Abstract
The northern clingfish, Gobiesox maeandricus, is able to adhere to slippery, fouled and irregular surfaces in the marine intertidal environment. We have found that the fish can adhere equally well to surfaces with a broad range of surface roughness, from the finest sandpaper (Ra = 15 µm) to textures suitable for removing finish from flooring (Ra = 269 µm). The fishes outperform man-made suction cups, which only adhere to the smoothest surfaces. The adhesive forces of clingfish correspond to pressures 0.2–0.5 atm below ambient and are 80–230 times the body weight of the fish. The tenacity appears related to hierarchically structured microvilli around the edges of the adhesive disc that are similar in size and aspect ratio to the setae found on the feet of geckoes, spiders and insects. This points to a possible biomimetic solution to the problem of reversibly adhering to irregular, submerged surfaces.
Keywords: Gobiesox maeandricus, hierarchical microstructure, rough surfaces, papillae
1. Introduction
The rocky intertidal is an extreme environment with high, variable forces from crashing waves and strong water currents. In near shore environments worldwide, a family of fishes (Gobiesocidae) has evolved an adhesive disc that allows them to adhere to rocks and even launch predatory attacks on attached molluscs. The adhesive event is fast, reversible and works on rugose surfaces fouled by algae and encrusting organisms. This group of fishes offers an unusual opportunity to understand the functional principles behind a reversibly adhesive disc capable of strong tenacity despite irregular, slippery and wet surfaces [1].
We examined the performance and morphology of the northern clingfish, Gobiesox maeandricus, a small (16 cm) species found commonly in the Pacific northwest of the United States. The adhesive disc with which the fish attaches to the substrate includes elements of the pectoral and pelvic girdles. The attachment organ is roughly circular with two posterolateral vents and a fimbriate edge. Unlike smooth-surfaced manufactured suction cups, this disc appears rough, with many small papillae evident to the naked eye [2]. When attaching to a surface, the fish rocks its pelvic girdle, forcing water out from under the disc and creating an area of sub-ambient pressure [3].
2. Material and methods
(a). Specimens
Twenty-two northern clingfish were caught in the rocky intertidal of San Juan Island, Washington, USA. The fishes were euthanized, weighed and photographed for later measurement of length and suction disc area. We also tested eight manufactured suction cups of different sizes. The surface area of the adhesive discs and the suction cups was measured from digital images in ImageJ (NIH, available at http://rsbweb.nih.gov/ij/).
(b). Surface generation
We created eight surfaces with different roughness. To isolate the effect of surface texture on adhesion, we removed confounding effects of material stiffness, wettability, surface chemistry and temperature by making moulds and casting the surfaces in resin [4]. We made dental wax (Coltene President light body) moulds of sandpapers with seven different grit sizes (Buehler Carbimet 2 assorted grits; table 1) that were then cast with an epoxy resin (SPI Supplies Low Viscosity Spurr Kit; [5]). The resin casts were baked at 70°C for 24 h. An eighth surface was cast using a mould made from glass, which is smooth at the nanometre scale. The cast surfaces were no smaller than 70 × 40 mm (much larger than the largest sucker discs) and were glued to the bottom of small watertight aquaria.
Table 1.
Successful adhesion of clingfish and manufactured suction cups to rough surfaces in fluids of different viscosity.
| medium (viscosity) | surface roughness (µm) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 15.3 | 21.8 | 35.0 | 52.2 | 78.0 | 127.0 | 269.0 | ||
| clingfish | sea water (1 cP) | √ | √ | √ | √ | √ | √ | √ | √ |
| manufactured suction cup | sea water (1 cP) | √ | √ | √ | — | — | — | — | — |
| methylcellulose (20 cP) | √ | √ | √ | — | — | — | — | — | |
| glycerine (1400 cP) | √ | √ | √ | √ | √ | √ | √a | — | |
aOnly five out of eight specimens adhered successfully.
(c). Force measurements
We measured maximum adhesive force with an MTS Synergie 100 materials testing machine at a pulling speed of 1 m min−1. It has previously been established that dead clingfish retain substantial suction tenacity (96% of that for live fishes) when tested in a less sophisticated, but similar rapid pull-off manner, so our experiments were all conducted on cadaveric material [3]. We attached freshly euthanized fish to the materials testing machine with suture thread that looped under the vertebral column near the caudal end of the suction disc and through the opercular gill openings. Manufactured suction cups were tested by looping a thread around their knobs (n = 6) or by clamping them directly to the materials testing machine (n = 2).
We filled the test aquaria with sea water to a depth of 5 cm and preconditioned each specimen with three adhesion events. The fish was depressed gently to evacuate water from under the disc (preliminary experiments showed that further pressure did not lead to larger forces). We generated a random order for the substrates for each specimen and this order was then used four times. We used only the highest load for a particular specimen–substrate pair. Maximum performance represents a lower bound on live fish performance, whereas the mean or range of performance of the dead fish will not be equivalent to any parameter of live fish because of variation owing to behaviour and motivation [6]. If a specimen became detached less than 30 s before a pulling force was applied by hand, the specimen–substrate pair was recorded as 0 N and not tested using the MTS. We refer to the adhesive force as ‘suction’ force because when a small hole was drilled in the substrate, adhesion was not measurable with the MTS system. Even dead, these fish were able to maintain adhesion on many of the smoother surfaces for an hour or more.
To determine the extent of the effect of viscosity on adhesion of commercial suction cups, we also performed experiments in liquids with two different viscosities: (i) a methylcellulose–sea water solution with a viscosity of 20 centipoise (cP) and (ii) 99 per cent glycerine with a viscosity of 1400 cP.
(d). Stress calculations
Sea water with impurities and microbubbles is not likely to bear a tensile load [7] and so maximum suction adhesion (F) depends on surface area of the suction cup (A) and the ambient pressure outside the suction cup (p):
| 2.1 |
To account for size differences, we compared the tensile stresses at adhesive failure based on equation (2.1):
| 2.2 |
pad stands for the tensile stress and Fad is the measured adhesive force.
(e). Statistics
Statistical analyses were calculated with R v. 2.13.1 (http://www.r-project.org/). We applied a two-way ANOVA with surface roughness and specimen type as independent variables and tensile stress as the dependant variable. We calculated one-way ANOVAs for the effect of surface roughness on adhesive stress in clingfish and suction cups, and performed a Tukey honestly significant difference (HSD) test.
(f). Imaging
We used scanning electron microscopy (SEM) to study the epithelial microstructure of the adhesive disc. Specimens were either prepared by dehydration with ethanol and hexamethyldisilazane and examined with a NeoScope JCM 5000 tabletop SEM or were studied with a Hitachi S-4800 SEM equipped with a Gatan ALTO 2500 cryo-preparation stage.
3. Results
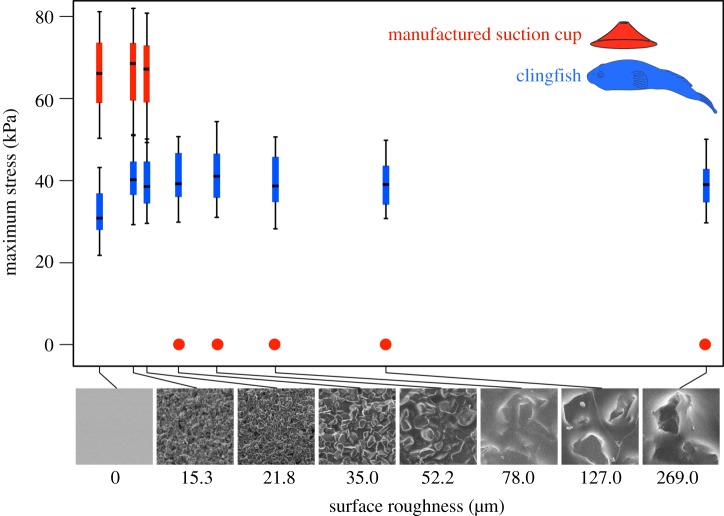
Clingfish ranged in standard length from 70 to 120 mm, in adhesive disc area from 3.9 to 12.3 cm2 and in mass from 8.2 to 42 g. Suction cups ranged in disc area from 3.6 to 15.2 cm2. We found that clingfishes can adhere equally well to all but the smoothest surface (figure 1; Tukey HSD, p > 0.98) where peak adhesive stress was significantly lower (figure 1; Tukey HSD, p < 0.0023). The force of clingfish adhesion varied from 80 to 230 times body weight, whereas peak stress ranged from 20 to 50 kPa. Manufactured suction cups showed 80 per cent higher average peak stress than clingfish (figure 1; two-way ANOVA, d.f. = 1, F-value = 304.1, p < 0.001) on the three smoothest surfaces (Ra = 0, 15.3 and 21.8 µm) but failed to adhere to the five surfaces with grit sizes of more than 21.8 µm.
Figure 1.
Plots of maximum adhesive stress for clingfishes (blue) and manufactured suction cups (red) on surfaces with different roughness in sea water. These boxplots show the distribution of force values; middle marks indicate medians, and each section of box and whisker indicates a quartile (25%) of the data. Suction cups only adhere to surfaces smoother than 35 µm. Clingfishes stick equally well across the entire range of roughness except on the nanoscale smooth surface where adhesion is slightly weaker (p < 0.05).
To simulate the effect of fish mucus, we repeated peak stress measurements for manufactured suction cups using viscous liquids. A 20-fold increase in viscosity, matching fish mucus [8], did not help commercial suction cups adhere to rougher surfaces. A 1400-fold increase in viscosity allowed commercial suction cups to adhere to more surfaces (up to Ra = 127 µm) but they still failed on the roughest surface (Ra = 269 µm; table 1). On the smooth surfaces that suction cups always adhere to (Ra = 0, 15.3 and 21.8 µm) there was no statistical difference in adhesive stress when tested in different viscosities (Tukey HSD test, p > 0.99).
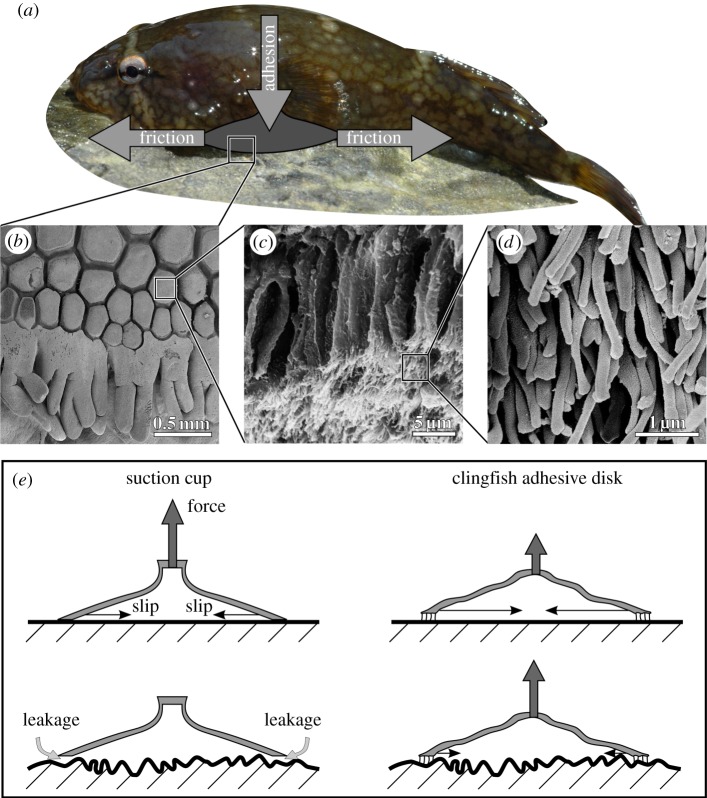
SEM of the clingfish adhesive disc revealed that the papillae on the ventral face of the suction disc are arranged as a tiled surface with narrow channels between them. When the mucus was removed, these papillae were identified as a hierarchically structured material. The papillae are subdivided into tightly packed rods with an aspect ratio of approximately 1 : 10 and a height of 15 µm. Each rod is then apically subdivided into cylindrical filaments approximately 3 µm long and 0.2 µm in diameter (figure 2b–d).
Figure 2.
Effectiveness of the clingfish adhesive disc. (a) A clingfish adhering to a rock surface. Two types of forces act on the clingfish adhesive disc. (i) Adhesion acts normal to the surface and holds the fish to the rock; and (ii) friction acts parallel to the surface and prevents sliding of the disc along the substrate. (b) SEM of the ventral surface of the adhesive disc showing the tiled papillae covered in mucus and the fimbriate edge of the adhesive disc. Papillae occur at the edges of the suction disc and other points of surface contact. (c) SEM of a papilla, consisting of multiple rods subdivided apically into fine filaments. (d) SEM of the filaments on the tips of the rod-like structures. (e) Schematic cross sections through a manufactured suction cup and a clingfish adhesive disc. Pulling on a suction cup causes its sides to slip inward. The filaments on the disc of clingfish cause higher friction and prevent slipping on rough surfaces. The flexibility of the disc and its filaments allow for sealing on rough surfaces, whereas the stiff manufactured suction cups fail owing to leakage.
4. Discussion
Using hierarchically structured microvilli on the ventral surface of the adhesive disc, clingfishes adhere equally well to a diversity of surfaces that differ greatly in roughness. The microvilli of clingfish have diameters around 0.2 µm, which are similar in size to the adhesive setae of salticid spiders (1 µm) and geckos (0.29 µm) and provide a striking case of convergent evolution [9,10]. It is unlikely that van der Waals forces play a substantial role because of the difficulty of removing water and mucus from between the microvilli and the substrate. Mucus, however, may help edges of the disc to conform to irregularities and maintain suction or viscous adhesion. In addition, we suggest the hierarchical structure and low stiffness of the disc allows the edges of the suction disc to interdigitate with the asperities of a rugose surface [11–13]. This interdigitation increases friction at the edge of the disc over surface irregularities, allowing exceptional adhesive performance on rough substrates.
This view is supported by the surprising finding that the poorest performance is on the nanoscale smooth surface. Friction plays an important role in the clingfish system; when a suction cup fails, the edges slide towards the centre of the cup, eventually buckling inwards and causing failure (figure 2e). On the smoothest surface, friction is reduced, causing the adhesive disc of the clingfish to fail at lower tensile stress. Because rubber manufactured suction cups may be built from stiffer materials than the clingfish disc, the edges do not slide towards the centre as easily.
The presence of a viscous fluid increases suction performance by increasing Stefan adhesion, which scales with viscosity [14]. The more viscous a liquid medium is, the more force required to pull it through gaps between the suction cup and surface asperities, allowing a poorer fit between suction cup and substrate to function with adequate tenacity. But mucus driven viscosity cannot explain the high performance of the clingfish relative to manufactured suction devices because, even in a medium with a viscosity 70 times that of fish mucus, suction cups could not adhere to the roughest surfaces. This fact, and the persistence of tenacity over 30 trials from a single fish indicate that suction performance depends more on disc microstructure than on mucus.
The measured adhesive stresses are 20–50% of the maximum suction adhesion that is possible at atmospheric pressure (approx. 101 kPa). The adhesive disc skeleton in clingfishes is connected to the pectoral girdle by extensive musculature [3] and attachment to the substrate is an active process, e.g. by pulling the adhesive disc dorsally, volume is increased and pressure under the disc is lowered. Although our experiment was performed with dead animals, previous work predicts only slightly higher suction performance (about 4%, [3]) in live fishes.
Despite the similarities in structure of the microvilli, there are two notable differences between geckos and arthropods versus clingfish: (i) clingfish adhere under water and (ii) clingfish microvilli lack spatulate termini that increase apical flexibility and are important in van der Waals adhesion [9,15]. Spatulate termini are found in systems that support locomotion on smooth surfaces [15], and their absence in clingfishes might be explained by the static nature of the clingfish system. The clingfish performance data and morphology presented here suggest a potential biomimetic avenue for improving suction performance on rough surfaces, by mimicking a compliant, hierarchical surface at the edges of an attachment device.
Acknowledgements
This project was financially supported by an NSF REU (DBI-1004193) to D.K.W., the funding initiative in evolutionary biology of the Volkswagen foundation (I/84 206) to T.K. and the Seaver Institute and the NSF (IOS-1256602) to A.P.S.
References
- 1.Eschmeyer WN, Herald ES, Hammann H. 1983. A field guide to Pacific coast fishes of North America 1st edn Order Gobiesociformes (ed. Peterson RT.), pp. 108–109 Boston, MA: Houghton Mifflin Harcourt [Google Scholar]
- 2.Green DM, Barber DL. 1988. The ventral adhesive disc of the clingfish Gobiesox maeandricus: integumental structure and adhesive mechanisms. Can. J. Zool. 66, 1610–1619 10.1139/z88-235 (doi:10.1139/z88-235) [DOI] [Google Scholar]
- 3.Arita GS. 1967. A comparative study of the structure and function of the adhesive apparatus of the Cyclopteridae and Gobiesocidae, pp. 1–90 Vancouver, Canada: The University of British Columbia [Google Scholar]
- 4.Scherge M, Gorb SN. 2001. Biological micro- and nanotribology. Berlin, Germany: Springer [Google Scholar]
- 5.Spurr AR. 1969. A low-viscosity epoxy resin embedding medium for electron microscopy. J. Ultrastruct. Res. 26, 31–43 10.1016/S0022-5320(69)90033-1 (doi:10.1016/S0022-5320(69)90033-1) [DOI] [PubMed] [Google Scholar]
- 6.Herrel A, O'Reilly JC. 2006. Ontogenetic scaling of bite force in lizards and turtles. Physiol. Biochem. Zool. 79, 31–42 10.1086/498193 (doi:10.1086/498193) [DOI] [PubMed] [Google Scholar]
- 7.Smith AM. 1991. Negative pressure generated by octopus suckers: a study of the tensile strength of water in nature. J. Exp. Biol. 157, 257–2711684615 [Google Scholar]
- 8.Roberts S, Powell M. 2005. The viscosity and glycoprotein biochemistry of salmonid mucus varies with species, salinity and the presence of amoebic gill disease. J. Comp. Physiol. 175, 1–11 10.1007/s00360-005-0473-5 (doi:10.1007/s00360-005-0473-5) [DOI] [PubMed] [Google Scholar]
- 9.Autumn K, Liang Y, Hsieh S, Zesch W, Chan W, Kenny T, Fearing R, Full R. 2000. Adhesive force of a single gecko foot-hair. Nature 405, 681–685 10.1038/35015073 (doi:10.1038/35015073) [DOI] [PubMed] [Google Scholar]
- 10.Kesel A, Martin A, Seidl T. 2003. Adhesion measurements on the attachment devices of the jumping spider Evarcha arcuata. J. Exp. Biol. 206, 2733–2738 10.1242/jeb.00478 (doi:10.1242/jeb.00478) [DOI] [PubMed] [Google Scholar]
- 11.Filippov A, Popov VL, Gorb SN. 2011. Shear induced adhesion: contact mechanics of biological spatula-like attachment devices. J. Theor. Biol. 276, 126–131 10.1016/j.jtbi.2011.01.049 (doi:10.1016/j.jtbi.2011.01.049) [DOI] [PubMed] [Google Scholar]
- 12.Persson B. 2003. On the mechanism of adhesion in biological systems. J. Chem. Phys. 118, 7614. 10.1063/1.1562192 (doi:10.1063/1.1562192) [DOI] [Google Scholar]
- 13.Persson B, Gorb SN. 2003. The effect of surface roughness on the adhesion of elastic plates with application to biological systems. J. Chem. Phys. 119, 11 437–11 444 10.1063/1.1621854 (doi:10.1063/1.1621854) [DOI] [Google Scholar]
- 14.Denny MW. 1988. Biology and the mechanics of the wave-swept environment. Princeton, NJ: Princeton University Press [Google Scholar]
- 15.Gorb SN, Varenberg M. 2007. Mushroom-shaped geometry of contact elements in biological adhesive systems. J. Adhes. Sci. Technol. 21, 1175–1183 10.1163/156856107782328317 (doi:10.1163/156856107782328317) [DOI] [Google Scholar]