] [Google Scholar]
- 22.Zeh JA, Bonilla MM, Su EJ, Padua MV, Anderson RV, Kaur D, Yang D-S, Zeh DW. 2012. Degrees of disruption: projected temperature increase has catastrophic consequences for reproduction in a tropical ectotherm. Glob. Change Biol. 18, 1833–1842 10.1111/j.1365-2486.2012.02640.x (doi:10.1111/j.1365-2486.2012.02640.x) [DOI] [Google Scholar]
- 23.Lopez-Vaamonde C, Wikström N, Kjer KM, Weiblen GD, Rasplus J-Y, Machado CA, Cook JM. 2009. Molecular dating and biogeography of fig-pollinating wasps. Mol. Phylogenet. Evol. 52, 715–726 10.1016/j.ympev.2009.05.028 (doi:10.1016/j.ympev.2009.05.028) [DOI] [PubMed] [Google Scholar]


