Abstract
While the ecological consequences of plant diversity have received much attention, the mechanisms by which intraspecific diversity affects associated communities remains understudied. We report on a field experiment documenting the effects of patch diversity in the plant Baccharis salicifolia (genotypic monocultures versus polycultures of four genotypes), ants (presence versus absence) and their interaction on ant-tended aphids, ants and parasitic wasps, and the mechanistic pathways by which diversity influences their multi-trophic interactions. Five months after planting, polycultures (versus monocultures) had increased abundances of aphids (threefold), ants (3.2-fold) and parasitoids (1.7-fold) owing to non-additive effects of genetic diversity. The effect on aphids was direct, as plant genetic diversity did not mediate ant–aphid, parasitoid–aphid or ant–parasitoid interactions. This increase in aphid abundance occurred even though plant growth (and thus aphid resources) was not higher in polycultures. The increase in ants and parasitoids was an indirect effect, due entirely to higher aphid abundance. Ants reduced parasitoid abundance by 60 per cent, but did not affect aphid abundance or plant growth, and these top-down effects were equivalent between monocultures and polycultures. In summary, intraspecific plant diversity did not increase primary productivity, but nevertheless had strong effects across multiple trophic levels, and effects on both herbivore mutualists and enemies could be predicted entirely as an extension of plant–herbivore interactions.
Keywords: ant-tended aphids, aphid-tending ants, Baccharis salicifolia, monocultures, parasitic wasps, polycultures
1. Introduction
Plant biodiversity has profound ecological consequences for the structure of their associated communities and ecosystem functions. Two decades of research have shown that high plant species diversity can lead to increased primary production [1,2], and the abundance and diversity of multi-trophic populations and communities that interact with plants [3,4]. More recent studies have shown that intraspecific plant genetic diversity can also affect community structure and govern ecosystem processes [5,6], with an effect size comparable with those of plant interspecific diversity [7]. Mechanistically, these effects of inter- and intraspecific plant diversity have been shown to occur through both sampling effects (diversity increases the likelihood of including exceptional individuals) and non-additive effects (diversity alters the traits of individuals; [5,6]).
Most studies on plant intra- and interspecific diversity have focused exclusively on the bottom-up effects of plant diversity within a single trophic level (herbivores; [8,9]), but plant diversity may also directly or indirectly affect the third trophic level, i.e. enemies and mutualists of herbivores (see [3,4,10], and the scheme represented in figure 1). The pathways for plant diversity to affect herbivore enemies or mutualists can be classified into two types [11]. First, there are density-mediated indirect interactions. In this case, plant diversity directly influences the density of herbivores and, in so doing, indirectly influence enemy/mutualist abundance (no changes in per capita interaction rates). Second, there are trait-mediated indirect interactions. In this case, plant diversity indirectly influences herbivore, enemy or mutualist traits and, in so doing, changes the per capita herbivore–enemy or herbivore–mutualist interaction. For example, plant diversity may modify (through changes in plant quality/resistance) either herbivore quality or herbivore susceptibility to enemies [11]. The distinction between these two mechanisms is in turn critical for predicting whether plant effects on higher trophic levels feedback to influence herbivores and plants; whereas trait-mediated effects alter the strength of such top-down effects, no such feedbacks are predicted where bottom-up effects are density-mediated [11,12].
Figure 1.
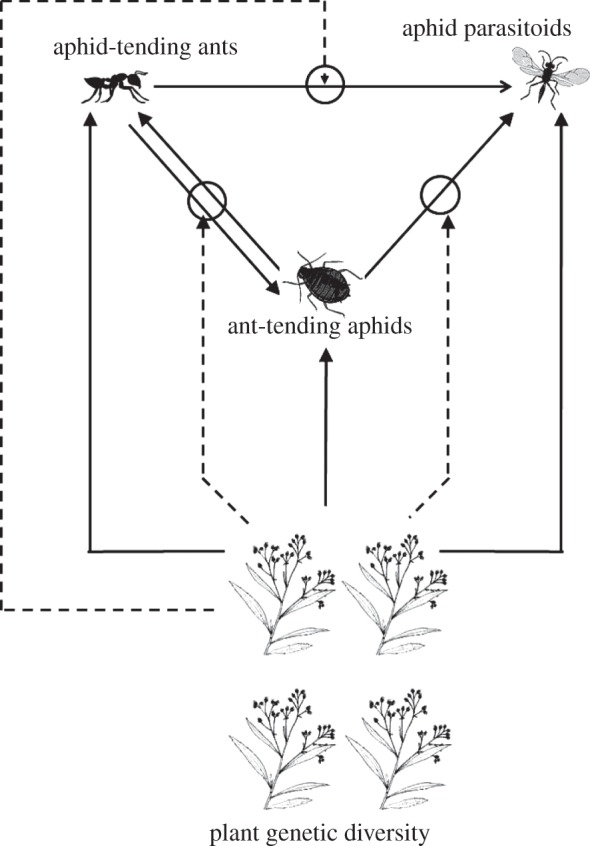
Food web associated with Baccharis salicifolia. Solid lines with arrowheads indicate direct effects among trophic levels. Dashed lines with circles indicate trait-mediated indirect effects, where plant diversity indirectly affects one species by directly affecting the traits of another and thus mediates their pairwise interaction (e.g. plant diversity influences the attractiveness of herbivores and, in so doing, indirectly influences enemy/mutualist abundance through changes in per capita interaction rates). Density-mediated indirect effects occur through the product of sequential direct effects (e.g. plant diversity influences the density of herbivores and, in so doing, indirectly influence enemy/mutualist abundance without changes in per capita interaction rates). Because we do not manipulate parasitoid presence/absence, the effects of parasitoids on aphids and ants were not quantified.
Despite recent advances in the study of plant diversity effects on food web dynamics [4,6,10], the relative importance of these two mechanisms remains understudied. Here, we investigated the bottom-up effects of plant genetic diversity on multi-trophic communities and the mechanistic pathways by which plant genetic diversity may vary in their influence on interactions between higher trophic levels.
2. Material and methods
(a). Study system
We studied the long-lived, dioecious woody shrub Baccharis salicifolia (Asteraceae) at the University of California Irvine's Arboretum (33.66° N, 117.85° E; Orange County, CA, USA). At this site, B. salicifolia is colonized by cotton aphid, Aphis gossypii Glover (Hemiptera: Aphididae) [13]. This aphid is commonly tended by the non-native ant Linepithema humile Mayr (Hymenoptera: Formicidae), which feeds upon the aphid's sugary waste (so-called ‘honeydew’) in exchange for protection from predators and parasitoids of aphids [13]. The most common natural enemies are parasitic wasps (Hymenoptera: Braconidae) [13].
(b). Experimental design and measurements
A common garden was established adjacent to the natural population from which the experimental plants were originally collected. On 1 March 2012 we planted one-year-old B. salicifolia plants (plant height = 101.1 ± 1.8 cm) that were propagated from cuttings of eight B. salicifolia genotypes (four male and four female). Plants were arranged in plots with two levels of plant genetic diversity: (i) 32 monoculture plots and (ii) 32 polyculture plots of four different genotypes (including two males and two females). Genotypes were randomly selected for inclusion in each polycultures. Each plot (genotypic combination hereafter) consisted of four plants in two parallel rows of two plants each. Plants within genotypic combinations were separated by 10 cm, and plots were separated by 1 m. On 21 June, we excluded ants from half of the plants (plant height = 250.3 ± 11.4 cm) by burying 20 cm-tall by 25 cm-diameter aluminium flashing rings into the soil 5 cm deep, and coating the outside surface with sticky paste (Tanglefoot Company, MI, USA) [12]. Control plants were surrounded by unburied aluminium rings without sticky paste. The experiment followed a randomized split-plot design replicated in eight blocks, with ant treatment (two levels: presence or absence) as the whole plot factor and genetic diversity (mono- and polycultures) as the split factor, with eight genotypic combinations in each block (four monocultures and four polycultures), and plant position within genotypic combinations being randomly assigned [10]. All blocks were separated by at least 2 m.
On 20 July, approximately five months after planting, we measured the total stem height of all the plants (plant height = 333.1 ± 14.3 cm) and censused all arthropods by visually surveying every plant. Plant size (a surrogate for growth rate) was taken an indicator of resource abundance for herbivores [10]. Arthropods were classified as: aphids (always A. gossypii), ants (always L. humile) and parasitic wasps (Braconidae spp.). Other arthropods were rare.
(c). Statistical analyses
Data analysis of plant growth and arthropod abundances (mean number per plant) was performed with mixed linear models using the mixed procedure in SAS (SAS v. 9.2 System, SAS, Cary, NC, USA). The main effects of ants, genetic diversity, their interaction and plant sex were treated as fixed factors. The effects of the genotypic combination nested within the diversity treatments, and genotypic combination × ant interaction were also included as fixed factors. The effects of block and block × ant interaction were treated as random factors. To account for size differences among plant genotypes, final height was included in analyses of arthropod abundance [10]. To test whether observed diversity effects were due to sampling versus non-additive effects, the approach of Loreau & Hector [14] was followed; observed polyculture values were compared with expected polyculture values based upon genotype measurements in monoculture according to Johnson et al. [6] (see the electronic supplementary material, appendix S1). Normality was achieved by log-transforming arthropod data.
3. Results
Five months after planting, we recorded 1256 arthropods, classified as 248 ants (20%), 770 ant-tended aphids (61%) and 238 parasitic wasps (19%).
We found that genetic polycultures (versus monocultures) increased the abundance of aphids threefold (F1,96 = 54.59; p < 0.001; electronic supplementary material, table S2), ants 3.2-fold (F1,48 = 21.74; p < 0.001; electronic supplementary material, table S3) and parasitic wasps 1.7-fold (F1,96 = 14.55; p < 0.001; electronic supplementary material, table S4; figure 2a,c,e respectively). In all cases, there were significant non-additive effects of diversity (see the electronic supplementary material, table S1). However, when aphid abundance was accounted for in the statistical model, the significant effect of plant diversity disappeared for ants (F1,47 = 1.71; p = 0.197; electronic supplementary material, table S3) and parasitic wasps (F1,95 = 0.29; p = 0.588; electronic supplementary material, table S4; figure 2d,f), suggesting that plant diversity effects on higher trophic levels were density-mediated indirect effects owing to direct effects on aphid abundance. Genetic monocultures (n = 8) did not differ significantly in arthropod abundance (aphids: F7,3 = 0.94, p = 0.578; ants: F7,1 = 0.42, p = 0.831; parasitoids: F7,3 = 1.52, p = 0.396; electronic supplementary material, figure S1).
Figure 2.
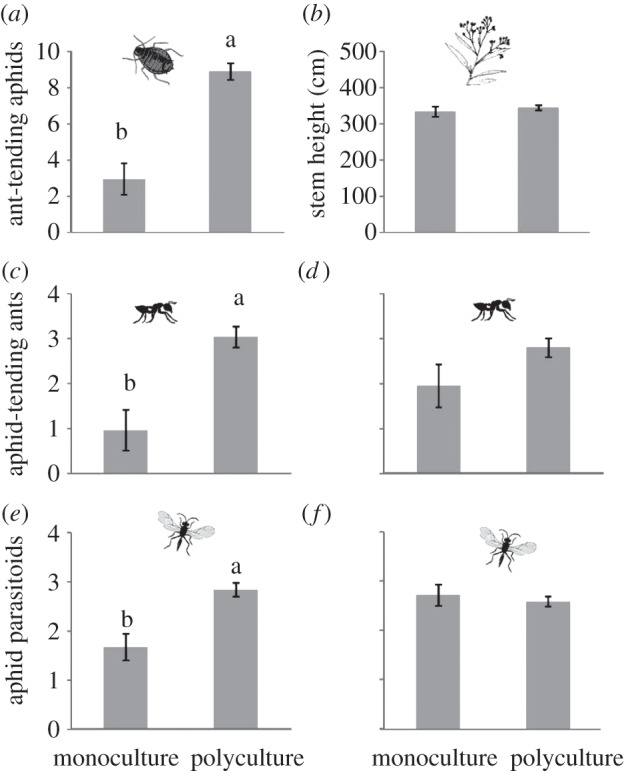
Effect of plant genetic diversity (monocultures versus polycultures) on (a) ant-tended aphids, (b) total stem height in cm, (c,d) aphid-tending ants and (e,f) aphid parasitoids. Total abundance (mean number per plant) was used to evaluate associated arthropods. To remove the density-mediated indirect effect of aphids on ants and parasitoids, we used aphid abundance as a covariate in the statistical model (d,f). Least-square means ± s.e. (n = 32) are shown, except for ants (n = 16). Different letters indicate significant differences (p < 0.05) among genetic diversity treatments.
Interestingly, the effect of plant diversity on aphids was not attributable to increased resource abundance, as diversity did not affect plant growth (F1,96 = 0.46; p = 0.501; electronic supplementary material, table S5; figure 2b), suggesting that instead higher aphid recruitment or retention on variable resource patches. Furthermore, ant effects were not contingent on plant diversity for either parasitoids (F1,96 = 0.20; p = 0.659) or aphids (F1,97 = 0.11; p = 0.741).
Similarly, plant diversity did not mediate the top-down effects; although the presence of ants (versus exclusion) reduced parasitoid abundance by 60 per cent (F1,6 = 10.43; p = 0.018; electronic supplementary material, table S4 and figure S2), ants did not affect aphid abundance (F1,6 = 1.49; p = 0.268; electronic supplementary material, table S2 and figure S2) or plant height (F1,49 = 3.52; p = 0.110; electronic supplementary material, table S5 and figure S2).
4. Discussion
Our results demonstrate that non-additive effects of plant genetic diversity strongly determined arthropod community structure from the bottom-up, but did not affect the interactions between higher trophic levels. Specifically, genetic diversity in B. salicifolia increased the abundance of aphids, aphid-tending ants and parasitic wasps. However, while plant genetic diversity exerted a direct influence over aphid abundance, the effect on the third trophic level (ants and parasitoids) was a density-mediated indirect effect owing to changes in aphid abundance; herbivore–mutualist, herbivore–enemy and mutualist–enemy interactions were not mediated by plant genetic diversity. Furthermore, these bottom-up effects were not due to changes in plant growth rate. Finally, ants had top-down effects on parasitoids but not aphids and plants, and these were consistent between monocultures and polycultures.
The direct positive effect of plant genetic diversity on herbivore abundance (here ant-tended aphids) has been commonly observed in previous studies [5,6,8]. Several mechanisms have been proposed in order to explain these diversity effects, for example: (i) complementarity in resource use among plant genotypes might increase plant growth/quality and thus aphid abundance [7]. However, we did not find greater plant growth in polyculture plots. (ii) Plant genetic diversity could increase the attraction of herbivores to airborne volatiles as has been reported elsewhere [15]. For example, Glinwood et al. [15] found that a mix of barley genotypes produced a more attractive combination of volatiles for an aphid species.
The most noteworthy result of our study, as we previously mentioned, was that plant genetic diversity effect on higher trophic levels (i.e. ant–aphid and parasitoid–aphid interactions) was a density-mediated indirect effect through changesin aphid abundance. Specifically, variation in aphid abundance caused parallel variation in ants and parasitoids. Past studies have investigated the mechanisms by which genetic diversity influence higher trophic levels in terms of sampling versus non-additive diversity effects [5,6], whereas the novelty of our work was in manipulating top-down control (ant presence/absence), and thus rigorously studying how genetic diversity mediates interactions among higher trophic levels [10]. Contrasting with our results, these previous works found that bottom-up effects of plant diversity increased the abundance of individuals from the third trophic level through trait-mediated indirect effects of herbivores [6,10]. For example, in similar work, Johnson et al. [6] found that plant genetic diversity of evening primrose (Oenothera biennis) increased the abundance and richness of predatory arthropods, independently of herbivore abundance. In parallel, Moreira et al. [10] found that pine species diversity increased ant abundance not only by increasing aphid number, but also by increasing ant recruitment per aphid. Whereas this study found density-mediated indirect interactions and no feedback, Moreira et al. [10] found trait-mediated indirect interactions and feedbacks to plant performance, probably due to suppression of untended herbivores by ants.
In conclusion, this study adds to the growing evidence for the community-wide consequences of population genetic diversity within plants species [5,6]. Intraspecific plant diversity had strong effects across multiple trophic levels. Yet, the effects on both herbivore mutualists and enemies could be predicted entirely as an extension of plant–herbivore interactions, and these bottom-up influences of diversity did not feedback to mediate the top-down effects of ants. These results thus underscore the importance of a mechanistic perspective for understanding and predicting the role of plant genetic diversity in structuring multi-trophic communities.
Acknowledgements
We thank Andrew Datu, Chelsea Hertler, Thanh T. Pham, Hong Chen, Shaun Hu, Luis Abdala-Roberts and Silvia Portela for their technical assistance. Comments and suggestions by two anonymous referees helped to improve the manuscript. This research was funded by National Science Foundation grants nos DEB-0919178 and DEB-1120794. X.M. received financial support from Postdoctoral Fulbright/Spanish Ministry of Education grant programme.
References
- 1.Isbell FI, Polley HW, Wilsey BJ. 2009. Biodiversity, productivity and the temporal stability of productivity: patterns and processes. Ecol. Lett. 12, 443–451 10.1111/j.1461-0248.2009.01299.x (doi:10.1111/j.1461-0248.2009.01299.x) [DOI] [PubMed] [Google Scholar]
- 2.Tilman D, Wedin D, Knops J. 1996. Productivity and sustainability influenced by biodiversity in grassland ecosystems. Nature 379, 718–720 10.1038/379718a0 (doi:10.1038/379718a0) [DOI] [Google Scholar]
- 3.Haddad NM, Crutsinger GM, Gross K, Haarstad J, Knops JMH, Tilman D. 2009. Plant species loss decreases arthropod diversity and shifts trophic structure. Ecol. Lett. 12, 1029–1039 10.1111/j.1461-0248.2009.01356.x (doi:10.1111/j.1461-0248.2009.01356.x) [DOI] [PubMed] [Google Scholar]
- 4.Haddad NM, Crutsinger GM, Gross K, Haarstad J, Tilman D. 2011. Plant diversity and the stability of foodwebs. Ecol. Lett. 14, 42–46 10.1111/j.1461-0248.2010.01548.x (doi:10.1111/j.1461-0248.2010.01548.x) [DOI] [PubMed] [Google Scholar]
- 5.Crutsinger GM, Collins MD, Fordyce JA, Gompert Z, Nice CC, Sanders NJ. 2006. Plant genotypic diversity predicts community structure and governs an ecosystem process. Science 313, 966–968 10.1126/science.1128326 (doi:10.1126/science.1128326) [DOI] [PubMed] [Google Scholar]
- 6.Johnson MT, Lajeunesse MJ, Agrawal AA. 2006. Additive and interactive effects of plant genotypic diversity on arthropod communities and plant fitness. Ecol. Lett. 9, 24–34 10.1111/j.1461-0248.2005.00833.x (doi:10.1111/j.1461-0248.2005.00833.x) [DOI] [PubMed] [Google Scholar]
- 7.Cook-Patton SC, McArt SH, Parachnowitsch AL, Thaler JS, Agrawal AA. 2011. A direct comparison of the consequences of plant genotypic and species diversity on communities and ecosystem function. Ecology 92, 915–923 10.1890/10-0999.1 (doi:10.1890/10-0999.1) [DOI] [PubMed] [Google Scholar]
- 8.Castagneyrol B, Lagache L, Giffard B, Kremer A, Jactel H. 2012. Genetic diversity increases insect herbivory on oak saplings. PLoS ONE 7, e44247. 10.1371/journal.pone.0044247 (doi:10.1371/journal.pone.0044247) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Utsumi S, Ando Y, Craig TP, Ohgushi T. 2011. Plant genotypic diversity increases population size of a herbivorous insect. Proc. R. Soc. B 278, 3108–3115 10.1098/rspb.2011.0239 (doi:10.1098/rspb.2011.0239) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moreira X, Mooney KA, Zas R, Sampedro L. 2012. Bottom-up effects of host-plant species diversity and top-down effects of ants interactively increase plant performance. Proc. R. Soc. B 279, 4464–4472 10.1098/rspb.2012.0893 (doi:10.1098/rspb.2012.0893) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mooney KA, Singer MS. 2012. Plant effects on herbivore–enemy interactions in natural systems. In Trait-mediated indirect interactions: ecological and evolutionary perspectives (eds Ohgushi T, Schmitz O, Holt RD.), pp. 107–130 Cambridge, UK: Cambridge University Press [Google Scholar]
- 12.Mooney KA, Agrawal AA. 2008. Plant genotype shapes ant-aphid interactions: implications for community structure and indirect plant defense. Am. Nat. 171, E195–E205 10.1086/587758 (doi:10.1086/587758) [DOI] [PubMed] [Google Scholar]
- 13.Mooney KA, Pratt R, Singer MC. 2012. The tri-trophic interactions hypothesis: interactive effects of host plant quality, diet breadth and natural enemies on herbivores. PLoS ONE 7, e34403. 10.1371/journal.pone.0034403 (doi:10.1371/journal.pone.0034403) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loreau M, Hector A. 2001. Partitioning selection and complementarity in biodiversity experiments. Nature 412, 72–76 10.1038/35083573 (doi:10.1038/35083573) [DOI] [PubMed] [Google Scholar]
- 15.Glinwood R, Ahmed E, Qvarfordt E, Ninkovic V, Pettersson J. 2009. Airborne interactions between undamaged plants of different cultivars affect insect herbivores and natural enemies. Arthropod Plant Interact. 3, 215–224 10.1007/s11829-009-9072-9 (doi:10.1007/s11829-009-9072-9) [DOI] [Google Scholar]


