Abstract
The hosts of brood parasitic birds are under strong selection pressure to recognize and remove foreign eggs from their nests, but parasite eggs may be too large to be grasped whole and too strong to be readily pierced by the host's bill. Such operating constraints on egg removal are proposed to force some hosts to accept parasite eggs, as the costs of deserting parasitized clutches can outweigh the cost of rearing parasites. By fitting microcameras inside nests, we reveal that the Neotropical baywing (Agelaioides badius), a host of the screaming cowbird (Molothrus rufoaxillaris) and shiny cowbird (Molothrus bonariensis), instead circumvents such constraints by kicking parasite eggs out of the nest. To our knowledge, this is the first report of a passerine bird using its feet to remove objects from the nest. Kick-ejection was an all-or-nothing response. Baywings kick-ejected parasite eggs laid before their own first egg and, if heavily parasitized, they ejected entire clutches and began again in the same nest. Few baywings were able to rid their nests of every parasite egg, but their novel ejection method allowed them to reduce the median parasitism intensity by 75 per cent (from four to one cowbird eggs per nest), providing an effective anti-parasite defence.
Keywords: brood parasitism, egg rejection, cowbird, host defence
1. Introduction
Many hosts of obligate brood parasitic birds defend themselves against parasitism by removing foreign eggs from their nest. Hosts can use their bills to perform such removals in one of two ways: either by grasping the egg whole, or first puncturing the egg and then gripping the broken shell [1]. For some hosts, however, neither will be a suitable technique.
Grasp-ejection is prevented when the parasite's egg is too large relative to the host's bill [2–4]. Similarly, puncture-ejection is impeded when the parasite egg's shell is too tough to pierce [5,6]. Facing such constraints, hosts could abandon the nest and build a new one or add new nest material to cover over the parasitized clutch [7–9], but both of these strategies delay reproduction and do not guarantee that the replacement clutch will escape parasitism [8–11]. Operating constraints on egg removal have thus been proposed to play an important role in determining whether or not hosts evolve this defence [2]. If the costs of abandoning or burying a parasitized clutch are greater than the costs of rearing parasitized broods, then egg acceptance will be maintained in evolutionary equilibrium [2,6,9–11].
In light of such constraints, however, the behaviour of South America's baywing (Agelaioides badius) is puzzling. Bill measurements indicate that baywings should be unable to grasp the eggs of their parasites, the screaming cowbird (Molothrus rufoaxillaris) and the shiny cowbird (Molothrus bonariensis) [12]. Nevertheless, they often eject cowbird eggs, which can be found just outside the rim of the nest cup or on the ground below the nest [12]. These ejected eggs are usually intact, excluding the possibility of puncture-ejection. Moreover, cowbird eggs have thick eggshells [5] and resist puncture well [13]. How then are baywings able to eject cowbirds eggs?
In this study we (i) use microcameras inside baywing nests to describe for the first time their egg ejection behaviour and (ii) assess whether their egg ejection constitutes a defence against parasitism.
2. Material and methods
The study was conducted at El Destino Reserve, Buenos Aires Province, Argentina (35°08′ S, 57°23′ W) between 2002 and 2012. Baywings breed principally in the old nests of other species, and sometimes in nest-boxes [14]. Annual rates of parasitism by screaming and shiny cowbirds vary between 92–100% and 16–23% of all nests, respectively [14]. To determine how baywings eject eggs, we placed infrared microcameras (Handykam) inside eight baywing nests, connected to digital video recorders below the nest (Lawmate PV-500). To increase our chances of filming ejection, we artificially parasitized five of these nests with one screaming cowbird egg, prior to baywing laying.
Egg ejection occurs in two forms [12]. The first is pre-laying ejection (the ejection of cowbird eggs laid before the baywing begins laying). Screaming and shiny cowbirds lay 31 per cent and 48 per cent of their eggs, respectively, before the first baywing egg [15]. We quantified baywings’ responses to these cowbird eggs from a sample of 116 nests found before host's laying (see the electronic supplementary material, table S1). We checked nests daily or every other day to record nest contents. Eggs were considered ‘ejected’ if they disappeared from an active nest or were found outside the nest cup, ‘buried’ if baywings covered them with new lining material, or ‘accepted’ if they remained in the nest cup after baywings began to lay.
The second form of ejection that may occur is the ejection of the entire clutch, including the baywing's own eggs, after which a new clutch is laid in the same nest [12]. We quantified baywing responses to clutches using a sample of 87 nests that were active at least until the baywing's clutch was completed, and where we did not manipulate clutch composition (see the electronic supplementary material, table S2). We considered a clutch ‘rejected’ if baywings did not incubate the eggs (assessed by egg temperature) and ‘accepted’ otherwise. We classified rejected clutches as ‘ejected’ if all eggs were missing or found outside the nest cup but the nest remained active, and ‘abandoned’ if eggs remained in the nest cup but parents no longer attended the nest. To confirm that clutch ejection was a response to parasitism, we analysed the effect of parasitism on clutch rejection by hosts using a generalized linear model (GLM) with binomial error distribution and logit link function with clutch rejection as the response variable. Explanatory variables were the number of cowbird eggs laid during host's laying (i.e. parasitism intensity) and clutch initiation date. The intensity of parasitism correlated positively with total clutch size (Spearman correlation: r = 0.86, p < 0.0001, n = 87 nests) and negatively with the number of host eggs at the end of laying (r =−0.39, p = 0.0002, as cowbirds sometimes destroy eggs prior to parasitism); thus, total clutch size and host egg number were not included in the model. We first fitted the full model and subsequently removed non-significant terms (p > 0.10) to obtain a minimal model. To calculate whether baywings benefited from clutch ejection, we compared parasitism rates and intensity, and survivorship of host eggs, between ejected and replacement clutches using non-parametric tests.
Finally, to calculate the net benefit of baywings’ rejection habits, we compared the number of cowbird eggs laid in baywing nests with the number of cowbird eggs found in incubated clutches using a Mann–Whitney U test. Statistical tests were two-tailed and completed in R v. 2.13.2.
3. Results
Video recordings showed that baywings ejected intact eggs by kicking them out of the nest cup using their feet (see the electronic supplementary material, video S1 and S2). This included seven nests where cowbird eggs were laid (n = 2) or artificially added (n = 5) before the baywing laid its first egg, and one nest where an entire naturally parasitized clutch was ejected. Baywings would stand in the nest, lean forward onto their breast and run rapidly on the spot until their feet made contact with the egg and propelled it out of the nest. Running-on-the-spot behaviour continued sometimes even after all eggs had been ejected. We did not observe baywings attempt to grasp or puncture intact eggs, but they did use their bills to remove sticks from the nest and eggs that had been broken by cowbirds.
From 116 nests found during pre-laying, 78 (67%) were parasitized before hosts began laying with 231 cowbird eggs (mean ± s.e., 3.0 ± 0.3 per nest; range, 1–14). Hosts ejected 210 of those eggs (91%, 72 nests), buried 17 by adding new nest material (7%, four nests), and accepted four (2%, four nests). Pre-laying ejection removed 36 per cent of all cowbird eggs laid in these nests (n = 605). Baywings rejected their entire clutch at 27 of 87 nests that survived until at least the baywings’ clutch completion. Twenty-four clutches were ejected and the other three abandoned, usually within 2 days from clutch completion (mean ± s.e., 1.10 ± 0.01 days; range, 0–5, n = 23 nests with known rejection dates). Clutch ejection was positively related to the intensity of parasitism during host's laying (GLM: Wald 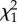 = 4.39, p < 0.0001, n = 84; figure 1), but unrelated to clutch initiation date (Wald
= 4.39, p < 0.0001, n = 84; figure 1), but unrelated to clutch initiation date (Wald 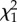 =−0.24, p = 0.81). Following clutch ejection, hosts laid a replacement clutch in the same nest in all but one case, where the nest was later abandoned. Ejected and replacement clutches did not differ in parasitism rates (22 of 23 and 19 of 23 parasitized nests, respectively; Fisher's exact test: p = 0.35), but replacement clutches received significantly fewer cowbird eggs (ejected: median = 4, interquartile range = 2–4.5; replacement: median = 1, interquartile range = 1–2; Wilcoxon signed-rank test: z =−3.16, p = 0.0016) and had significantly more baywing eggs survive to clutch completion (ejected: median = 3, interquartile range = 3–3.5; replacement: median = 4, interquartile range = 3.5–4; Wilcoxon: z = 3.21, p = 0.0013).
=−0.24, p = 0.81). Following clutch ejection, hosts laid a replacement clutch in the same nest in all but one case, where the nest was later abandoned. Ejected and replacement clutches did not differ in parasitism rates (22 of 23 and 19 of 23 parasitized nests, respectively; Fisher's exact test: p = 0.35), but replacement clutches received significantly fewer cowbird eggs (ejected: median = 4, interquartile range = 2–4.5; replacement: median = 1, interquartile range = 1–2; Wilcoxon signed-rank test: z =−3.16, p = 0.0016) and had significantly more baywing eggs survive to clutch completion (ejected: median = 3, interquartile range = 3–3.5; replacement: median = 4, interquartile range = 3.5–4; Wilcoxon: z = 3.21, p = 0.0013).
Figure 1.
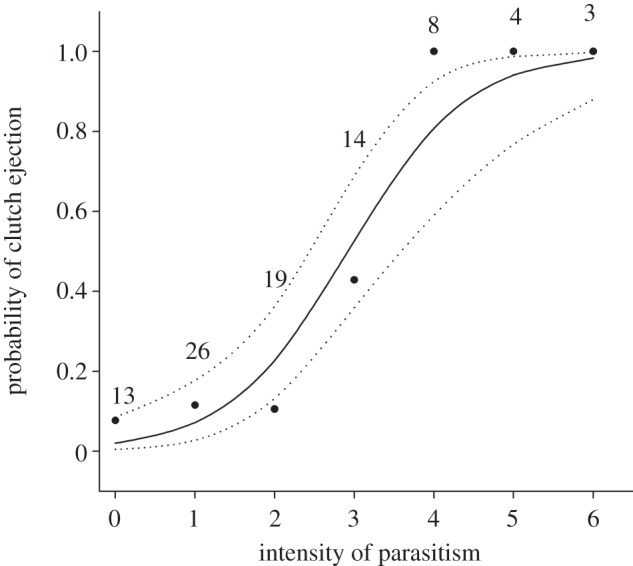
Probability of entire clutch ejection (solid line) and 95% CI (dotted line) as a function of the intensity of parasitism during host's laying as predicted from a GLM. Black circles indicate the observed proportion of ejected clutches, with sample sizes above.
Considering the effects of both pre-laying ejections and clutch ejections, baywings reduced the median parasitism intensity of their clutches by 75 per cent, from four to one cowbird egg per nest (Mann–Whitney U test: z =−7.03, p < 0.0001; figure 2).
Figure 2.
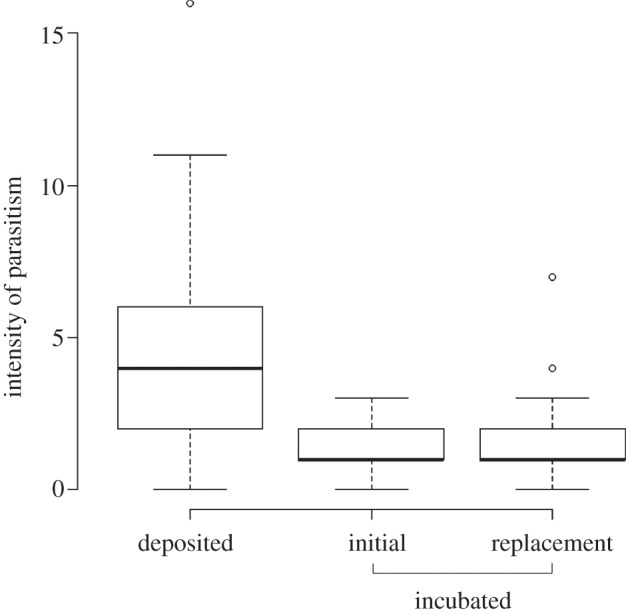
Boxplots of the number of cowbird eggs deposited in baywing nests (n = 116 nests) and the number that remained in incubated clutches, including initial clutches (n = 60) and replacement clutches (n = 23).
4. Discussion
We show that baywings eject their brood parasites’ eggs by kicking them out of the nest cup with their feet. In doing so, they circumvent the physical constraints of removing parasite eggs with the bill [12], without abandoning the nest or partially rebuilding it.
While the use of the bill to eject parasite eggs probably derives from nest sanitation behaviours widespread among birds [16], we know of no precedent for passerines actively using their feet to remove objects from the nest. The movements associated with kick-ejection were, however, highly stereotyped and repeated during the pre-laying phase (whether or not eggs were present), only ceasing when baywings began to lay. Perhaps then, the baywing's singular ejection method has its origins in nest-building behaviours used to shape the nest cup before laying, where these behaviours have become greatly exaggerated under the selection pressure of parasitism.
Unlike ejection with the bill, baywing's kick-ejection appears to preclude the selective removal of only foreign eggs, as they cannot see the eggs they are kicking. Ejection was instead all-or-nothing, with baywings ejecting almost all eggs laid before their own first egg, and then if heavily parasitized, ejecting entire clutches (after which they laid a new clutch in the same nest). Whether baywings can recognize parasite eggs in their nest is unclear. Recognition is not necessary, however, to account for their rejection habits, which may be triggered simply by the timing and intensity of parasitism [12].
Kick-ejection rarely resulted in baywings rearing unparasitized broods, but did greatly reduce the number of cowbirds eggs incubated, from four to one per nest (75%). Such a reduction in parasitism intensity would lead parents to waste less energy on rearing unrelated offspring [17], and probably reduces the risk that their young are outcompeted by cowbirds for food (given that baywing chicks sometimes perish in parasitized broods, even when cowbirds number just one or two [12,14]). Also, in the case of whole-clutch ejections, replacement clutches typically had not only fewer cowbird eggs but more surviving baywing eggs (owing to reduced egg-puncturing by cowbirds). Thus, while the net costs and benefits of kick-ejection remain to be investigated, we propose it is an effective anti-parasite defence, alternative to those described so far in hosts of avian brood parasites.
Acknowledgements
This study complies with Argentinian Law.
We thank Fundación Elsa Shaw de Pearson for permitting our research at Reserva El Destino. M.C.D.M. and J.C.R. are Research Fellows of Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET). C.A.U. was supported by a CONICET fellowship, and R.G. by the Cogito Foundation. This study was supported by grants of Agencia Nacional de Promoción Científica y Tecnológica and the University of Buenos Aires.
References
- 1.Davies N. 2000. Cuckoos, cowbirds and other cheats. London, UK: T & A.D. Poyser [Google Scholar]
- 2.Rohwer S, Spaw CD. 1988. Evolutionary lag versus bill-size constraints: a comparative study of the acceptance of cowbird eggs by old hosts. Evol. Ecol. 2, 27–36 10.1007/BF02071586 (doi:10.1007/BF02071586) [DOI] [Google Scholar]
- 3.Underwood TJ, Sealy SG. 2006. Grasp-ejection in two small ejecters of cowbird eggs: a test of bill-size constraints and the evolutionary equilibrium hypothesis. Anim. Behav. 71, 409–416 10.1016/j.anbehav.2005.06.004 (doi:10.1016/j.anbehav.2005.06.004) [DOI] [Google Scholar]
- 4.Rasmussen JL, Underwood TJ, Sealy SG. 2010. Functional morphology as a barrier to the evolution of grasp ejection in hosts of the brown-headed cowbird (Molothrus ater). Can. J. Zool. 88, 2010–2017 10.1139/Z10-088 (doi:10.1139/Z10-088) [DOI] [Google Scholar]
- 5.Mermoz ME, Ornelas JF. 2004. Phylogenetic analysis of life-history adaptations in parasitic cowbirds. Behav. Ecol. 15, 109–119 10.1093/beheco/arg102 (doi:10.1093/beheco/arg102) [DOI] [Google Scholar]
- 6.Antonov A, Stokke BG, Moksnes A, Røskaft E. 2009. Evidence for egg discrimination preceding failed rejection attempts in a small cuckoo host. Biol. Lett. 5, 169–171 10.1098/rsbl.2008.0645 (doi:10.1098/rsbl.2008.0645) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petit LJ. 1991. Adaptive tolerance of cowbird parasitism by prothonotary warblers: a consequence of nest-site limitation? Anim. Behav. 41, 425–432 10.1016/S0003-3472(05)80843-7 (doi:10.1016/S0003-3472(05)80843-7) [DOI] [Google Scholar]
- 8.Guigueno MF, Sealy SG. 2010. Clutch abandonment by parasitized yellow warblers: egg burial or nest desertion? Condor 112, 399–406 10.1525/cond.2010.090135 (doi:10.1525/cond.2010.090135) [DOI] [Google Scholar]
- 9.Hosoi SA, Rothstein SI. 2000. Nest desertion and cowbird parasitism: evidence for evolved responses and evolutionary lag. Anim. Behav. 59, 823–840 10.1006/anbe.1999 (doi:10.1006/anbe.1999) [DOI] [PubMed] [Google Scholar]
- 10.Hoover JP, Yasukawa K, Hauber ME. 2006. Spatially and temporally structured avian brood parasitism affects the fitness benefits of hosts’ rejection strategies. Anim. Behav. 72, 881–890 10.1016/j.anbehav.2006.02.011 (doi:10.1016/j.anbehav.2006.02.011) [DOI] [Google Scholar]
- 11.Krüger O. 2011. Brood parasitism selects for no defence in a cuckoo host. Proc. R. Soc. B 278, 2777–2783 10.1098/rspb.2010.2629 (doi:10.1098/rspb.2010.2629) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fraga RM. 1998. Interactions of the parasitic screaming and shiny cowbirds (Molothrus rufoaxillaris and M. bonariensis) with a shared host, the bay-winged cowbird (M. badius). In Parasitic birds and their hosts (eds Rothstein SI, Robinson SK.), pp. 173–193 Oxford, UK: Oxford University Press [Google Scholar]
- 13.Gloag R, Fiorini VD, Reboreda JC, Kacelnik A. 2012. Brood parasite eggs enhance egg survivorship in a multiply parasitized host. Proc. R. Soc. B 279, 1831–1839 10.1098/rspb.2011.2047 (doi:10.1098/rspb.2011.2047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Mársico MC, Mahler B, Reboreda JC. 2010. Reproductive success and nestling growth of the baywing parasitized by screaming and shiny cowbirds. Wilson J. Ornithol. 122, 417–431 10.1676/09-140.1 (doi:10.1676/09-140.1) [DOI] [Google Scholar]
- 15.De Mársico MC, Reboreda JC. 2008. Egg-laying behavior in screaming cowbirds: why does a specialist brood parasite waste so many eggs? Condor 110, 143–153 10.1525/cond.2008.110.1.143 (doi:10.1525/cond.2008.110.1.143) [DOI] [Google Scholar]
- 16.Guigueno M, Sealy SG. 2012. Nest sanitation in passerine birds: implications for egg rejection in hosts of brood parasites. J. Ornithol. 153, 35–53 10.1007/s10336-011-0731-0 (doi:10.1007/s10336-011-0731-0) [DOI] [Google Scholar]
- 17.Ursino CA, De Mársico MC, Sued M, Farall A, Reboreda JC. 2011. Brood parasitism disproportionately increases nest provisioning and helper recruitment in a cooperatively breeding bird. Behav. Ecol. Sociobiol. 65, 2279–2286 10.1007/s00265-011-1238-7 (doi:10.1007/s00265-011-1238-7) [DOI] [Google Scholar]


