Abstract
When resources are spatially and temporally variable, consumers can increase their foraging success by moving to track ephemeral feeding opportunities as these shift across the landscape; the best examples derive from herbivore–plant systems, where grazers migrate to capitalize on the seasonal waves of vegetation growth. We evaluated whether analogous processes occur in watersheds supporting spawning sockeye salmon (Oncorhynchus nerka), asking whether seasonal activities of predators and scavengers shift spatial distributions to capitalize on asynchronous spawning among populations of salmon. Both glaucous-winged gulls and coastal brown bears showed distinct shifts in their spatial distributions over the course of the summer, reflecting the shifting distribution of spawning sockeye salmon, which was associated with variation in water temperature among spawning sites. By tracking the spatial and temporal variation in the phenology of their principal prey, consumers substantially extended their foraging opportunity on a superabundant, yet locally ephemeral, resource. Ecosystem-based fishery management efforts that seek to balance trade-offs between fisheries and ecosystem processes supported by salmon should, therefore, assess the importance of life-history variation, particularly in phenological traits, for maintaining important ecosystem functions, such as providing marine-derived resources for terrestrial predators and scavengers.
Keywords: marine-derived nutrients, subsidy, phenological tracking, ecosystem-based management, phenological diversity, resource wave
1. Introduction
Phenological diversity in plants has been shown to have critical effects on the migration, foraging and performance of mobile herbivores. For example, gazelles (Eudorcas thomsonii) in the Serengeti ecosystem compensate for high local temporal variation in the availability of their principal forage by moving to exploit the spatial variation in grass growth, driven by spatial variation in rainfall [1]. Similarly, deer (Odocoileus hemionus) and geese (Anser albifrons albifrons) coordinate their seasonal movements to exploit the ‘green wave’ of vegetation that develops in response to elevation, precipitation and latitudinal gradients in plant growth [2,3]. A similar ‘brown wave’ of roots and shoots is also apparent in plants that are key food for digging herbivores such as inland grizzly bears (Ursus arctos) [4]. Phenological tracking by consumers on seasonally ephemeral prey enables them to extend the duration of high-quality foraging opportunities, particularly if consumers can integrate across finer-scale patterning in the spatial and temporal variation of their prey. Though similar spatial and temporal interactions must certainly occur between carnivores and their prey, examples of these relationships remain distinctly unappreciated.
Anadromous Pacific salmon return to coastal watersheds to spawn after accumulating most of their growth in the ocean. A diverse community of freshwater and terrestrial predators and scavengers feeds heavily on salmon during their annual spawning migrations [5]. Coastal brown bears are particularly dependent on anadromous salmon, with body size, fecundity and densities all strongly positively correlated with salmon abundance [6]. Recognition of the importance of the annual pulse of salmon-derived resources for coastal watersheds has prompted efforts to assess the trade-offs between allocating salmon to commercial fisheries versus a variety of ecosystem processes such as providing a high-quality resource subsidy to coastal bear populations [7,8]. However, such efforts have focused specifically on the trade-offs associated with allocating salmon abundance between fisheries and ecosystems, and have not assessed whether the life-history variation within salmon species affects their profitability to terrestrial consumers.
Near the northern end of their range, the salmon migration and spawning season within individual watersheds typically lasts only two to four months, thereby placing substantial temporal constraints on predators and scavengers that achieve most of their annual growth by consuming salmon. These temporal constraints are magnified because salmon are most vulnerable to consumers only once they have moved into shallow habitats to spawn. In total, an individual salmon population is active on the spawning grounds for three to five weeks, and carcasses decompose rapidly after death, thereby presenting consumers with an extremely restricted period of time to feed on this high-quality and superabundant subsidy. However, because there is substantial variation in the timing of salmon spawning among individual populations using individual tributaries of rivers, mobile consumers are presented with a much longer season of potential foraging opportunity than if salmon populations all spawned simultaneously within river basins [9].
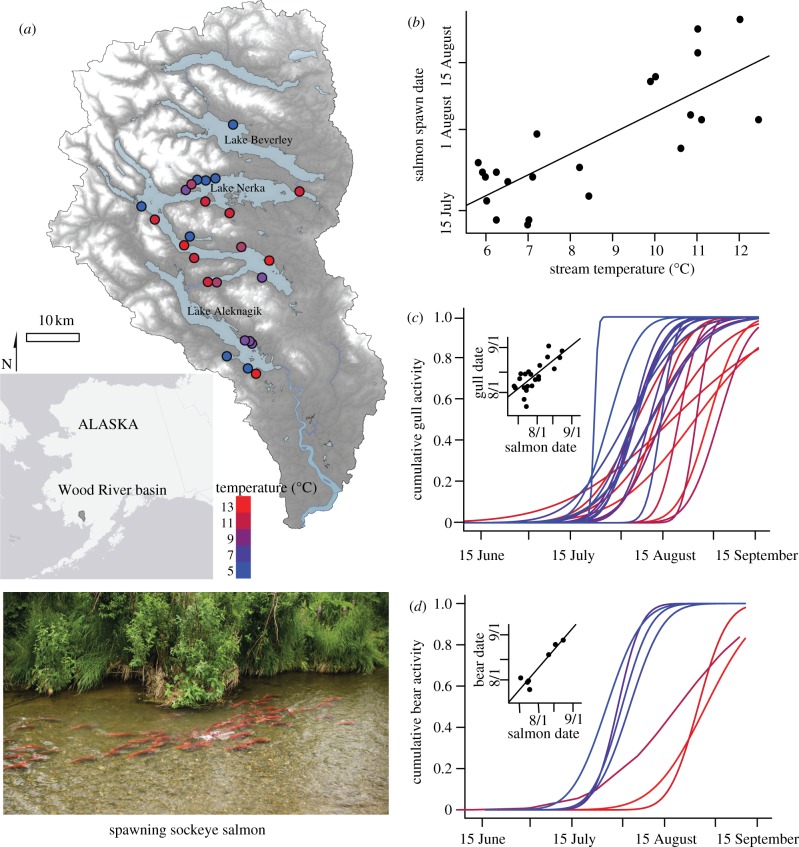
Here, we quantified the seasonal changes in the spatial distributions of glaucous-winged gulls (Larus glaucescens) and coastal brown bears within a single river basin to quantify their responses to the changing seasonal spatial distribution of spawning sockeye salmon (Oncorhynchus nerka). Sockeye salmon populations spawn early in cold water systems where embryo development is slow, and spawn later in the season at sites characterized by warm water [10]. We tested the hypothesis that the spatial and temporal variation in the distribution of two terrestrial consumers are associated with the changing spatial distributions of salmon resources that track the variation in water temperatures among spawning sites (a ‘crimson tide’, figure 1).
Figure 1.
(a) Map of Wood River basin showing sockeye salmon spawning locations and corresponding average summer water temperature as indicated by dot colour. (b) Relationship between water temperature and sockeye salmon spawning date. (c,d) Cumulative distribution functions (cdf), representing the proportion of the cumulative seasonal activity observed at any site on a specific date, for (c) gulls and (d) bears at sockeye salmon spawning locations. Colours of lines correspond to water temperatures, and insets show relationship between the mean of the cdf for gulls and bears, and sockeye salmon spawn timing among study sites (see the electronic supplementary material, table S1).
2. Material and methods
This work was performed in the Wood River watershed (figure 1a; 59o20′ N, 158o40′ W), a major river draining to Bristol Bay, Alaska, USA. The Wood River drains five large lakes that are fed by dozens of tributaries and connecting rivers, most of which provide spawning habitat for genetically distinct populations of sockeye salmon, the dominant anadromous fish species in this watershed. Among the many traits that show population-specific phenotypic variation, spawning date is highly variable across this system [9,10].
We monitored seasonal changes in the foraging activity of coastal brown bears and glaucus-winged gulls in the watersheds of lakes Aleknagik, Nerka and Beverley (figure 1a) during 2011. Water temperatures in 24 streams, rivers and lake beaches were monitored with i-buttons (Dallas Semiconductor, Dallas, TX, USA) between 1 June and early September (2011) according to the methods described in [10]. The date when sockeye salmon initiated spawning was documented by visually assessing all spawning sites at least twice weekly during July and August. Gull distributions were monitored visually from a boat at the outflow of streams, on lake beaches, and along the length of the Agulowak and Agulukpak rivers. Most spawning sites were surveyed for gulls 1–3 times per week from 1 June to 14 September 2011, except at remote sites where this was not logistically possible (see the electronic supplementary material, figure S1). Tributaries were surveyed by counting the number of gulls located within a 100 m radius of the stream outflow. The Agulowak and Agulukpak rivers were surveyed by travelling up the length of the river in a jet boat and counting the number of gulls in the stream channel, on gravel bars and in riparian trees.
Bear activity on streams was monitored with remote camera traps using methods similar to Shardlow & Hyatt [11]. One camera with an infrared triggering mechanism (Wildgame Innovations N6 Game Camera) was installed in a tree 1.5–5 m above water level along each of four streams and two lake beaches. All cameras were operational from 20 June to 12 September, except for at the Anvil Bay site from 5–18 July. At the Agulukpak River, bear activity was monitored visually on a daily basis at the head of the river.
Changes in foraging activity of gulls and bears at each site were quantified by fitting a cumulative normal distribution (cnd), defined by a mean and standard deviation, to the cumulative number of bears or gulls observed at each site from 20 June to 12 September. Bears were quantified at a daily temporal resolution from cameras and visual surveys, whereas gulls were quantified at a more coarse resolution, depending on how often surveys were taken at each site. All data were deposited in the Dryad repository http://dx.doi.org/10.5061/dryad.jj0fg [12].
3. Results
Average summer water temperatures varied from 5.6°C to 12.4°C among the 24 sockeye salmon spawning sites (figure 1a). The date sockeye salmon initiated spawning varied by 43 days among individual populations, and was positively correlated with average summer water temperature (r2 = 0.63, p < 0.0001; figure 1b). Sockeye salmon initiated spawning on the coldest sites between 12 and 22 July. At these sites, bear and gull activity increased substantially on approximately 15 July, and the mean of the cnd describing seasonal consumer activity was achieved 9 days later for bears and 18 days later for gulls (figure 1c,d; electronic supplementary material, table S1). Sockeye salmon initiated spawning at the warmest sites around 13 August, and the means of the seasonal bear and gull activity were both achieved about 7 days later. Overall, the seasonal timing of bear and gull foraging activity was positively correlated with the seasonal timing of sockeye spawn timing among sites (gulls: r2 = 0.57, n = 24, p < 0.0001, figure 1c; bears: r2 = 0.89, n = 7, p < 0.01, figure 1d). Using least-squares regressions of the date at which bears and gulls first initiated activity on streams with different temperatures and salmon spawn timing (see the electronic supplementary material, table S1), we estimate that bears and gulls were actively feeding on salmon for at least 65 and 60 days, respectively, across the study sites. Had there been no variation in spawn timing of salmon among populations, consumers would have had access to salmon for about 33 days, or about half the time period observed across the sites we surveyed in this watershed.
4. Discussion
Our data suggest that predators and scavengers of salmon seasonally shift their spatial distributions to exploit the variation in spawning sockeye salmon across a single river basin. In mid-July, activity of gulls and bears was concentrated on cold streams with early-spawning populations of salmon, and by late August had shifted to concentrate on lake beaches and large rivers to coincide with salmon spawning at these sites with warmer water temperatures. Because salmon are an ephemeral resource at any single location in space, individual consumers that can shift their distributions to track the phenological variation in salmon substantially extend (approx. 2×) the period of time over which they can capitalize on this critical resource. Further, because the abundance of salmon in any given population is extremely variable among years and population dynamics across the watershed do not tend to be synchronous through time [9], variation in spawn timing among populations probably enables mobile consumers to effectively locate particularly profitable foraging opportunities over the course of the salmon spawning season. If spawning were perfectly synchronous across the watershed, consumers would have about half as much time to locate and capitalize on locally profitable foraging opportunities. Further, because much of the stream temperature variation is expressed at relatively small spatial scales in this river basin [10], mobile consumers do not have to travel far to exploit the spatial and temporal variation in salmon. Given our data, we are unable to conclude that individual consumers actively move to exploit variation in run timing of salmon, and identify the mechanisms they use to track variation in salmon availability in the watershed. However, given that we rarely observe bears and gulls at other sites in the watershed at times when salmon are not actively spawning, we are confident that the trends observed are caused by coordinated movements of individual consumers across the landscape. At present, the magnitude of these benefits to mobile consumers such as bears and gulls has yet to be quantified.
While the fitness benefits of phenological variation in salmon spawning to terrestrial consumers are not quantified, it is apparent that aquatic predators benefit from this phenological diversity in salmon. For example, temperature variation between the tributaries of a third-order stream was associated with variation in the spawn timing of genetically distinct populations of sockeye salmon [13]. Rainbow trout (Oncorhynchus mykiss), a species that depends heavily on salmon eggs in coastal ecosystems, performed seasonal movements to capitalize on the time-dependent spatial variation in salmon eggs and, therefore, achieved higher seasonal growth rates by moving to exploit the extended foraging season produced by variation in spawn timing of its principal prey [13].
Ecosystem-based fisheries management (EBFM) seeks to balance requirements of ecosystems and benefits to humans in harvest strategies [14], yet there remains a poor understanding of which ecosystem attributes to consider in such policy. Here, we show that life-history variation within species may have important effects on ecosystem functions and is probably a key attribute to consider in EBFM efforts. While there is an increasing call for EBFM of salmon to satisfy their roles in watersheds [7], current efforts have focused specifically on total abundance as the relevant metric for allocating salmon to fisheries and ecosystems [8]. Our results suggest that additional emphasis should be placed on the role of life-history diversity in key traits of salmon in ecosystem processes. In the case of sockeye salmon in Bristol Bay, changing harvest rates to increase salmon abundance in watersheds without maintaining variation in spawn timing among populations may not succeed in benefitting terrestrial and aquatic consumers that depend on salmon resources to support their annual growth, but must do so in a very constricted time window. Further, human development of watersheds that reduces spatial variation in water temperatures or the diversity of spawn timing among salmon populations will erode the range of foraging opportunities for terrestrial consumers such as bears and gulls across the landscape.
While scientific and policy attention has focused on conserving species diversity, it is becoming increasingly apparent that distinct populations are under substantially more threat from human activities than are species [15]. For example, salmon watersheds of the Pacific Northwest have lost approximately 29 per cent of the population diversity of Pacific salmon [16], whereas no single species is at imminent risk of extinction. We suggest that future work should focus on understanding how biological diversity within individual taxa affects species' interactions, and ecosystem processes and services, to better inform conservation and management policy.
Acknowledgements
We thank the staff of the WTSP, Bill Berkhahn, Alison Eskelin and Gene Shepherd. Financial support was provided by the US NSF, the Gordon and Betty Moore Foundation, and the US Fish and Wildlife Service through their Western Alaska Landscape Conservation Cooperative.
References
- 1.Fryxell JM, Wilmshurst JF, Sinclair ARE, Haydon DT, Holt RD, Abrams PA. 2005. Landscape scale, heterogeneity, and the viability of Serengeti grazers. Ecol. Lett. 8, 328–335 10.1111/j.1461-0248.2005.00727.x (doi:10.1111/j.1461-0248.2005.00727.x) [DOI] [Google Scholar]
- 2.Sawyer H, Kauffman MJ. 2011. Stopover ecology of a migratory ungulate. J. Anim. Ecol. 80, 1078–1087 10.1111/j.1365-2656.2011.01845.x (doi:10.1111/j.1365-2656.2011.01845.x) [DOI] [PubMed] [Google Scholar]
- 3.van Wijk RE, Kolzsch A, Kruckenberg H, Ebbinge BS, Muskens G, Nolet BA. 2012. Individually tracked geese follow peaks of temperature acceleration during spring migration. Oikos 121, 655–664 10.1111/j.1600-0706.2011.20083.x (doi:10.1111/j.1600-0706.2011.20083.x) [DOI] [Google Scholar]
- 4.Coogan SCP, Nielsen SE, Stenhouse GB. 2012. Spatial and temporal heterogeneity creates a ‘brown tide’ in root phenology and nutrition. ISRN Ecol. 2012, 10. 10.5402/2012/618257 (doi:10.5402/2012/618257) [DOI] [Google Scholar]
- 5.Gende SM, Edwards RT, Willson MF, Wipfli MS. 2002. Pacific salmon in aquatic and terrestrial ecosystems. Bioscience 52, 917–928 10.1641/0006-3568(2002)052[0917:PSIAAT]2.0.CO;2 (doi:10.1641/0006-3568(2002)052[0917:PSIAAT]2.0.CO;2) [DOI] [Google Scholar]
- 6.Hilderbrand GV, Schwartz CC, Robbins CT, Jacoby ME, Hanley TA, Arthur SM, Servheen C. 1999. The importance of meat, particularly salmon, to body size, population productivity, and conservation of North American brown bears. Can. J. Zool. 77 10.1139/cjz-77-1-132 (doi:10.1139/cjz-77-1-132) [DOI] [Google Scholar]
- 7.Darimont CT, et al. 2010. Salmon for terrestrial protected areas. Conserv. Lett. 3, 379–389 10.1111/j.1755-263X.2010.00145.x (doi:10.1111/j.1755-263X.2010.00145.x) [DOI] [Google Scholar]
- 8.Levi T, Darimont CT, MacDuffee M, Mangel M, Paquet P, Wilmers CC. 2012. Using grizzly bears to assess harvest-ecosystem tradeoffs in salmon fisheries. PLoS Biol. 10, 10. 10.1371/journal.pbio.1001303 (doi:10.1371/journal.pbio.1001303) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schindler DE, Hilborn R, Chasco B, Boatright CP, Quinn TP, Rogers LA, Webster MS. 2010. Population diversity and the portfolio effect in an exploited species. Nature 465, 609–612 10.1038/nature09060 (doi:10.1038/nature09060) [DOI] [PubMed] [Google Scholar]
- 10.Lisi PJ, Schindler DE, Bentley KT, Pess GR. 2013. Association between geomorphic attributes of watersheds, water temperature, and salmon spawn timing in Alaskan streams. Geomorphology 185, 78–86 [Google Scholar]
- 11.Shardlow TF, Hyatt KD. 2013. Quantifying associations of large vertebrates with salmon in riparian areas of British Columbia streams by means of camera-traps, bait stations, and hair samples. Ecol. Indicator 27, 97–107 10.1016/j.ecolind.2012.11.011 (doi:10.1016/j.ecolind.2012.11.011) [DOI] [Google Scholar]
- 12.Schindler DE, Armstrong JB, Bentley KT, Jankowski K, Lisi PJ, Payne LX. 2013. Riding the crimson tide: mobile terrestrial consumers track phenological variation in spawning of an anadromous fish. 10.5061/dryad.jj0fg (doi:10.5061/dryad.jj0fg) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruff CP, Schindler DE, Armstrong JB, Bentley KT, Brooks GT, Holtgrieve GW, McGlauflin MT, Torgersen CE, Seeb JE. 2011. Temperature-associated population diversity in salmon confers benefits to mobile consumers. Ecology 92, 2073–2084 10.1890/10-1762.1 (doi:10.1890/10-1762.1) [DOI] [PubMed] [Google Scholar]
- 14.Pikitch EK, et al. 2004. Ecosystem-based fishery management. Science 305, 346–347 10.1126/science.1098222 (doi:10.1126/science.1098222) [DOI] [PubMed] [Google Scholar]
- 15.Hughes JB, Daily GC, Ehrlich PR. 1997. Population diversity: its extent and extinction. Science 278, 689–692 10.1126/science.278.5338.689 (doi:10.1126/science.278.5338.689) [DOI] [PubMed] [Google Scholar]
- 16.Gustafson RG, Waples RS, Myers JM, Weitkamp LA, Bryant GJ, Johnson OW, Hard JJ. 2007. Pacific salmon extinctions: quantifying lost and remaining diversity. Conserv. Biol. 21, 1009–1020 10.1111/j.1523-1739.2007.00693.x (doi:10.1111/j.1523-1739.2007.00693.x) [DOI] [PubMed] [Google Scholar]