Abstract
Pseudosuchia, one of the two main clades of Archosauria (Reptilia: Diapsida), suffered a major decline in lineage diversity during the Triassic–Jurassic (TJ) mass extinction (approx. 201 Ma). Crocodylomorpha, including living crocodilians and their extinct relatives, is the only group of pseudosuchians that survived into the Jurassic. We reassess changes in pseudosuchian morphological diversity (disparity) across this time interval, using considerably larger sample sizes than in previous analyses. Our results show that metrics of pseudosuchian disparity did not change significantly across the TJ boundary, contrasting with previous work suggesting low pseudosuchian disparity in the Early Jurassic following the TJ mass extinction. However, a significant shift in morphospace occupation between Late Triassic and Early Jurassic taxa is recognized, suggesting that the TJ extinction of many pseudosuchian lineages was followed by a major and geologically rapid adaptive radiation of crocodylomorphs. This marks the onset of the spectacularly successful evolutionary history of crocodylomorphs in Jurassic and Cretaceous ecosystems.
Keywords: disparity, adaptive radiation, Triassic–Jurassic mass extinction, Pseudosuchia, Crocodylomorpha
1. Introduction
Pseudosuchia is one of the two major subdivisions of Archosauria [1,2], along with the ‘bird-line archosaurs’ Avemetatarsalia (which includes dinosaurs, pterosaurs and birds). Early pseudosuchians, the ‘crocodile-line’ archosaurs (crocodilian stem-lineage), have often been characterized as the potential competitors of early dinosaurs during the Late Triassic [3,4], approximately 235–201 Ma. The high morphological diversity and species richness achieved by Triassic pseudosuchians suffered dramatically during the Triassic–Jurassic (TJ) extinction (one of the ‘big five’ Phanerozoic mass extinctions), with only one lineage surviving into the Jurassic, the Crocodylomorpha. However, a reported dramatic decrease in pseudosuchian morphological diversity (disparity) in the Early Jurassic [5] has been proposed to be potentially an artefact of low sample sizes and incomplete taxonomic sampling [6]. In order to test previous hypotheses of pseudosuchian disparity decline across the TJ boundary [5], we compiled a new dataset with substantially increased taxon sampling among Early Jurassic taxa.
Here, we provide evidence that pseudosuchian disparity in the Early Jurassic equalled that of the Late Triassic when corrected for sample size differences, and that the TJ extinction was followed by a geologically rapid adaptive radiation of crocodylomorphs. This radiation marks the beginning of the spectacular evolutionary history of crocodylomorphs in post-Triassic Mesozoic ecosystems, which saw the clade evolve an astonishing range of body sizes, habitats and niches [7,8].
2. Material and methods
Our dataset combines four morphological cladistic studies [1,2,9,10] and includes 298 discrete cranial characters scored across 36 Late Triassic and Early Jurassic pseudosuchians. The 36 taxa were chosen to represent all major morphologies and evolutionary lineages of pseudosuchians across the TJ boundary (see the electronic supplementary material). Taxa were binned according to stratigraphic age based upon the Paleobiology Database (http://paleodb.org/). Analyses included three separate comparisons. First, disparity was compared for all Late Triassic versus Early Jurassic pseudosuchians (two-bin analysis). Second, the Late Triassic and Early Jurassic intervals were each divided into two subequal length time bins (four-bin analysis). Finally, comparisons were made between Triassic and Jurassic Crocodylomorpha.
The dataset were transformed using the software Matrix [11] to derive a Euclidean distance matrix, which was then subjected to principal coordinates analysis (PCoA) in the multivariate package Ginkgo [12] using a Calliez negative eigenvalue correction. PCoA produced a taxon-defined empirical morphospace (figure 1). PCoA outputs were analysed using the program Rare [11] to produce the disparity metrics and rarefied disparity profiles. Four disparity metrics were calculated: the sum and product of the ranges and variances on the first 26 axes, which encompass 90 per cent of the cumulative variance. Bootstrapping re-sampling was carried out with 1000 replicates and 95% CIs were used to assess statistical significance of differences in disparity between time bins and taxonomic groupings. Rarefaction profiles were reconstructed using a minimum of five taxa (in all analysis except for disparity within Crocodylomorpha, where a minimum of three was used owing to an overall smaller sample size), and a maximum corresponding to the total number of taxa being analysed in each run. Range measures indicate the entire spread of morphological variation (morphospace size), whereas variance measures denote average dissimilarity among forms (spread of taxa in morphospace) [13,14]. Rarefaction curves [15] are used to standardize disparity measures according to sample size, and help visualize the rate at which mean disparity values change with increasing numbers of taxa. In order to assess statistical significance of the separation in morphospace between Late Triassic and Early Jurassic taxa a non-parametric multivariate analysis of variance (npMANOVA [16]) was used. Calculations were performed in PAST [17].
Figure 1.
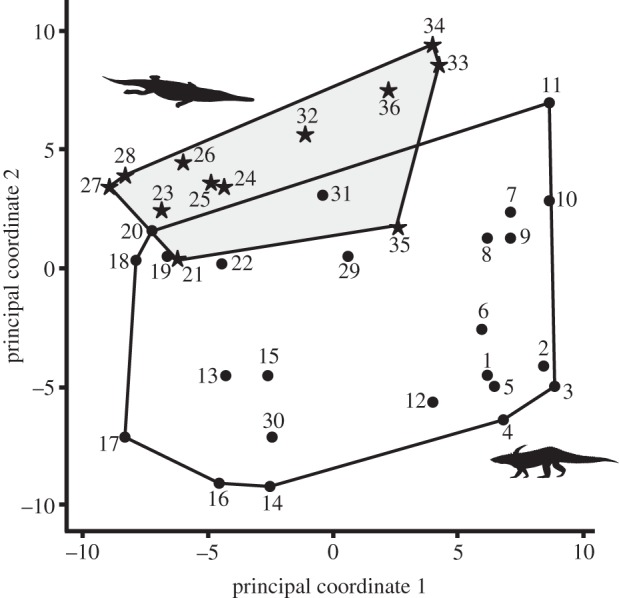
Two-dimensional morphospace for all 36 pseudosuchian taxa based on principal coordinate axes 1 and 2. Principal coordinate axes 1 and 2 represent cumulatively 26.68% of overall variance (PCo1, 16.69%; PCo2, 9.99%). Black circles, Late Triassic; black stars, Early Jurassic. Numbers for taxa are provided in the electronic supplementary material. Silhouettes taken from Wikipedia and www.phylopic.org.
3. Results
Late Triassic species occupy a significantly different area of morphospace from Early Jurassic species, with only a small overlap (figure 1; npMANOVA test, F = 3.827, p = 0.0019*). Early Jurassic taxa occupy a more restricted area of morphospace than do Late Triassic taxa; however, this may be related to sample size differences (12 Early Jurassic taxa compared with 24 Late Triassic taxa) that stem from the relatively small number of fossiliferous terrestrial rock sequences of Early Jurassic age worldwide.
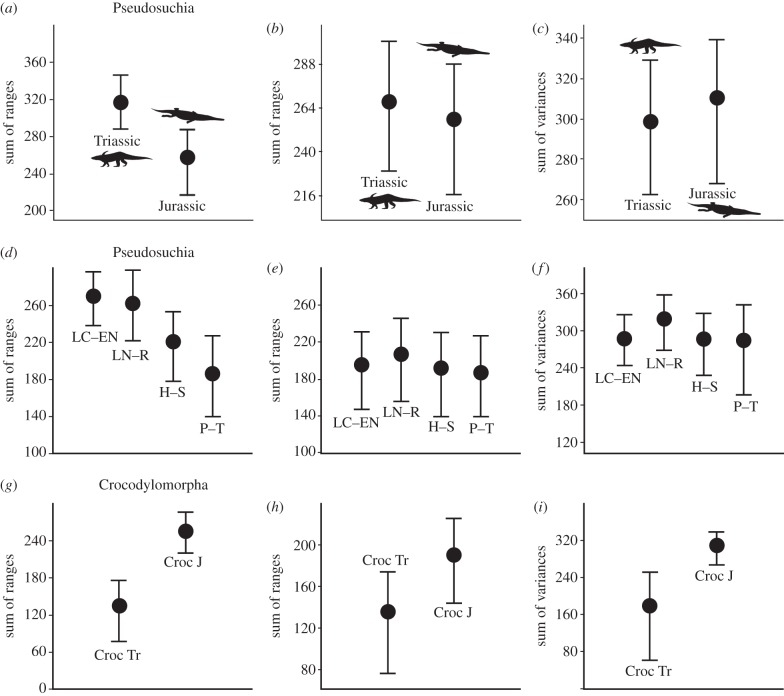
Consistent results were obtained with all four disparity metrics; we present here two of these metrics, the sum of ranges and sum of variances (see the electronic supplementary material for additional results). When complete sample sizes are considered, a significant decrease in disparity from the Late Triassic to Early Jurassic is seen in sum of ranges data (figure 2a,d) for both two-bin and four-bin analyses. These decreases disappear almost completely when rarefaction is used to correct for sample size, and the difference between the Triassic and Jurassic is no longer significant in either analysis (figure 2b,e). No significant differences are observed in the sum of variances for either two-bin or four-bin analyses (figure 2c,f). Comparisons of Late Triassic and Early Jurassic crocodylomorphs show significantly higher disparity for Early Jurassic taxa for both sum of ranges and sum of variances (figure 2g,i). When corrected for unequal sample sizes, the increase in disparity is still visible but is non-significant (figure 2h). Rarefaction profiles show that Jurassic crocodylomorphs are more disparate than Triassic crocodylomorphs at all sample sizes (see the electronic supplementary material), suggesting that this pattern is robust.
Figure 2.
Morphological disparity for different subsets of the pseudosuchian dataset. Mean disparity values, based on the sum of ranges and sum of variances are shown with error bars representing 95% CIs, obtained with 1000 bootstrap replicates. Comparisons of mean disparity values are illustrated for: (a,b,c) Late Triassic and Early Jurassic (b, corrected to an equal sample size); (d,e,f) four time intervals (e, corrected to an equal sample size; abbreviations: LC, late Carnian; EN, early Norian; LN, late Norian; R, Rhaetian; H, Hettangian; S, Sinemurian; P, Pliensbachian; T, Toarcian); (g,h,i) Late Triassic and Early Jurassic crocodylomorphs (h corrected to equal sample size).
4. Discussion
The most striking result of our study is that, when corrected for sample size, metrics of morphological disparity for pseudosuchians as a whole remain essentially unchanged across the TJ boundary, despite the decimation of the clade at that boundary, with only crocodylomorphs surviving into the Jurassic. However, we also show that Late Triassic pseudosuchian taxa occupy a significantly different area of morphospace from Early Jurassic taxa (with only a small overlap), and that crocodylomorph disparity increases across the boundary. The shift in morphospace indicates that the body plans shown by pseudosuchians changed significantly across the TJ boundary, at least when cranial characters are analysed. This suggests that the lineages of crocodylomorphs that survived the TJ extinction radiated rapidly into different morphologies from those represented by pseudosuchians during the Triassic. Our results contrast with the previous work of Brusatte et al. [5], who reported that pseudosuchian disparity was significantly lower in the Early Jurassic than in the Late Triassic. It is likely that, as previously proposed [6], the results reported by those authors are biased by low taxonomic sampling (four Early Jurassic pseudosuchian species in contrast with the 12 analysed here) and exclusion of morphologically divergent taxa such as thalattosuchians.
The highly derived phylogenetic position of the Early Jurassic goniopholid crocodylomorph Calsoyasuchus valliceps (Kayenta Formation of Arizona; [18]) implies that numerous and substantial missing lineages (‘ghost lineages’) are present around the base of Crocodylomorpha [9,18], supporting the hypothesis that a major crocodylomorph radiation occurred in the earliest Jurassic. Diverse crocodylomorph assemblages have been described from the Early Jurassic of Arizona, China and southern Africa [18–21]. However, Early Jurassic terrestrial vertebrate assemblages are generally poorly sampled worldwide, and recognition and documentation of this adaptive radiation likely remains incomplete. Furthermore, low sample sizes and poor temporal sampling require the use of relatively coarse time bins in disparity and richness analyses, making it difficult to pinpoint the exact timing of a pseudosuchian extinction and subsequent crocodylomorph radiation. Nevertheless, our documentation of both a significant shift in pseudosuchian morphospace occupation, and an increase in crocodylomorph disparity across the TJ boundary is consistent with a geologically rapid adaptive radiation of crocodylomorphs potentially triggered by the TJ decimation of pseudosuchian and other tetrapod lineages.
Our results support the hypothesis that the impact of the TJ extinction on some vertebrate groups was relatively short-lived and that for some clades the main extinctions were closely followed by new evolutionary radiations, which allowed the disparity of affected groups to recover. For pseudosuchians, any impact of the end-Triassic extinction on measures of disparity was apparently short-lived, by contrast with the marine ichthyosaurs in which disparity was severely reduced and never recovered to pre-extinction levels [22]. In both cases, however, there were significant shifts in morphospace occupation across the TJ boundary. Dinosaur disparity appears to have been largely unaffected by the TJ extinction [5], and no change in disparity of pterosaurs has been detected (although this may be an artefact of a large gap in Early Jurassic taxon sampling [23]). In summary, the impact of the TJ extinction on vertebrates appears to have been complex and clade specific, but for some clades such as crocodylomorphs the extinction may have played a critical role in triggering their subsequent evolutionary success.
Acknowledgements
We thank members of the Mesozoic Vertebrate (BSPG, Munich) and Archosauromorph (GeoBio-Center, LMU, Munich) research groups and John Parsch (LMU, Munich) for discussion. Steve Brusatte and Matthew Wills provided data and methodological advice. We thank the reviewers Steve Brusatte and Marcello Ruta for helpful review comments on an earlier version of this manuscript. R.J.B. is supported by an Emmy Noether Programme Award from the DFG (BU 2587/3-1).
References
- 1.Nesbitt SJ. 2011. The early evolution of archosaurs: relationships and the origin of major clades. Bull. Am. Mus. Nat. Hist. 352, 1–292 10.1206/352.1 (doi:10.1206/352.1) [DOI] [Google Scholar]
- 2.Brusatte SL, Benton MJ, Desojo JB, Langer MC. 2010. The higher-level phylogeny of Archosauria (Tetrapoda: Diapsida). J. Syst. Palaeont. 8, 3–47 10.1080/14772010903537732 (doi:10.1080/14772010903537732) [DOI] [Google Scholar]
- 3.Bakker RT. 1971. Dinosaur physiology and the origin of mammals. Evolution 25, 636–658 10.2307/2406945 (doi:10.2307/2406945) [DOI] [PubMed] [Google Scholar]
- 4.Brusatte SL, Benton MJ, Ruta M, Lloyd GT. 2008. Superiority, competition, and opportunism in the evolutionary radiation of dinosaurs. Science 321, 1485–1488 10.1126/science.1161833 (doi:10.1126/science.1161833) [DOI] [PubMed] [Google Scholar]
- 5.Brusatte SL, Benton MJ, Ruta M, Lloyd GT. 2008. The first 50 Myr of dinosaur evolution: macroevolutionary pattern and morphological disparity. Biol. Lett. 4, 733–736 10.1098/rsbl.2008.0441 (doi:10.1098/rsbl.2008.0441) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Irmis RB. 2011. Evaluating hypotheses for the early diversification of dinosaurs. Earth Environ. Sci. Trans. Roy. Soc. Edinburgh 101, 397–426 10.1017/S1755691011020068 (doi:10.1017/S1755691011020068) [DOI] [Google Scholar]
- 7.O'Connor PM, et al. 2010. The evolution of mammal-like crocodyliforms in the Cretaceous Period of Gondwana. Nature 466, 748–751 10.1038/nature09061 (doi:10.1038/nature09061) [DOI] [PubMed] [Google Scholar]
- 8.Sereno PC, Larsson HCE. 2009. Cretaceous crocodyliforms from the Sahara. ZooKeys 28, 1–143 10.3897/zookeys.28.325 (doi:10.3897/zookeys.28.325) [DOI] [Google Scholar]
- 9.Pol D, Turner AH, Norell MA. 2009. Morphology of the Late Cretaceous crocodylomorph Shamosuchus djadochtaensis and a discussion of neosuchian phylogeny as related to the origin of Eusuchia. Bull. Am. Mus. Nat. Hist. 324, 1–103 [Google Scholar]
- 10.Young MT, de Andrade MB. 2009. What is Geosaurus? Redescription of Geosaurus giganteus (Thalattosuchia: Metriorhynchidae) from the Upper Jurassic of Bayern, Germany. Zoo. J. Linn. Soc. 157, 551–585 10.1111/j.1096-3642.2009.00536.x (doi:10.1111/j.1096-3642.2009.00536.x) [DOI] [Google Scholar]
- 11.Wills MA. 1998. Crustacean disparity through the Phanerozoic: comparing morphological and stratigraphic data. Biol. J. Linnean Soc. 65, 455–500 10.1111/j.1095-8312.1998.tb01149.x (doi:10.1111/j.1095-8312.1998.tb01149.x) [DOI] [Google Scholar]
- 12.Bouxin G. 2005. Ginkgo, a multivariate analysis package. J. Veg. Sci. 16, 355–359 10.1111/j.1654-1103.2005.tb02374.x (doi:10.1111/j.1654-1103.2005.tb02374.x) [DOI] [Google Scholar]
- 13.Foote M. 1993. Contributions of individual taxa to overall morphological disparity. Paleobiology 19, 403–419 [Google Scholar]
- 14.Wills MA, Briggs DEG, Fortey RA. 1994. Disparity as an evolutionary index: a comparison of Cambrian and Recent arthropods. Paleobiology 20, 93–130 10.2307/2401014 (doi:10.2307/2401014) [DOI] [Google Scholar]
- 15.Foote M. 1992. Rarefaction analysis of morphological and taxonomic diversity. Paleobiology 18, 1–16 [Google Scholar]
- 16.Anderson MJ. 2001. A new method for non-parametric multivariate analysis of variance. Aust. Ecol. 26, 32–46 10.1111/j.1442-9993.2001.01070.pp.x (doi:10.1111/j.1442-9993.2001.01070.pp.x) [DOI] [Google Scholar]
- 17.Hammer Ø, Harper DAT, Ryan PD. 2001. PAST: paleontological statistics software package for education and data analysis. Pal. Electronica 4, 9 See http://palaeo-electronica.org/2001_1/past/issue1_01.htm [Google Scholar]
- 18.Tykoski RS, Rowe TB, Ketchum RA, Colbert MW. 2002. Calsoyasuchus valliceps, a new crocodyliform from the Early Jurassic Kayenta formation of Arizona. J. Vert. Paleontol. 22, 593–611 10.1671/0272-4634(2002)022[0593:CVANCF]2.0.CO;2 (doi:10.1671/0272-4634(2002)022[0593:CVANCF]2.0.CO;2) [DOI] [Google Scholar]
- 19.Sues H-D, Clark JM, Jenkins FA. 1994. A review of the Early Jurassic tetrapods from the Glen Canyon Group of the American Southwest. In In the shadow of the dinosaurs: Early Mesozoic tetrapods (eds Fraser NC, Sues H-D.), pp. 284–294 Cambridge, UK: Cambridge University Press [Google Scholar]
- 20.Luo Z, Wu X-C. 1994. The small tetrapods of the Lower Lufeng formation, Yunnan, China. In In the shadow of the dinosaurs: Early Mesozoic tetrapods (eds Fraser NC, Sues H-D.), pp. 251–270 Cambridge, UK: Cambridge University Press [Google Scholar]
- 21.Knoll F. 2005. The tetrapod fauna of the Upper Elliot and Clarens formations in the main Karoo Basin (South Africa and Lesotho). Bull. Soc. géol. Fr. 176, 81–91 10.2113/176.1.81 (doi:10.2113/176.1.81) [DOI] [Google Scholar]
- 22.Thorne PM, Ruta M, Benton MJ. 2011. Resetting the evolution of marine reptiles at the Triassic-Jurassic boundary. Proc. Natl Acad. Sci. USA 108, 8339–8344 10.1073/pnas.1018959108 (doi:10.1073/pnas.1018959108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Butler RJ, Brusatte SL, Andres B, Benson RBJ. 2012. How do rock record biases affect studies of disparity in deep time? A case study of the Pterosauria (Reptilia: Archosauria). Evolution 66, 147–162 10.1111/j.1558-5646.2011.01415.x (doi:10.1111/j.1558-5646.2011.01415.x) [DOI] [PubMed] [Google Scholar]